Control of PNG kinase, a key regulator of mRNA translation, is coupled to meiosis completion at egg activation
Figures
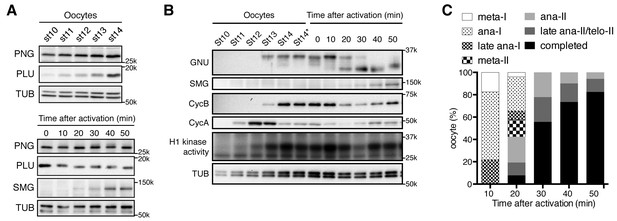
PAN GU (PNG) kinase activation coincides with GIANT NUCLEI (GNU) dephosphorylation after the completion of meiosis.
(A) Dynamics of PNG and PLU proteins during oocyte maturation and after egg activation. Protein levels of PNG and PLU in oocytes in the indicated stages of oocyte development and in in vitro activated oocytes at the indicated times after hypotonic buffer treatment (activation) were examined by immunoblotting. Alpha Tubulin (TUB) was examined as a loading control. SMAUG (SMG) was examined as the indicator of PNG activation. (B) Dynamics of GNU and mitotic Cyclin proteins during oocyte maturation and after egg activation. GNU and the mitotic CycA and B in oocytes at the indicated stages of oocyte development and in in vitro activated oocytes at the indicated times after hypotonic buffer treatment (activation) were examined by immunoblotting. Total CDK1 activity was measured as H1 kinase activity. Alpha Tubulin (TUB) was examined as a loading control. SMG was examined as the indicator of PNG activation. Stage 14 oocytes before or after being held in the female and thus dehydrated (St14, St14*) were examined (Mahowald et al., 1979), because held stage 14 oocytes were used for in vitro egg activation (0 min; see Materials and methods). (C) Meiotic progression in in vitro activated oocytes. In vitro activated oocytes were fixed at specific time points after hypotonic buffer treatment (activation), the DNA stained with DAPI, and the meiotic stage in the activated oocytes was determined by DNA morphology and quantified. All of the results shown were repeated in two biological replicates except the PLU immunoblot in the bottom of panel 1A, which was done one time.
-
Figure 1—source data 1
Timing of each stage of meiosis following egg activation.
- https://doi.org/10.7554/eLife.22219.004

twine is required for accumulation of PNG kinase complex component proteins in oocytes.
PNG, PLU and GNU protein levels in stage 14 oocytes (st14) from control twineHB5/CyO (twine/+) or mutant twineHB5/ twineHB5 (twine/ twine) females were examined by immunoblotting. CycB protein levels were unchanged as previously reported (Von Stetina et al., 2008). Alpha tubulin (TUB) was used as a loading control. Although some amount of GNU in st14 oocytes showed a faster mobility, this arose due to dephoshorylation during sample preparation (see Figure 1—figure supplement 2). There was one biological replicate of this immunoblot.
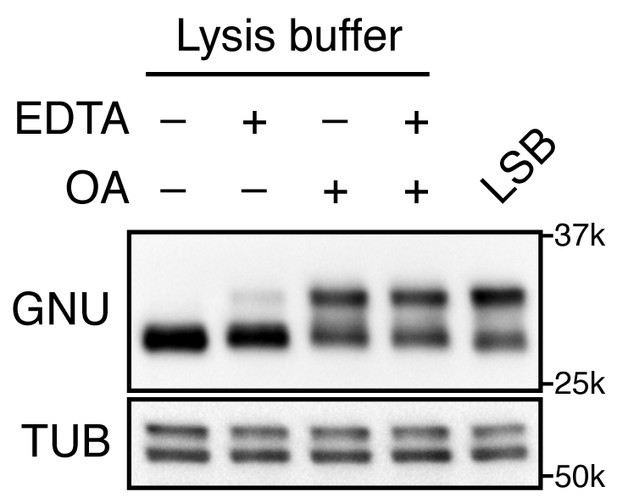
GNU mobility shift is sensitive to phosphatase activity.
