Cap-proximal nucleotides via differential eIF4E binding and alternative promoter usage mediate translational response to energy stress
Figures
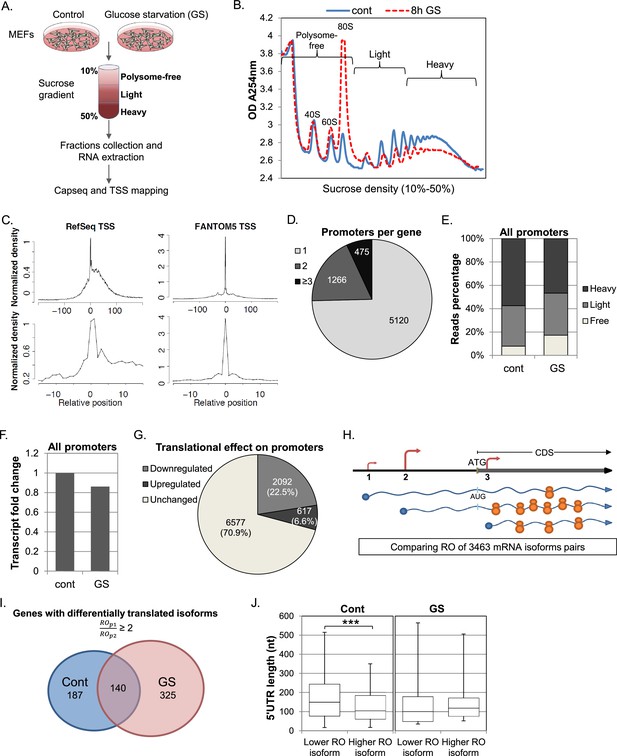
Experimental design and general analysis of the impact of TSS selection on translation efficiency.
(A) A schematic flowchart of the biological experiment and sample preparation for the CapSeq analysis. (B) Polysomal profiling of MEF cells subjected to glucose starvation (GS) for 8 hr (dashed red) or untreated (blue). (C) Metagene analysis of CapSeq reads relative to the annotated TSS of Refseq and the summit of FANTOM5 TSSs at low and high resolutions. Only TSS regions (−200..200) with at least ten bases covered by reads were considered, and the coverage in each region was normalized to mean zero and standard deviation of one. The normalized coverage was then summed across all regions. (D) The relative distribution of genes with the indicated number of promoters per gene. (E) The relative global sum of reads for all promoters (9286 promoters with >500 reads) in the polysome-free, light and heavy polysomal fractions in basal (cont) and GS conditions. The presented data are the mean of two independent replicates. (F) The fold change of the global mRNA levels between the basal (cont) and GS conditions. The presented data are the mean of two independent replicates. (G) The number and percentage of promoters translationally affected by GS. Promoters that had ribosome occupancy (RO) change of two-fold or more in both repeats were considered affected. (H) A scheme demonstrating the differential translation and transcription of transcripts with alternative TSSs/promoters. The TSSs are shown as arrows and the size of the arrow denotes the strength of the TSS relative to other TSSs of the same gene. The number of ribosomes occupying each mRNA represents the extent of its translation. (I) Promoters from the same gene were paired and their ROs were compared. Pairs of promoters that had an RO difference of two-fold or more in control or GS conditions in both repeats, independently, were considered as differentially translated promoters. The numbers of genes with at least one pair of differentially translated isoforms in control and GS conditions in both repeats are presented in a Venn diagram. (J) Boxplot presentation of the distributions of the 5′UTR lengths of differentially translated isoforms in each promoter pair (as presented in I) in control and GS conditions. The bottom and the top whiskers represent 5% and 95% of the distribution, respectively.
