Symmetry broken and rebroken during the ATP hydrolysis cycle of the mitochondrial Hsp90 TRAP1
Figures
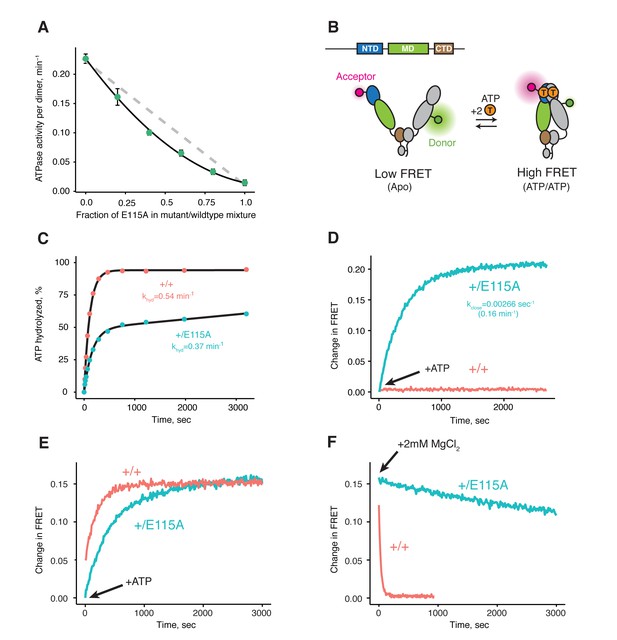
Both ATPs need to be hydrolyzed for efficient cycling.
(A) Steady-state ATPase assay with constant dimer concentration and varying ratios of wild-type and catalytically-dead E115A mutant shows that ATP hydrolysis by each protomer is not independent. Each point and error bar are one standard deviation and averaged from triplicate experiments. Black line is a fit to a binomial distribution of wild-type:mutant:heterodimer, solved for only the heterodimeric activity. The gray dashed line shows the expected activity for independent ATP hydrolysis. (B) Covalently linked heterodimers for the FRET assay with fluorescent labels on the NTD and MD (E140C, K413C respectively). (C) Single-turnover ATPase kinetics of wild-type (+/+, red) and heterodimeric, hemi-hydrolyzing (+/E115A, blue), show activity of the remaining site is not compromised. Black curves are exponential fits with an additional linear term to account for a slow steady-state activity in the +/E115A. (D) FRET assay looking at build up of closed state (high FRET) in the +/E115A heterodimer (+/E115A, blue) and wild-type (+/+, red) in presence of MgCl2 to allow ATP turnover. The +/E115A data were fit to an exponential with the indicated rate, kclose. (E) FRET assay showing closed state build up in both +/+ (red) and +/E115A (blue) heterodimers after addition of 2 mM ATP in absence of MgCl2. (F) Addition of excess MgCl2 triggers efficient dimer reopening, as measured by FRET, in +/+ (red) but not +/E115A (blue) heterodimers in reactions pre-incubated with ATP without Mg2+.
Raw fluorescence intensities of FRET data in Figure 1D–1F.
Donor intensities are colored orange and acceptor intensities are colored red. A–B) Raw fluorescence intensities of wild-type (+/+, A) and hemi-hydrolyzing heterodimer (+/E115A, B) in presence of ATP/Mg added at t = 0 s from Figure 1D. (C–D) Raw fluorescence intensities of +/+ (C) and +/E115A (D) showing dimer closure after addition of ATP at t = 0 s in absence of MgCl2 (with EDTA added) in Figure 1E. (E–F) Raw fluorescence intensities of +/+ (E) and +/E115A (F) showing dimer reopening after addition of 2 mM of MgCl2 at t = 0 s.