Stage 14 oocytes were lysed in a native lysis buffer in the presence or absence of EDTA and/or okadaic acid (OA). The mobility shift of GNU was examined by immunoblotting. Stage 14 oocytes lysed in LSB were the positive control for the mobility shifts, although GNU was slightly dephosphorylated under the lysate preparation conditions. Alpha tubulin (TUB) was used as a loading control. This exact experiment was done once, although the phosphatase sensitivity of GNU has been shown in many experiments.
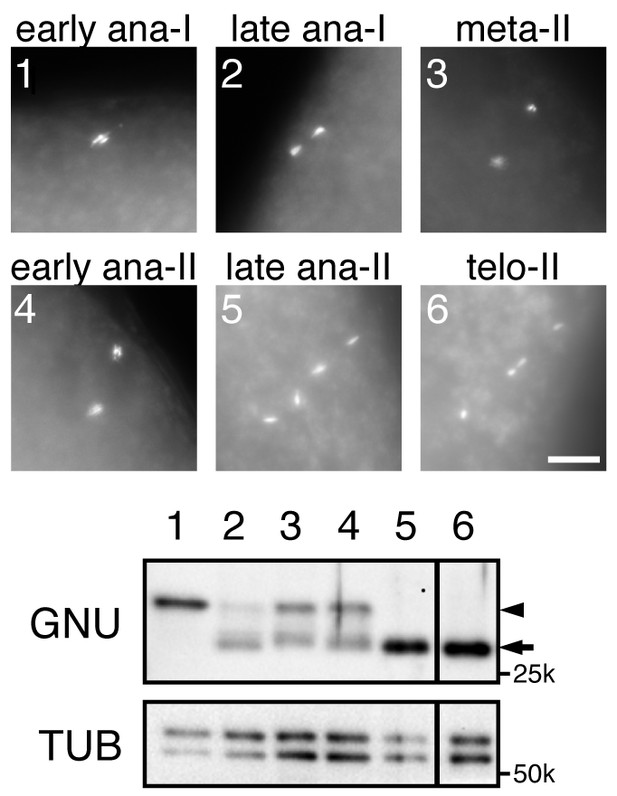
The hyperphosphorylated form of GNU disappears at the end of meiosis II.
In vitro activated oocytes were fixed, the DNA stained with Hoechst 33342, and their meiotic stage was determined by fluorescence microscopy. Individual staged oocytes were lysed and examined by immunoblotting for GNU. The numbers in the Hoechst 33342 staining panels correspond to the lane numbers in the immunoblot. Alpha Tubulin (TUB) was examined as a loading control. The arrowhead and arrow indicate the hyper-phosphorylated form and the hypophosphorylated form of GNU, respectively. The presence of some hypophosphorylated GNU in lanes 2–4 is a consequence of it not being possible to prepare lysates adequately rapidly on isolated activated eggs. This experiment was repeated in two biological replicates.
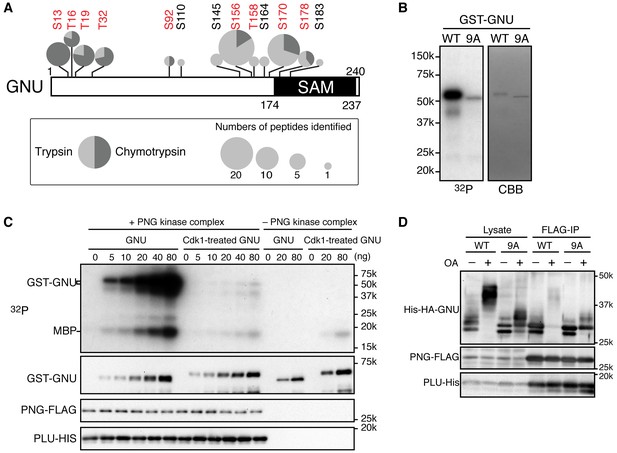
Cyclin B (CycB)/CDK1 phosphorylates GNU and in vitro inhibits its ability to activate PNG kinase.
