Cryo-electron tomography reveals novel features of a viral RNA replication compartment
Figures
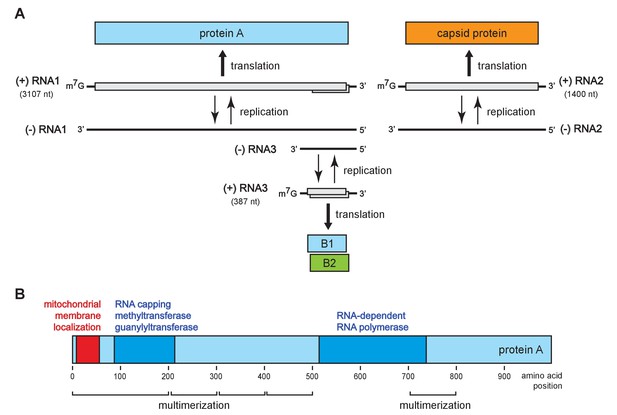
Flock house virus genome organization and functional map of replicase protein A.
(A) The bipartite FHV RNA genome encodes the viral replicase protein A on RNA1 and the capsid protein precursor on RNA2. Protein A mediates synthesis of a negative strand copy of the genomic RNAs which serves as template for synthesis of progeny positive strand RNAs, and an additional partial negative strand copy of RNA1 to template a 3’ co-terminal subgenomic RNA3. RNA3 potentially encodes protein B1, co-terminal with the C-terminus of protein A, and protein B2 which has anti-RNAi function. (B) A linear representation of protein A annotated to show membrane-association and enzymatic domains, and several regions independently capable of homotypic multimerization.
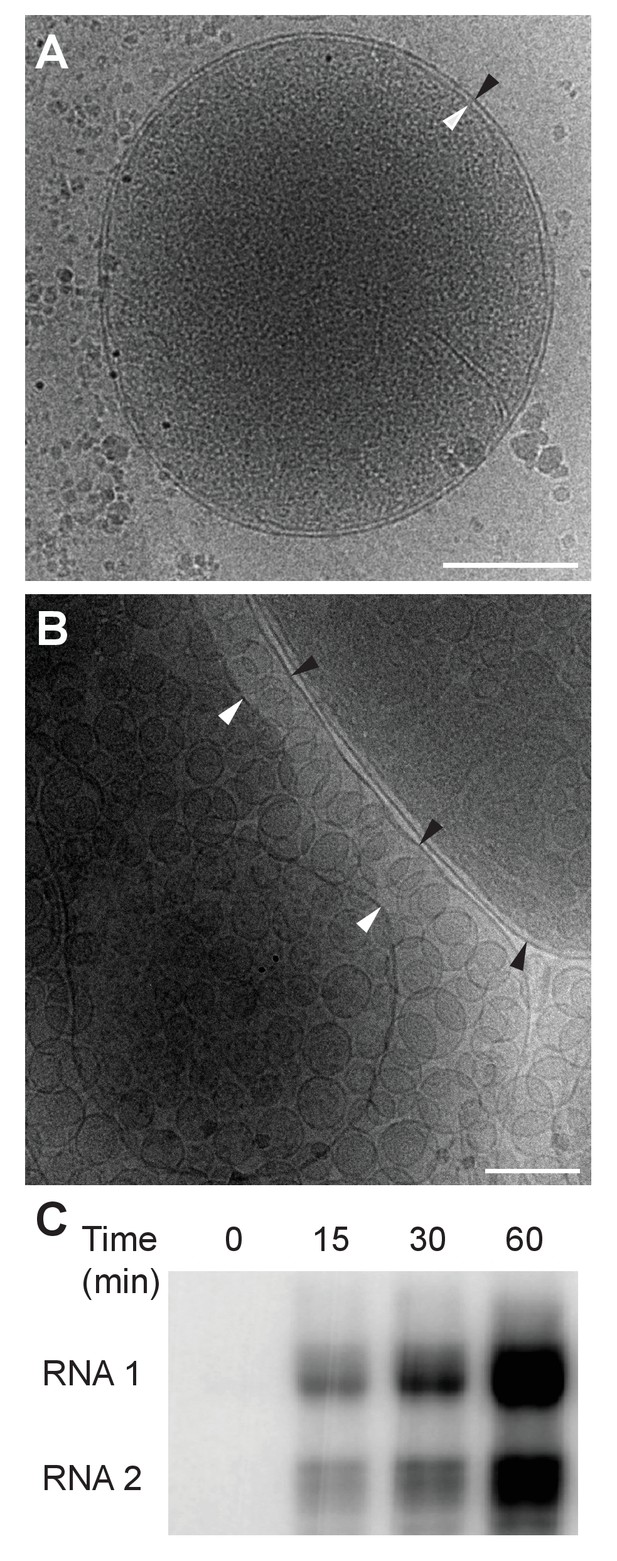
Cryo-EM images of Drosophila S2 cell mitochondria.