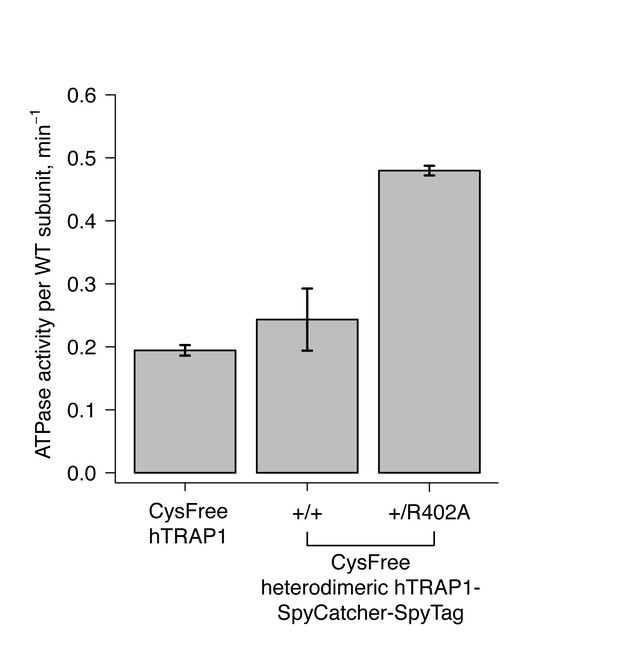
Steady-state ATPase assays of cysteine-free hTRAP1 and heterodimeric hTRAP1 (+/+and + /R402A) at 30 ˚C.
Each bar and error bar are one standard deviation and averaged from triplicate experiments. ATPase activities displayed are normalized per wild-type subunit.
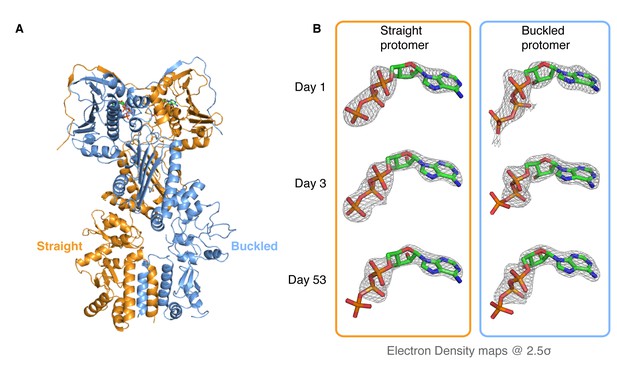
Kinetic crystallography indicates that the buckled arm hydrolyzes ATP first.
(A) 2.3 Å crystal structure of zTRAP1 closed with ATP obtained from a 3-day-old crystal showing minimal conformational changes without Mg2+. Buckled protomer is in blue and straight protomer is in orange. (B) ATP electron density maps for the buckled and straight protomers from crystals of different ages showing the evolution of in-crystal hydrolysis.
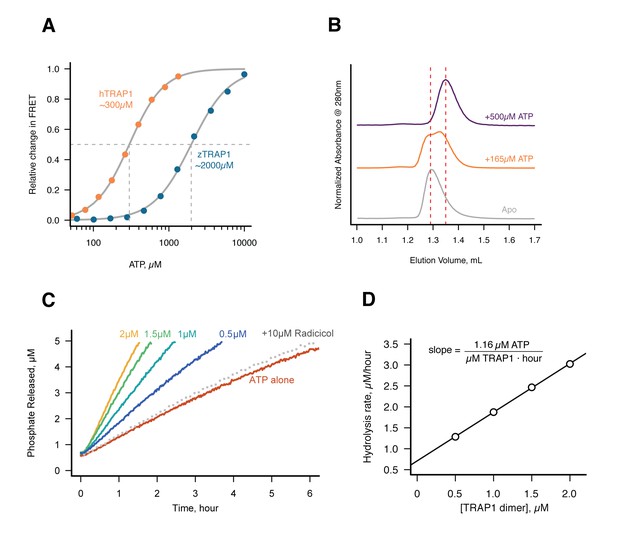
Without Mg2+hTRAP1 adopts the closed state and slowly hydrolyzes ATP in solution.