(A) A schematic representation of GNU protein and the phosphorylated residues identified in st14 oocytes. Overall coverage was 88%. In total, 93 phosphopeptides from tryptic and chymotryptic digests were assigned with >90% certainty based on Ascore. 62 out of the 93 phosphopeptides were with >99% certainty (see also supplemental figure). The region between amino acids 116 and 185 was poorly covered in the chymotrypsin analysis (supplement MS data). Phosphorylation sites in red match the S/T-P motif and were considered in further experiments. The size of the pie charts shows numbers of phosphopeptides assigned with >90% certainty. The light and dark gray indicate tryptic and chymotryptic peptides, respectively. The sterile alpha motif (SAM) in the C terminus of the protein is shown. (B) CDK1 directly phosphorylates GNU. GST-GNU WT or a mutant with alanine substitution of the nine S/T-P sites (9A) shown in red in (A) were incubated with purified active CycB/CDK1 in the presence of radioactive ATP. Phosphorylation of GST-GNU WT or 9A was detected by autoradiography (left panel, 32P). GST-GNU protein levels were examined by coomassie staining (right panel, CBB). This experiment was done twice. (C) CDK1 phosphorylation inhibits the ability of GNU to activate the PNG kinase. PNG kinase activity was measured by phosphorylation of myelin basic protein (MBP) (upper panel, 32P). GST-GNU treated or untreated with CycB/CDK1 was added into the kinase reaction in the nanogram (ng) amounts indicated. Note that GNU is also phosphorylated by PNG kinase. GST-GNU was incubated with MBP in the absence of PNG kinase complex as a negative control. The protein levels of the PNG kinase complex components in the reaction were examined by immunoblotting, confirming the phosphorylation state of GNU (bottom panels). (D) Phosphorylation at CDK1 sites in GNU prevents the interaction between GNU and the PNG-PLU subcomplex. Wild-type GNU binding to the PNG-PLU subcomplex was inhibited in okadaic acid (OA) -treated Sf9 cells, but alanine substitution in the nine S/T-P sites (9A) of GNU restores the binding. PNG-FLAG and PLU-His were expressed in Sf9 insect cells with His-HA-GNU WT or 9A (Lysate). The PNG complex expressing cells were incubated with or without OA, a PP2A inhibitor. PNG kinase complex formation was examined by immunoprecipitation of PNG-FLAG (FLAG-IP) followed by immunoblotting. Three biological replicates of this experiment were done.
-
Figure 2—source data 1
Phosphopeptides recovered by mass spec and their mass spec properties.
- https://doi.org/10.7554/eLife.22219.009
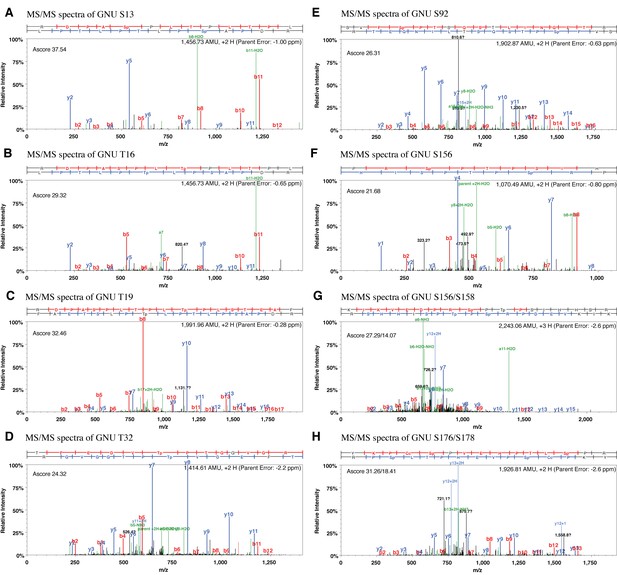
MS/MS spectra resulting from HCD fragmentation of phosphopeptides containing GNU.