Mitochondria were isolated from mock-infected or FHV-infected S2 cells, applied to carbon-coated grids, plunge-frozen, and imaged using a TF-30 electron microscope under cryogenic conditions. (A) Intact mitochondrion with inner (white arrowhead) and outer (black arrowhead) membranes approximately 10 nm apart. Scale bar = 200 nm. (B) Mitochondria isolated from FHV-infected cells were closely associated with numerous round vesicular compartments in the extensively dilated lumen between inner and outer membranes. Scale bar = 100 nm. (C) Mitochondria preparations from FHV-infected cells show active continued viral RNA synthesis when incubated in the presence of radiolabeled [32P]UTP. Results are representative of three independent experiments.
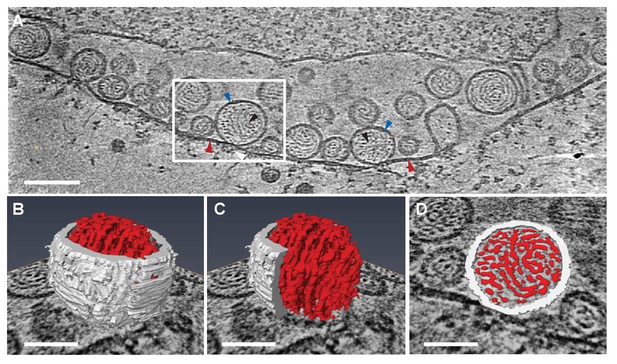
Viral RNA and protein complex association with FHV spherules.
(A) Cryo-ET image of a mitochondrion associated with FHV spherules, taken from a reconstructed tomogram obtained at 41,000 x magnification, defocus of −6. Mitochondrial outer membrane, spherule membrane, interior spherule filaments, and spherule aperture structures are indicated with red, blue, black, and white arrowheads, respectively, consistent with arrowhead colors used in Figures 5 and 6. Scale bar is 100 nm. (B–D) Segmentation of the white-outlined area in panel A. The Amira program (FEI) was used to trace and structurally segment spherule membrane in white and interior spherule density in red. Panels B and C provide three dimensional renditions, panel D segments the structure in a single plane. Scale bars are 50 nm. See Video 2 for complete tilt series.
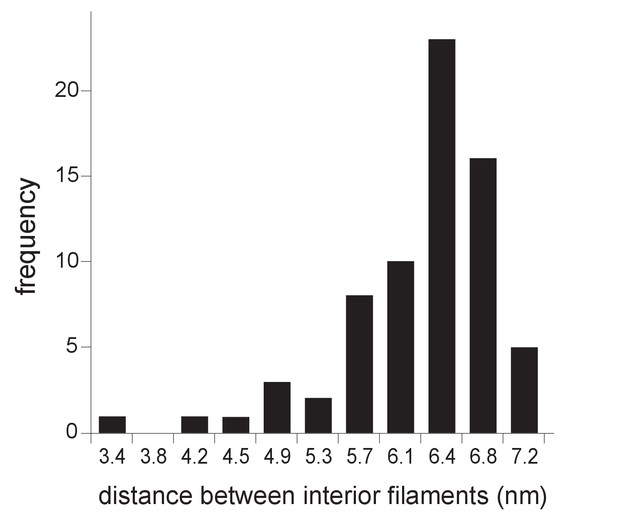
Distribution of center-to-center distance between interior spherule RNA filaments.