(A) Equilibrium titration of closure in response to ATP in presence of excess EDTA hTRAP1 (orange) and zTRAP1 (dark blue) using FRET. The indicated half-max concentrations are obtained from fits to the Hill equation (gray lines). (B) Size-exclusion chromatography of cysteine-free TRAP1 under apo (gray), and after 1.5 hr incubation at 30˚C with 165 µM ATP (partial closure, orange), and 500 µM ATP (full closure, purple). Red dashed lines are guides for apo and closed state peak positions. (C) Ultra sensitive assay of ATP hydrolysis using fluorescent phosphate-release assay with PBP-MDCC with ATP alone (red) and varying dimer concentrations of cysteine-free TRAP1, and 2 µM TRAP1 + 10 µM radicicol (gray dotted line). (D) Initial rates from phosphate-release kinetics plotted against TRAP1 dimer concentration confirming that the rate above baseline is TRAP1 dependent. The ATPase hydrolysis rate per TRAP1 dimer is 1.16 µM ATP· µM TRAP1−1 · hr−1.
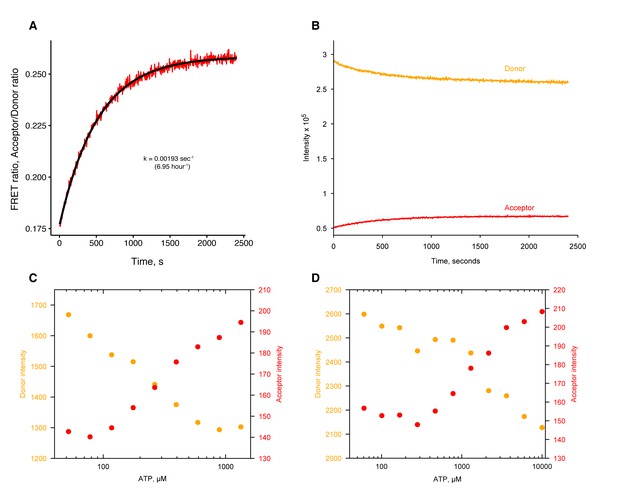
ATP-induced dimer closure in absence of Mg2+by FRET in human and zebrafish TRAP1.
(A) hTRAP1 dimer closure as seen by FRET after addition of 500 µM ATP at 30 ˚C. Closure rate is much faster than hydrolysis rate in absence of Mg2+. Black line is the fit to an exponential function with the given rate constant. (B) Raw donor (orange) and acceptor (red) fluorescence intensities of FRET data in A. (C) Raw donor (orange) and acceptor (red) intensities of ATP titration to human TRAP1 from Figure 3A. (D) Raw donor (orange) and acceptor (red) intensities of ATP titration to zebrafish TRAP1.
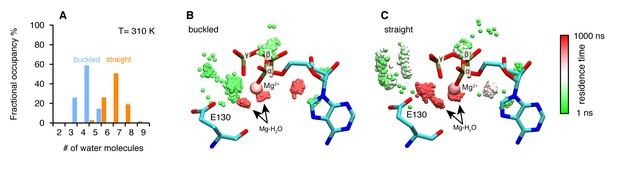
Microsecond all-atom molecular dynamics simulations of zTrap1 reveal asymmetric water dynamics near the ATP γ-phosphate.
(A) Histogram of water molecules counted near the ATP β- and γ-phosphates (<5 Å) throughout the simulation (each frame is three ns) in the buckled protomer (blue) and the straight protomer (orange) at T = 310 K. (B) and (C) Fractional residence time of each individual water molecule in the ATP-binding pocket for the buckled and straight protomers at T = 310 K, showing significant differences in solvation near the E130. Only the oxygen of water molecules near the ATP β- and γ-phosphates (<5 Å) are shown. Points are accumulated from all frames along the trajectory after aligning the system based on the ATP. Black arrows point to the persistent magnesium-coordinated water molecules, Mg-H2O, which are conserved for the two conformers. Water molecules were colored based on their residence time as indicated by the color bar.
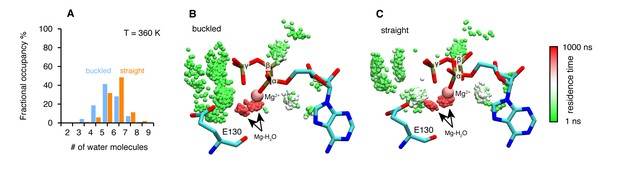
Microsecond all-atom molecular dynamics simulations of zTrap1 reveal asymmetric water dynamics near the ATP γ-phosphate at 360 K.