Example spectra are shown that led to the identification of phosphorylated Ser13 m/z 1,456.732+ (A), Thr16 m/z 1,456.732+ (B), Thr19 m/z 1,991.962+ (C), Thr32 m/z 1,414.612+ (D), Ser92 m/z 1,902.872+ (E), Ser156 m/z 1,070.472+ (F), Ser156 and 158 2,243.062+ (G), Ser170and Ser178 m/z 1,926.812+ (H). The phosphopeptides recovered were _RDPAS(ph)PLTPLTPL_, _RDPASPLT(ph)PLTPL_, _RDPASPLTPLT(ph)PLSTEAF_, _TFEDVT(ph)PTGGVGR_, _SVFS(ph)PTSQSTLINGETR_, _PRS(ph)PTPSIH_ and _KIKVEDPRS(ph)PT(ph)PSIHHSR_. The ScaffoldPTM Viewer (version 3.0.0) was used to illustrate the spectra. The corresponding Ascore is indicated. An Ascore of 20 (p=0.01) should result in the site being localized with 99% certainty (Beausoleil et al., 2006).
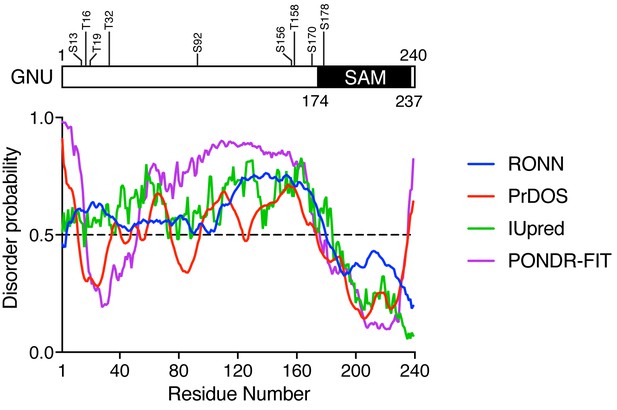
CDK1 phosphorylation sites of GNU are in a predicted disordered region.
GNU was analyzed for disordered regions using four prediction tools: RONN v3.2 (Yang et al., 2005), PrDOS (Ishida and Kinoshita, 2007), IUpred (Dosztányi et al., 2005) and PONDR-FIT (Xue et al., 2010). In contrast to the sterile alpha motif (SAM) in the C terminus of GNU, which is predicted to be ordered, the remaining protein appears disordered. The disordered region contains eight of the nine CDK phosphorylation sites.
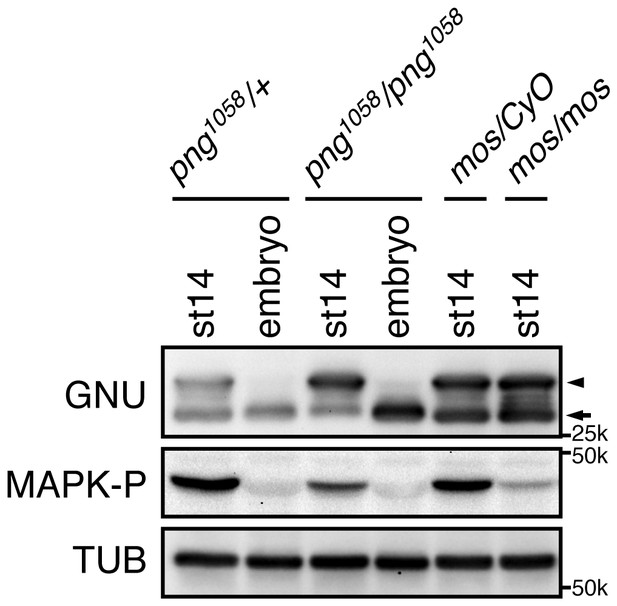
PNG and MAPK activity are dispensable for GNU hyperphosphorylation in mature oocytes.