Interior densities of FHV spherules were traced and segmented using the Amira program. Distances were measured as the shortest center-to-center spans on tomographic reconstructions such as in Figure 3A using IMOD software (Nicastro et al., 2006).
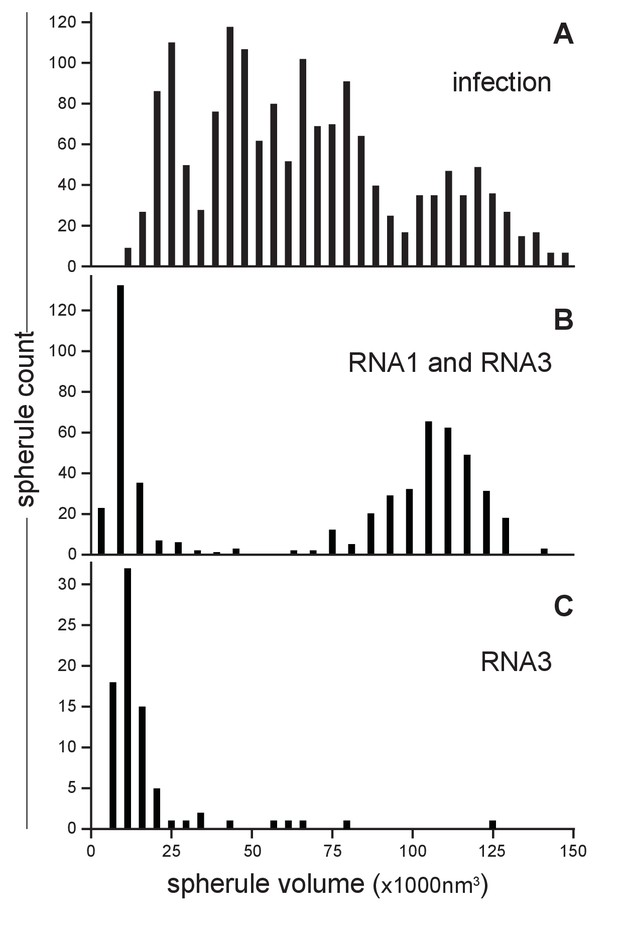
FHV RNA template length determines replication spherule vesicle volume.
(A) FHV infection involves the simultaneous replication of RNA1, RNA2, and RNA3 resulting in a range of spherule volumes from 13,600 to 145,000 nm3. Spherule volumes were calculated from measurements of spherule radius at the widest circumference using IMOD software (Nicastro et al., 2006). (B) Cells that transiently express protein A from a non-replicating RNA in addition to RNA1 template have spherule volumes that sort into two pronounced populations suggesting a volume range for spherules containing either RNA1 or RNA3. (C) Cells expressing protein A and RNA3 show only the smaller spherule volume population.
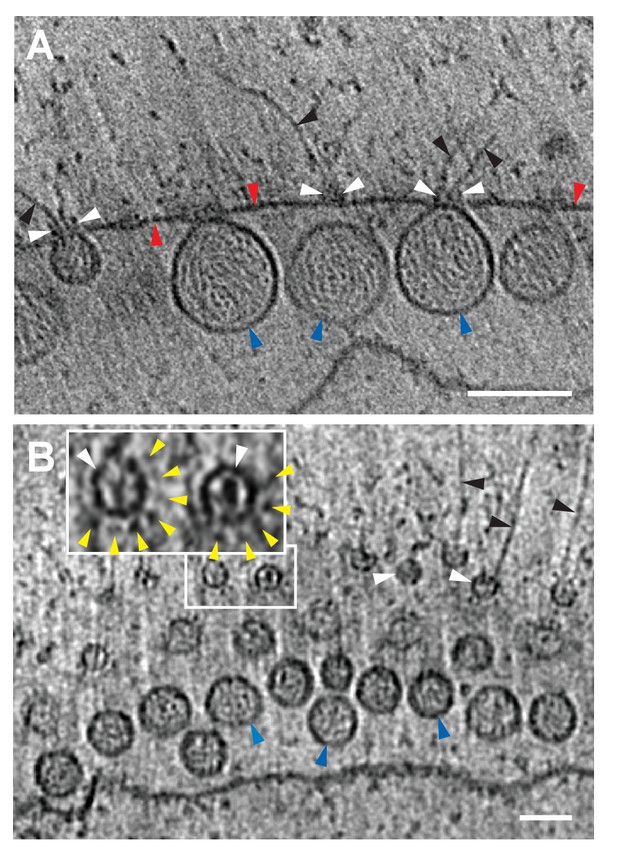
FHV spherule aperture densities are ordered protein complexes associates with viral RNA extrusions.