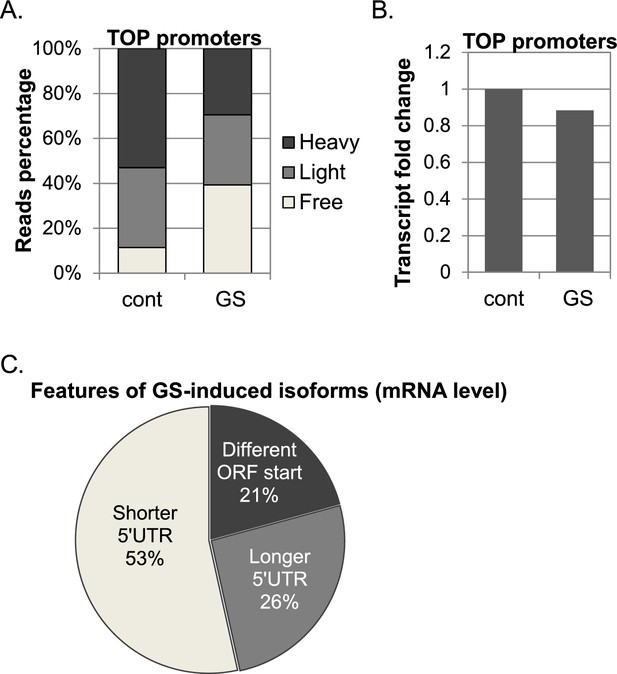
The response 5’TOP promoters to GS.
(A) The cumulative CapSeq reads of 414 identified promoters with TSS TOP element in the polysome-free, light and heavy fractions in basal (cont) and GS conditions. The presented data are the mean of two independent CapSeq replicates. (B) The change of the mRNA levels of TOP promoters in basal (cont) and GS conditions. The presented data are the mean of two independent CapSeq replicates. (C) The impact of the GS-induced isoforms on 5’UTR length and ORF start.
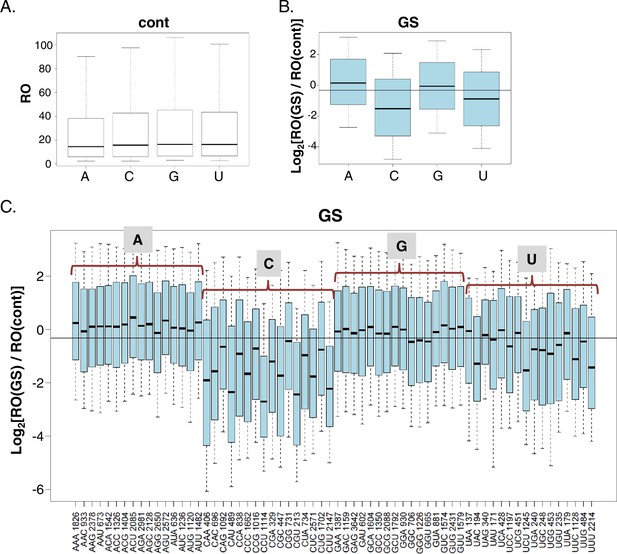
The impact of the TSS nucleotides on mRNA translation.
(A) The RO distributions of transcripts that initiate with the indicated nucleotide in basal conditions. (B) The distribution of the RO effect (log transformed) of transcripts that initiate with the indicated nucleotide. The horizontal line indicates the overall median value. (C) The effect of GS on the RO for each initiating trinucleotide. The horizontal line indicates the overall median value. All the data presented in this figure are the mean of the two independent replicates. The bottom and the top whiskers represent 5% and 95% of the distribution, respectively. The number of TSSs starting with the indicated trinucleotide is indicated near each of the trinucleotide sequence.
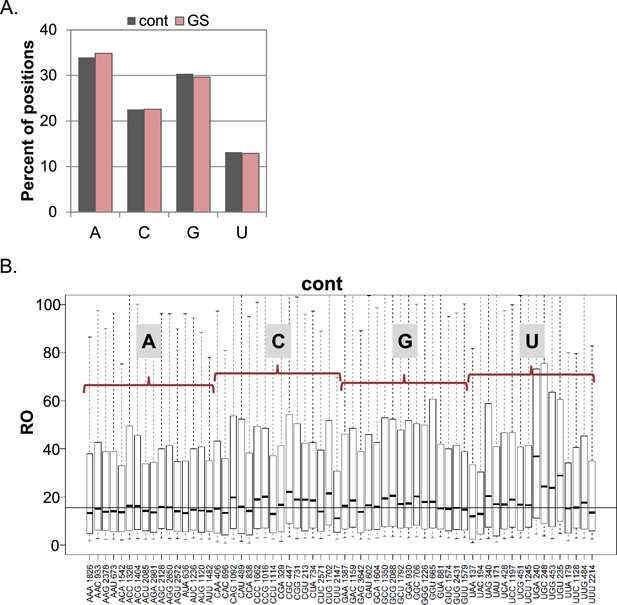
The frequency of the initiating nucleotides and their impact on basal translation.