Mature oocytes (st14) were isolated from females of the indicated genotypes: control png1058/FM6 (png1058/+), mutant png1058/png1058, control mos/CyO (mos/+) or mutant mos/mos. Fertilized embryos from png1058/+ or png1058/png1058 females were collected for 2 hr (embryo). GNU proteins in the st14 oocytes and the embryos were examined by immunoblotting. Phosphorylated MAPK (MAPK-P) shows the active form of MAPK. Alpha Tubulin (TUB) was examined as a loading control. The hyper-phosphorylated form of GNU (arrowhead) was seen in st14 oocytes in mutant png1058/png1058, and it was present in homozygous mos/mos mutants. MAPK activation has been shown to be defective in homozygous mos/mos oocytes but not in the mos/CyO sibling controls (Ivanovska et al., 2004). The dephosphorylated GNU in both mos/mos and mos/CyO control oocytes (arrow), most likely arose during sample preparation, as lysis was not sufficiently rapid. This immunoblot is from one biological replicate.
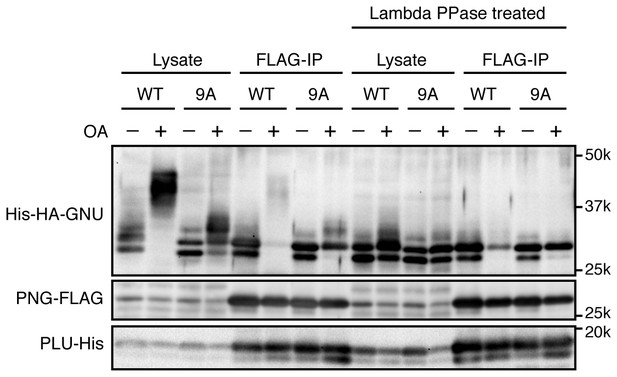
Phosphorylation at CDK1 sites in GNU blocks PNG kinase complex formation in Sf9 cells.
The samples in Figure 2D also were treated with λ phosphatase (Lambda PPase treated) to compare the protein levels of PNG kinase complex components precisely by collapsing the mobility forms. PNG-FLAG and PLU-His were expressed in Sf9 insect cells with His-HA-GNU WT or 9A (Lysate). The PNG complex expressing cells were incubated with or without okadaic acid (OA), a PP2A inhibitor. PNG kinase complex formation was examined by immunoprecipitation of PNG-FLAG (FLAG-IP). The Lysate and FLAG-IP samples were treated with λ phosphatase as described in Materials and methods. The left side of the panel is the same as shown in Figure 2D. This experiment is representative of three biological replicates, except the lambda phosphatase treatment was done in only this replicate.
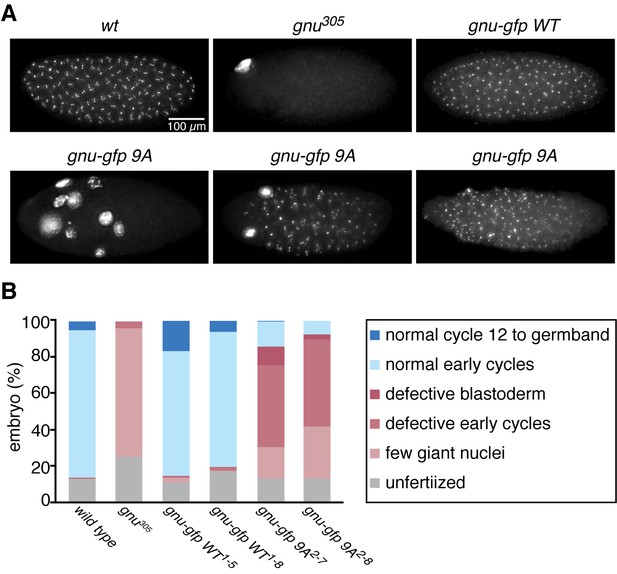
The GNU-9A protein can partially rescue gnu305/gnu305 mutants.