(A) A single tomographic slice perpendicular to the outer membrane of a mitochondrion from an FHV-infected cell, showing multiple spherule cross sections. The image was obtained from a reconstructed tomogram at 19,500x magnification, defocus of −6. The protein complex at the spherule neck aperture is indicated with white arrowheads and extruding filaments (likely product RNA strands - see main text) with black arrowheads. The mitochondrial outer membrane and the spherule membrane are indicated with red and blue arrowheads, respectively. (B) Tomographic slice nearly parallel to the outer membrane of an FHV-modified mitochondrion. The upper portion shows multiple spherule apertures (two of which are boxed in white) viewed from above the cytoplasmic side of the membrane. Filamentous protusions associated with these apertures again are marked with black arrowheads. Inset: magnified view to show the aperture-surrounding concentric circular arrangement of additional smaller densities (yellow arrowheads). In the lower portion, the plane of sectioning passes below the outer mitochondrial outer membrane to section multiple spherule vesicles. Scale bars are 100 nm. Arrowhead colors are consistent with those used in Figures 3 and 6. Tomograms were gauss-filtered to remove noise. See Videos 1 and 4 for complete tilt series and tomogram reconstructions.
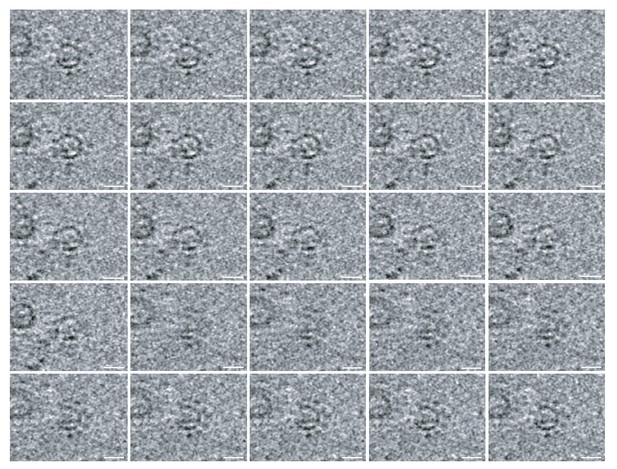
The 12-fold ‘teeth’ at the necks of FHV spherules.
A series of z-plane slices of a cryo-tomogram focusing on FHV crowns shows that even without the greatly-increased resolution from subtomogram averaging, subsets of the 12 teeth densities are readily visible surrounding the central turret structure in the original tomograms. Scale bar = 10 nm.

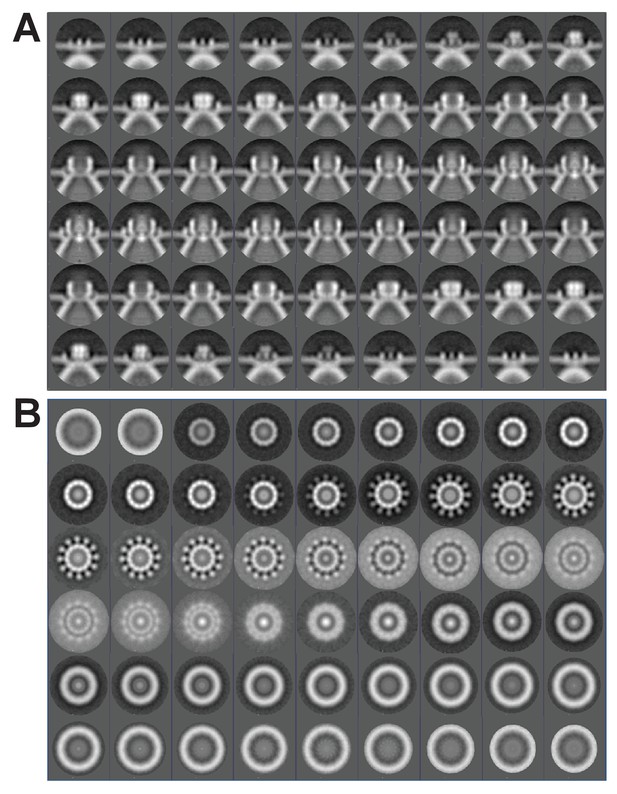
Subtomogram average of FHV crown structure.