(A) The percentage of each initiating nucleotide position as non-redundant TSSs in the CapSeq analysis in control and GS as indicated. (B) Boxplot presentation of the RO of each initiating trinucleotide in basal conditions. The horizontal line indicates the overall median value. The bottom and the top whiskers represent 5–95% of the population, respectively. The presented data are the mean of the two CapSeq experiments.
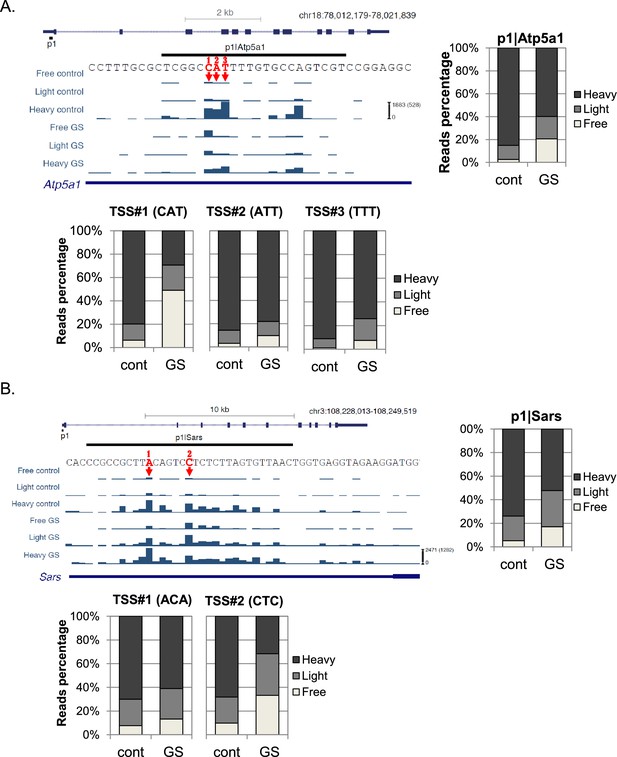
Examples of the effect of the first nucleotide on translation efficiency.
(A, B) Upper left panel – The chromosomal location, genomic structure and CapSeq data of the indicated genes in each fraction in control and GS conditions. The scale of the normalized and row reads (in parentheses) is shown. The nucleotide sequence of the major alternative TSSs within the promoter are marked by arrows and numbered. The upper right panel shows the relative levels of all reads for the indicated promoter and the lower panels for the designated specific TSSs in basal (cont) and GS conditions. The presented data are the mean of the two independent replicates.
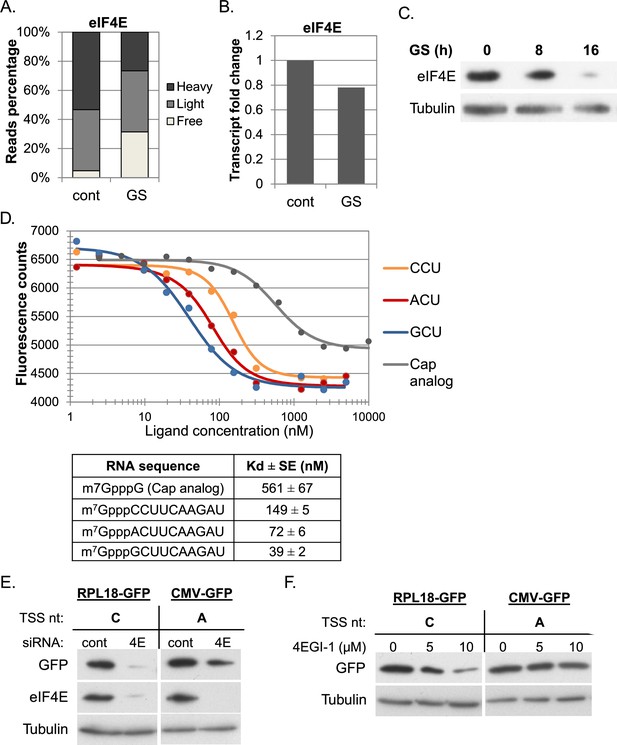
The effect of cap-proximal nucleotides on eIF4E binding affinity and activity.