(A,B) Fertilized embryos were collected for 2 hr from females of genotype wt, gnu305/gnu305 (gnu305), gnu305/gnu305;[gnu-gfp WT] (gnu-gfp WT) or gnu305/gnu305;[gnu-gfp 9A] (gnu-gfp 9A), fixed and stained with DAPI. The embryos from the wt and gnu-gfp WT females show normal early cycles, and the gnu305 embryo is classified as having a few giant nuclei. Several phenotypes are shown for gnu-gfp 9A, from left to right: few giant nuclei, defective early cycles, and defective blastoderm. (B) Quantification of the embryo phenotypes. Two independent lines for each transgene were tested (gnu-gfp WT1-5 and WT1-8 and 9A2-7 and 9A2-8). At least 300 embryos were scored for each of the transgene lines, and 150 were scored for the wild-type and gnu305 controls. The data are the sum of two technical replicates. A second biological replicate experiment was scored and comparable rescue results observed, but the embryos were not quantified.
-
Figure 3—source data 1
The numbers of embryos in each phenotypic class scored for each genotype.
- https://doi.org/10.7554/eLife.22219.015
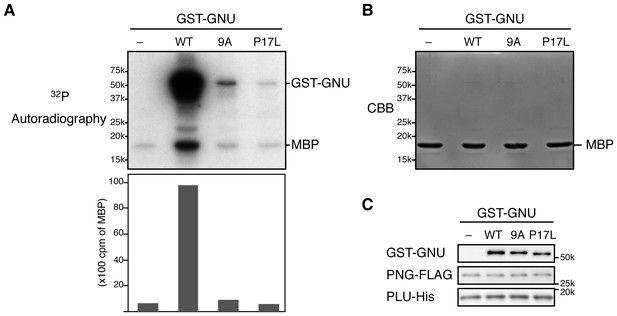
Alanine substitution in the nine S/T-P sites in GNU prevents its ability to activate PNG kinase in in vitro.
(A–C) Recombinant PNG kinase activity was measured with or without wild-type GST-GNU, the alanine mutant in the S/T-P sites (9A), or the GNUZ3-0591 mutant protein whose proline at residue 17 is changed to leucine (P17L; (Lee et al., 2003). PNG activity was examined by 32P incorporation into myelin basic protein (MBP; upper panel in A). GNU is a known in vitro PNG substrate (Lee et al., 2003), and it is phosphorylated by PNG in these experiments. 32P incorporated into MBP was quantified (bottom panel in A) by liquid scintillation counting. Protein levels in the reaction were examined by CBB staining for MBP (B) and immunoblotting for GST-GNU, PNG-FLAG and PLU-His (C). Representative of two independent experiments.
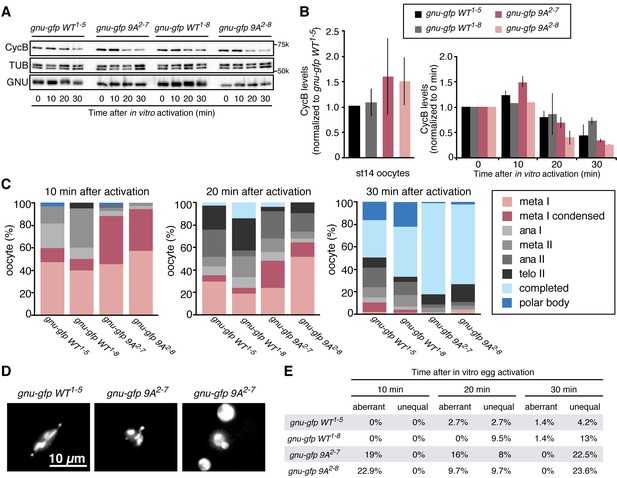
Phosphorylation-resistant GNU increases CycB protein levels in mature oocytes and affects meiotic progression after egg activation.