(A) Fractional occupancy of water molecules near the ATP β- and γ-phosphate (<5 Å) within each 3 ns window in the buckled protomer A (blue) and the straight protomer B (orange). (B) and (C) Dwell time of each individual water molecule in ATP-binding pocket for buckled protomer and straight at T = 360 K. The figures were rendered by showing the water molecules (only oxygen for clarity) near the ATP β- and γ-phosphate (<5 Å) of all frames along the trajectory after aligning the system based on ATP. Black arrows point to magnesium-coordinated water molecules, Mg-H2O. The water molecules were colored based on how long they stay in the vicinity of the ATP.
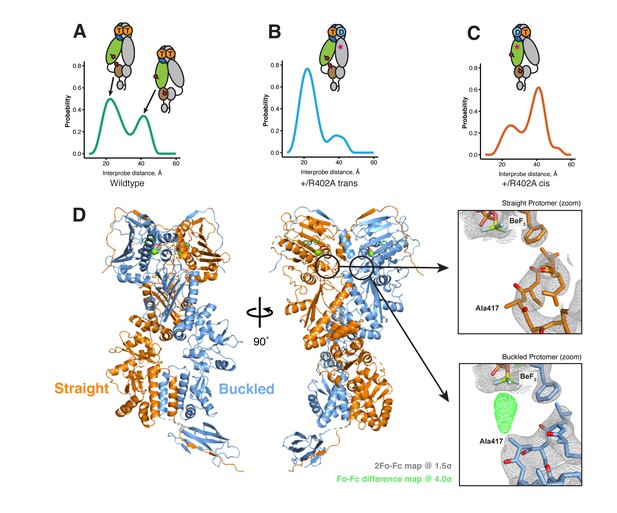
The asymmetry is flipped in the ATP/ADP state as revealed by DEER on hemi-hydrolyzed (ATP/ADP) heterodimers.
The cartoons depict hTRAP1 heterodimers (one protomer colored by domains and the other in gray; NTD, blue; MD, green; CTD, brown) with spin-labels (red circles) and the relevant nucleotide state (D or T, for ADP or ATP, respectively) as well as the R402A mutation (ADP state mimic; magenta star). The SpyCatcher-SpyTag is shown attached to the CTD tails. (A) +/+heterodimers (green line) partition roughly equally between the buckled (left, 22 Å) and straight (right, 41 Å) conformations. (B) Spin-labels on the opposite (trans) protomer of +/R402A heterodimers (blue line) show that nearly all molecules are buckled on the ATP arm. (C) +/R402A heterodimers (orange line) carrying spin-labels on the same (cis) protomer as the R402A mutation, showing that the protomer prefers the straight conformation. (D) Crystal structure of +/R417A heterodimeric zTrap1 with the SpyCatcher-SpyTag fusion in the asymmetric closed state showing buckled (blue) and straight (orange) protomers. The dashed line indicates disordered residues. Insets show that the γ-phosphate-sensing R417 is only present on the buckled arm. Phases come from a model having Ala on both protomers. 2Fo-Fc density (gray mesh) and Fo-Fc difference map (green mesh) around the position of the asymmetric R417A mutation. Strong positive density (green mesh) on the difference map is observed only at the buckled protomer.
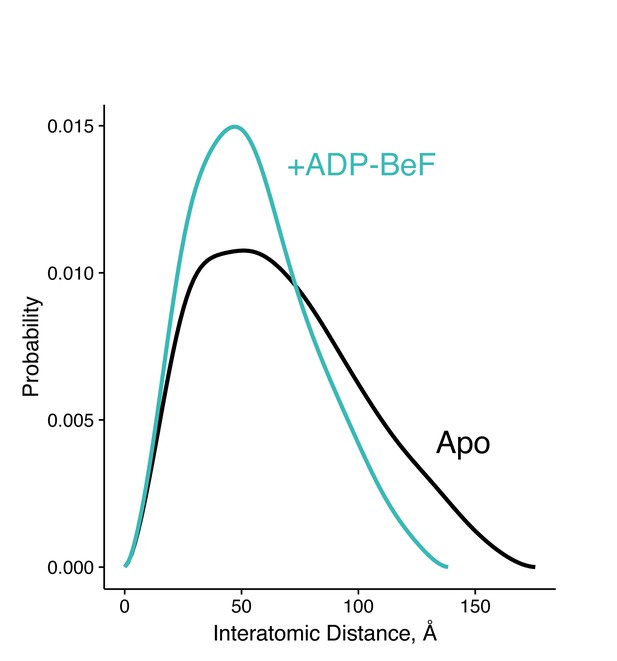
Interatomic distance distribution, P(r), from SAXS experiments of heterodimeric +/R402A human TRAP1.