(A) The relative level of eIF4E reads (mean of two independent replicates) in the indicated fractions in basal (cont) and GS conditions. (B) The relative mRNA levels (mean of two independent replicates) of eIF4E in basal (cont) and GS conditions. (C) Representative immunoblot of total lysate using eIF4E and Tubulin antibodies following GS of MEFs for the indicated times. (D) Upper panel – the change in the intrinsic fluorescence of eIF4E (300 nM) in response to increasing concentrations of capped RNA ligands (1.25 nM–5 μM) or cap analog (1.25 nM–10 μM). The graphs represent the mean of three independent experiments with two different protein preparations. The bottom panel shows the calculated dissociation constant values (Kd) of eIF4E binding affinity to the indicated RNA ligands. (E) The effect of eIF4E knockdown on GFP expression driven by mRNA with C or A as the initiating nucleotides. HEK293T cells were transfected with either eIF4E siRNA or a non-targeting siRNA (10 nM). 48 hr later cells were transfected again with GFP reporter genes driven either by RPL18 (C) or CMV (A) promoter. Cells were harvested 24 hr after the second transfection and analyzed by western blot with GFP, eIF4E and Tubulin antibodies as indicated. (F) HEK293T cells were transfected RPL18 and CMV driven GFP reporter gene. Six hours later, increasing amounts of 4EGI-1 were added to the media as indicated. Cells were harvested 24 hr after transfection and subjected to western blot using GFP and Tubulin antibodies.
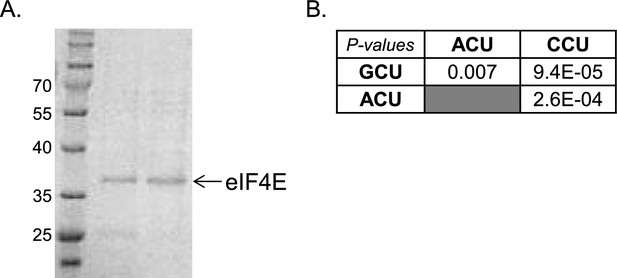
Complementary data for the analysis of eIF4E binding affinity.
(A) The purified eIF4E protein used for the binding studies was subjected to 10% SDS-PAGE and Coomassie blue staining. (B) The p-values of the differences in binding affinities between the indicated capped RNAs that differ by their first nucleotide.
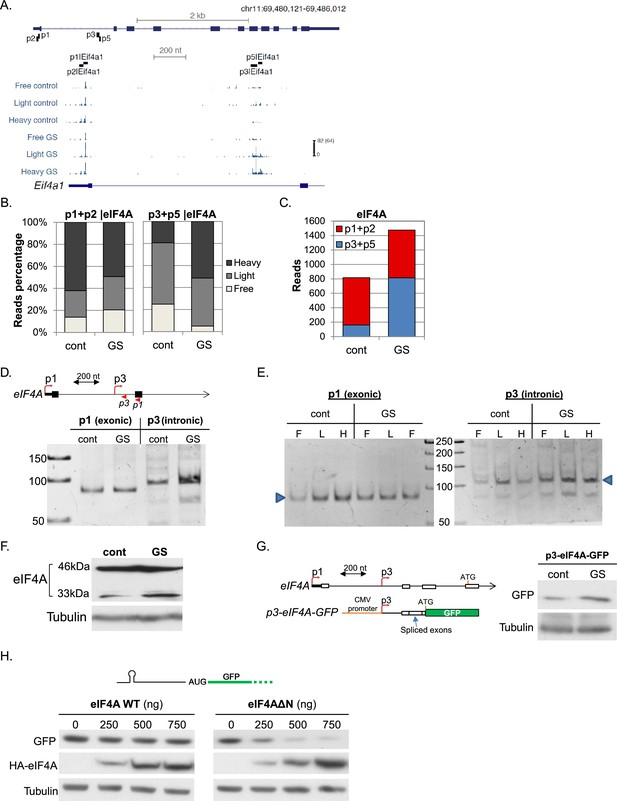
Characterization of the GS-induced AP of eIF4A.