(A–B) FHV crown subtomogram averages viewed from the X/Y plane (A), or from the Z-plane looking towards the spherule interior (B). Scale bars = 10 nm. (C–F) Density maps of FHV crown structures. (C) View of crown density map from the X/Y plane or as depicted in panel A. (D) View of FHV crown from the Z-plane towards the spherule interior as depicted in panel B. (E) Cut-away view of FHV crown. Arrowheads indicate the central turret (white) with interior cone-shaped central density, the teeth surrounding the central turret (yellow), the outer mitochondrial membrane (red), and the spherule membrane (blue). (F) The crown density map angled to show the entirety of all crown features. (G) Fourier shell correlation curve indicating the commonly used 0.5 and 0.143 cut-off intersections indicating the resolution of the reconstruction to be ~3.3–3.8 nm. (H) Averaged power spectra derived from Fourier transformed intensity profiles along the angular direction of all individually analyzed crown particles, strongly indicating 12-fold symmetry.
The 12-fold-symmetric FHV crown.
The subtomogram-averaged FHV crown structure represented as a series of slices imaged through the plane perpendicular to the outer mitochondrial membrane (A, ‘side view’) or perpendicular to the axis passing through the center of the crown to the center of the spherule (B, ‘top view’). The images start at upper left as viewed from the cytoplasmic side above the crown, and progress toward the pherule center.

FHV protein A replicase is a major component of spherule crown structures.
Immunogold labeling of a mitochondrion from FHV-infected cells. (A) Mitochondrial extracts were incubated with anti-protein A polyclonal serum and a secondary antibody conjugated to 6 nm gold, loaded onto grids, plunge-frozen, and imaged under cryogenic conditions. White arrows indicate 6 nm gold conjugated to a goat-anti rabbit antibody, black arrows indicate 15 nm fiducial gold used to align the tilt series. (B) Image from a reconstructed tomogram of the mitochondrion in panel A. Gold-conjugated antibodies accumulate at the outer mitochondria membrane at sites with spherules, and not in areas where spherules are absent. Inset provides a magnified view and indicate the inner (white arrowhead) and outer (black arrowhead) mitochondrial membranes. Scale bar (A–B) = 100 nm, inset scale bar = 50 nm. See Video 6 for complete tilt series.
Lack of immune-gold labeling of FHV spherules using a non-specific antibody control.
Under experimental conditions essentially the same as used for the results shown in Figure 7 in the main text of the paper, no specific immunogold labeling was observed using non-FHV-specific negative control IgG rabbit polyclonal antibody. (A) Single image from an acquired tilt-series. Spherules are apparent on the outer mitochondrial membrane. A white arrow indicates a sporadic 6 nm gold particle conjugated to the antibody. A black arrow indicates a smaller gold particle used to align the tilt series. (B) A tomographic slice from the reconstructed tomogram from panel A. Scale bars = 200 nm.