(A,B) Stage 14 oocytes (0 min) were isolated from gnu305/gnu305 females expressing gnu-gfp WT or 9A (gnu-gfp WT or gnu-gfp 9A) transgenes and were activated in vitro by hypotonic buffer treatment. CycB, TUB and GNU-GFP (GNU) protein levels were examined by immunoblotting at the indicated times after hypotonic buffer treatment (activation). The two independent lines for each transgene were tested (gnu-gfp WT1-5 and WT1-8 and 9A2-7 and 9A2-8). (B) CycB levels in (A) were quantified and normalized with TUB levels. The left graph in (B) shows the CycB levels in each line relative to the levels of gnu-gfp WT1-5. The error bars correspond to five biological replicates. The CycB protein levels are significantly higher in the gnu-gfp-9A oocytes from both transgene lines compared to gnu-gfp WT oocytes by a T test. The right graph in (B) shows the dynamics of CycB levels after egg activation in each line relative to their levels at 0 min. These data are from immunoblots from two biological replicates, except for the 10 min time point for lines 1–8 and 2–8, which were done once. (C) Meiotic progression is delayed in gnu-gfp-9A oocytes compared to gnu-gfp WT oocytes. Stage 14 oocytes (0 min) were isolated from gnu-gfp WT or 9A females and were activated in vitro by hypotonic buffer treatment. The activated oocytes were fixed at indicated times after hypotonic buffer treatment (activation) and were stained with DAPI to define their meiotic stages. The data for the 10 min time point are compiled from three biological replicates. The 20 and 30 min time points were done once. At least 30 but up to 140 oocytes were scored for each genotype for each data point. (D) Phospho-resistant GNU causes defects in chromosome alignment and segregation after egg activation. Chromosomes and post-meiotic nuclear morphology were examined in activated oocytes expressing the indicated transgene in a gnu305 mutant background. Representative images show a normal meiosis I (left: gnu-gfp WT1-5), aberrant meiosis I (middle: gnu-gfp 9A2-7) and interphase nuclei resulting from unequal segregation during meiosis (right gnu-gfp 9A2-7). (E) The percentage of oocytes with aberrant chromosome morphology or unequal chromosome segregation was scored at each time point for the indicated genotypes. The quantification is from one biological replicate. At least 20 but up to 140 oocytes were scored for each data point.
-
Figure 4—source data 1
Quantification of Cyclin B protein levels, the number of eggs in each meiotic stage following egg activation, and the number of eggs with aberrant or unequal meiotic divisions.
- https://doi.org/10.7554/eLife.22219.018
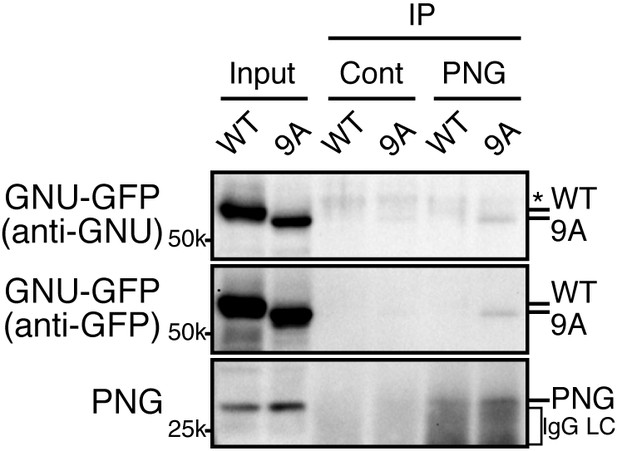
The GNU-9A mutant forms a complex with PNG prematurely in mature oocytes despite active CycB/CDK1.