(A) The chromosomal location, genomic structure and CapSeq data of eIF4A in each fraction in basal (cont) and GS conditions (uniquely mapped reads). The scale of the normalized and row reads (in parentheses) is shown. The positions of the FANTOM5 promoters are also indicated. (B) The relative levels of the reads from the indicated promoters (mean of two independent replicates) in the three polysomal fractions in control and GS conditions. (C) The number of CapSeq reads of the indicated promoters in control and GS conditions (mean of two independent replicates). (D) Analysis of the p1 and p3 promoters of eIF4A by 5’RACE. Upper panel shows schematic presentation of the relevant eIF4A genomic region, the positions of the analyzed promoters and the 5’RACE reverse primers (shown by arrowheads). The lower panel is the analysis of the 5’RACE PCR products by 6% PAGE. (E) Analysis of p1 and p3 transcript isoforms levels by 5’RACE in the indicated fractions of the polysome profile of control and glucose starved cells. (F) Representative immunoblot of total lysate of MEFs with eIF4A (C-terminal epitope) and Tubulin antibodies in control and GS. (G) The 5’UTR of the eIF4A from the p3 TSS to Met121 was cloned downstream of the CMV promoter and upstream of the GFP reporter gene as shown in the scheme. This construct was transfected into MEFs, which were then subjected to GS. Expression of GFP was monitored by western blot with GFP antibody. (H) Representative immunoblot of HEK293T cells that were co-transfected with GFP reporter having hairpin structure within the 5’UTR together with increasing amounts of WT or ΔN-eIF4A as indicated.
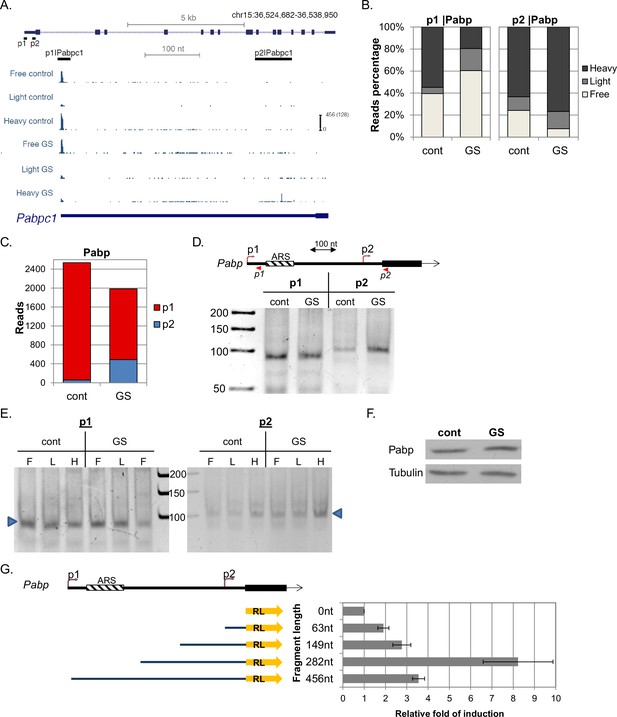
Characterization of Pabp APs.
(A) The chromosomal location, genomic structure and CapSeq data of Pabp in each fraction in basal (cont) and GS conditions. The scale of the normalized and row reads (in parentheses) is shown. The positions of the FANTOM5 promoters are also shown. (B) The relative levels of the indicated promoter reads of the polysomal fractions in control and GS conditions (mean of two independent replicates). (C) The total CapSeq reads of the indicated promoters in control and GS conditions (mean of two independent replicates). (D) 5’RACE analysis of p1 and p2 promoters of Pabp. Upper panel shows a schematic presentation of Pabp region containing the p1 and p2 promoters, their positions, 5’RACE reverse primers (shown as arrowheads) and adenine-rich autoregulatory sequences (ARSs). The lower panel is the analysis of the 5’RACE PCR products by 6% PAGE. (E) Analysis of p1 and p2 transcript isoforms levels by 5’RACE in the indicated fractions of the polysome profile of control and glucose starved cells. (F) Representative immunoblot of total lysate of MEFs with anti-Pabp and anti-Tubulin in control and GS conditions. (G) Characterization of Pabp p2 upstream region as a promoter. A scheme of p2 regulatory sequences of the indicated lengths (starting from the AUG) that were cloned upstream to a promoter-less Renilla luciferase (RL) reporter gene is shown on the left. These constructs were transfected into MEFs together Firefly luciferase reporter gene that served as an internal control. Renilla and Firefly luciferase activities were measured 24 hr after transfection. The results represent average ± SE of 4 transfection experiments.