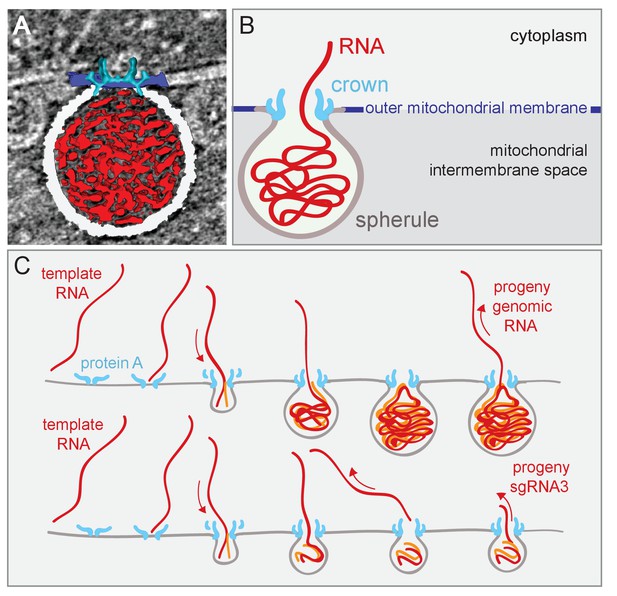
Model of FHV replication complex structure and function.
(A) Amira-assisted 3D segmentation of a single virus replication compartment in FHV-infected cells. The spherule membrane (white) encloses the viral RNA (red) and is anchored to the mitochondrial outer membrane (dark blue), by the FHV protein A crown (light blue, derived from the subtomogram average). (B) Cartoon rendering of the image in (A). Viral RNA is shown as exiting the spherule to include the observation of fibrillary extrusions in many other tomograms. (C) Model of the FHV RNA complex and RNA synthesis, where positive-strand RNA (red) associates with protein A on the mitochondrial membrane to initiate the synthesis of negative-strand RNA (orange) and progeny positive-strand RNA accommodated by increasing volume of the spherule as if blowing up a balloon. In this model, subgenomic RNA3 results from attenuated negative strand synthesis and subse- quent positive-strand production in small spherules.
Videos
Cryo-EM Tomogram of a Mitochondrion from FHV-infected cells.
Complete tomographic reconstruction of a mitochondrion bearing numerous FHV spherules, at 19,500x magnification, and represented as a series of Z-stack images. Scale bar = 200 nm.
Higher Magnification View of a Mitochondrion from FHV-infected cells.
Tomographic reconstruction of the mitochondrion with FHV spherules shown in Figure 3. Note particularly the closely packed fibrillar material in the spherule interiors. At 41,000x magnification, represented as a series of Z-stack images. Scale bar = 100 nm.
Tomographic View of Crowns and Cytoplasmic Filaments on a Mitochondrion from FHV-infected cells.
Detailed view of a portion the mitochondrion used in Figure 5 and Video 1. Viral RNA exits FHV spherules in close proximity to the ‘crown’ protein complexes at the spherule necks. Scale bar = 100 nm.
Subtomogram-averaged FHV crown structure.
Rotating views of the highly symmetrical subtomogram-averaged structure at the FHV spherule aperture.
Cryo-EM tomographic view of a mitochondrion from mock-infected cells.
Tomogram of mock-infected mitochondrion as a Z-stack series. In the absence of FHV infection, mitochondria isolated from 2 cells contain no apparent spherules or crown protein complex densities. The inner and outer mitochondrial membranes are closely appressed with little or no membrane dilation. Scale bar = 100 nm.
Immunogold-labeled mitochondrion from FHV-infected cells.
Tomogram of the mitochondrion from Figure 7 represented as a Z-stack series. Note the high accumulation of gold-conjugated anti-FHV protein A antibodies that occurs just outside of the outer mitochondrial membrane in the immediate vicinity of the FHV spherule necks, but is absent from portions of the outer membrane lacking spherules. Scale bar = 200 nm.
Three-dimensional image segmentation illustrating multiple features of an FHV spherule.
The outer spherule membrane (white) is contiguous with the outer mitochondrial membrane (dark blue). In the first half of the video, a section of the spherule membrane and the underlying spherule interior are purposely removed to show the internal fibrils (red). On the outer mitochondrial membrane at its junction with the invaginated spherule membrane, the crown (light blue) is shown facing toward the cytoplasmic space and surmounting the membrane neck connecting the spherule interior to the extra-mitochondrial cytoplasmic space. The crown density shown is derived from the subtomogram-averaged crown structure of Figure 6 and Video 4. In the final views, the spherule membrane is removed from the image to reveal more detail of the inner fibrillar contents. Segmentation was carried out using the Amira software package (FEI).