The GNU-9A mutant, but not wild-type GNU, associates with PNG in meta-I arrested mature oocytes. PNG was immunoprecipitated from extracts of stage 14 oocytes, which expressed GNU-GFP WT or the 9A mutant. The immunoprecipitates with PNG antibody were examined by immunoblotting. Asterisk indicates a nonspecific signal. IgG LC shows bands of the IgG antibody light chain. This experiment was repeated in two biological replicates.
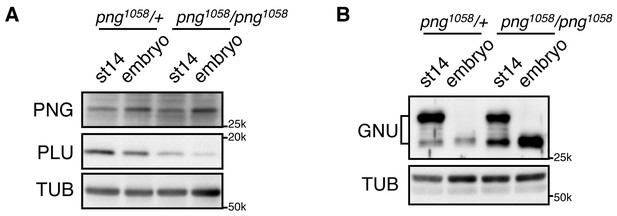
GNU protein levels decrease in embryos in a PNG-dependent manner.
(A) PNG, and PLU levels in stage 14 oocytes (st14) or 0–2 hr collections of fertilized embryos (embryo) from png1058/FM6 (png1058/+) or png1058/png1058 females were examined by immunoblotting. Alpha Tubulin (TUB) was used as a loading control. PNG and PLU levels were hardly changed during the first two hours of embryo development. Note that PLU levels are reduced in png mutant oocytes. (B) GNU levels in stage 14 oocytes (st14) or 0–2 hr collections of fertilized embryos (embryo) from png1058/+ or png1058/png1058 females were examined by immunoblotting. TUB was used as a loading control. GNU becomes dephosphorylated after egg activation in both the control and mutant, but is stabilized in the absence of functional png. This experiment was repeated in two biological replicates.
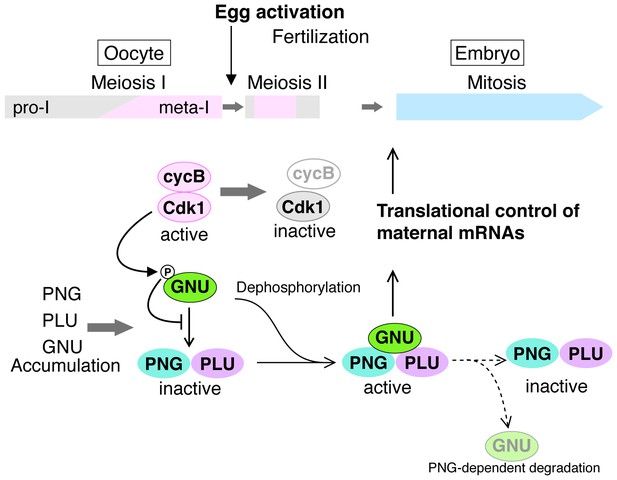
A model for developmental regulation of PNG kinase activity during the oocyte-to-embryo transition.
Although mature stage 14 oocytes, which are arrested at metaphase of meiosis I, have the PNG kinase complex components, CycB/ CDK1 phosphorylates and inhibits GNU, preventing binding to PNG-PLU and active PNG kinase. Egg activation triggers resumption and completion of meiosis, which result in CDK1 inactivation. Subsequently, GNU becomes dephosphorylated, binds to PNG-PLU and activates PNG kinase, leading to translational control of maternal mRNAs, essential for initiation and progression of the mitotic cycle in embryos. Thus, phosphorylation of GNU by CDK1 couples cell cycle progression and maternal mRNA translation during the oocyte-to-embryo transition. PNG kinase activity also reduces GNU protein levels after egg activation. Together with the PNG activation mechanism, the negative feedback causing GNU protein reduction limits PNG kinase activation to this short time window, important for the precise oocyte-to-embryo transition.