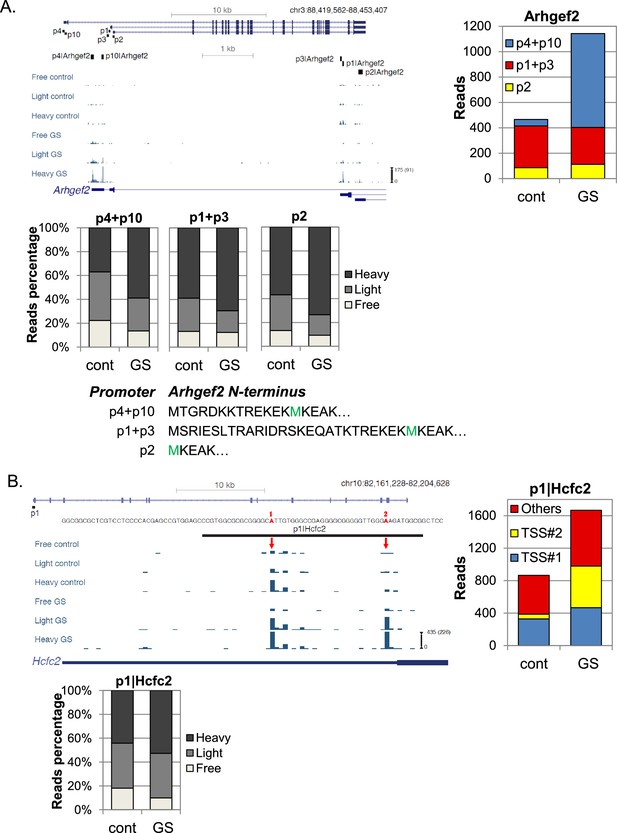
Examples of differential transcription and translation response to GS of APs.
(A, B) Upper left panel - The chromosomal location, genomic structure, the positions of the FANTOM5 promoters and CapSeq data of the indicated genes in each fraction in basal (cont) and GS conditions. The scale of the normalized and row reads (in parentheses) is shown. Upper right panel - The total number of CapSeq reads of the indicated promoters in cont and GS conditions. Lower panels - The relative CapSeq reads of the indicated APs/TSSs for the polysome-free, light and heavy polysomal fractions in basal (cont) and GS conditions. The presented data are the mean of the two independent replicates.
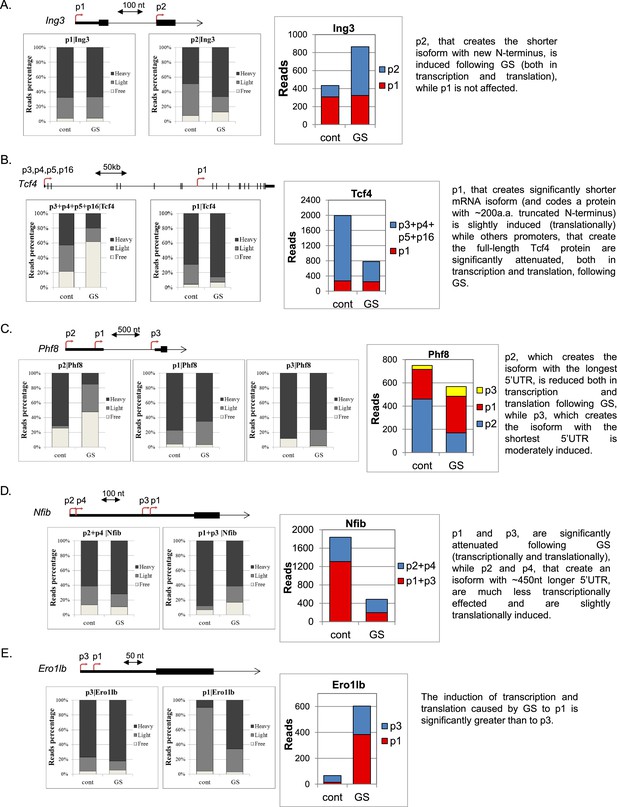
Additional examples of differential transcriptional and translational response to GS of APs.
(A–E) Upper panel – A schematic representation of the APs of the indicated genes and their locations. The CapSeq reads of the indicated APs of the polysome-free, light and heavy polysomal fractions in basal (cont) and GS conditions (mean of two independent replicates). Right panel - The CapSeq reads of the indicated promoters in control and GS conditions. Each example is accompanied by a brief summary of the expected outcome of the APs.
Additional files
-
Supplementary file 1
An Excel file containing gene lists corresponding to Figure 1 as indicated in the name of each sheet.
- https://doi.org/10.7554/eLife.21907.014
-
Supplementary file 2
A Java script for extraction the first base in each transcript.
- https://doi.org/10.7554/eLife.21907.015