The heterodimer is fully capable of closing, shown as overall reduction in the width and maximum dimension of the P(r) distribution upon incubation with an ATP analog (ADP-BeF, teal). The black curve is the P(r) of the apo dimer.
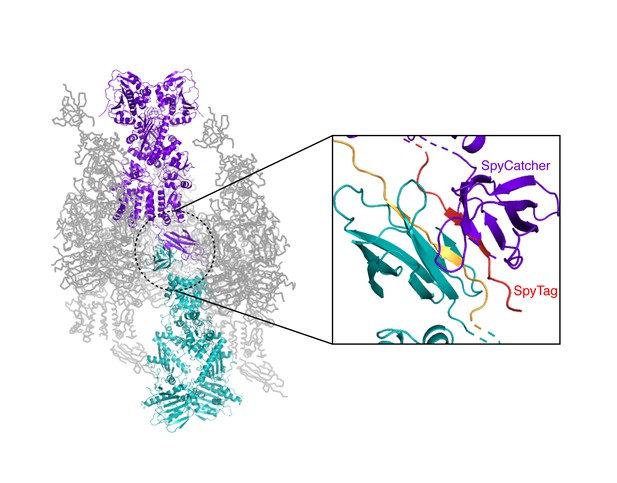
Crystal packing interactions for the heterodimeric (+/R417A) zTRAP1 fused to the SpyCatcher-Tag domains.
A pair of symmetry mates is colored purple and teal whose SpyCatcher-Tag domains pack against each other (black inset, a close-up of the dotted circle). The SpyCatcher and SpyTag belonging to one dimer are colored purple and red, respectively. The corresponding SpyCatcher-SpyTag symmetry mate is colored teal and yellow, respectively.
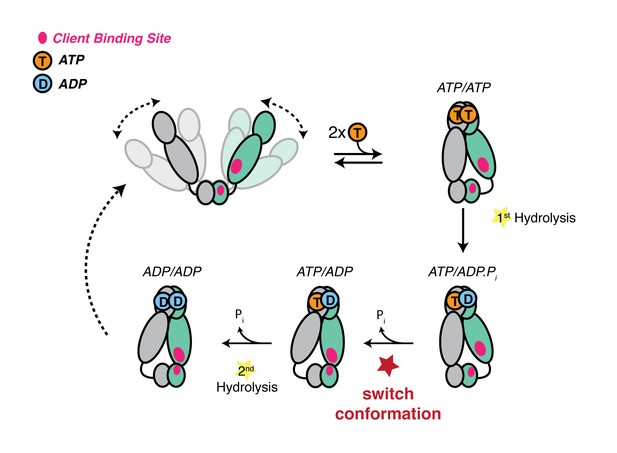
Revised model of the TRAP1 ATPase cycle showing the obligatory sequential hydrolysis and conformational switching.
The protomers are colored teal and gray. The dynamic apo state (upper left) binds two ATPs, which stabilize a strained asymmetric NTD-dimerized closed state. Within this closed state, ATP is hydrolyzed first by the buckled protomer. Release of Pi likely drives the observed conformational switch of the straight protomer (ATP) to a buckled conformation, while the previously buckled protomer (now ADP), straightens. Concomitant with the flip, the client-binding sites (magenta ellipses) are rearranged, to facilitate client remodeling. Now in a buckled conformation, the second ATP is set up to be hydrolyzed. Finally, the ADP/ADP dimer re-opens, releasing nucleotides and resetting TRAP1 to the apo state.
Additional files
-
Supplementary file 1
Data collection and refinement statistics for zebrafish TRAP1 crystals.
Each dataset was collected from a single crystal. Values in parentheses are for highest-resolution shell.
- https://doi.org/10.7554/eLife.25235.014





