Multiple short windows of calcium-dependent protein kinase 4 activity coordinate distinct cell cycle events during Plasmodium gametogenesis
Figures
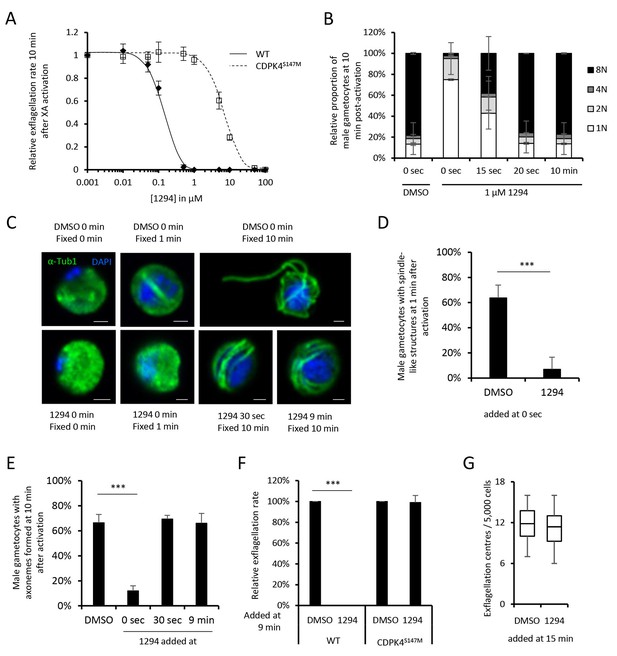
A chemical genetic approach reveals CDPK4 activity is required to initiate the first round of DNA replication, assemble the first mitotic spindle and initiate axoneme motility.
(A) A line expressing a CDPK4S147M allele is 50 times more resistant to compound 1294. Error bars show standard deviations, n = 3. (B) Effect of 1 μM 1294 addition at multiple time points after XA activation on microgametocytes ploidy. CDPK4 activity is required between 10 and 20 s to initiate the first round of DNA replication and the second genome replication. CDPK4 activity is however not required for DNA replication per se. Error bars represent standard deviations, n = 2. (C) Immunofluorescence assays showing the effect of 1294 at different time points after activation. Addition of 1 μM 1294 at the time of activation prevents the formation of mitotic spindles as observed 1 min after activation in presence of DMSO. Addition of 1294 at 30 s or at 9 min post-activation does not inhibit axoneme formation but blocks initiation of axoneme motility and condensation of chromatin. Absence of 1294 treatment leads to the exflagellation of male gametes 10 min after activation. Scale bars = 1 µm. (D) Quantification of macrogametocytes showing mitotic spindles at 1 min post-activation in DMSO or 1294 treated parasites. Error bars show standard deviations, n = 2, *** Student's T-test, p≤0.001. (E) Quantification of macrogametocytes showing axonemes formed at 10 min after activation. Error bars show standard deviations, n = 2, *** Student's T-test, p≤0.001. (F) Quantification of exflagellation events in the WT and CDPK4S147M lines when DMSO or 1294 were added 9 min after activation. Error bars show standard deviations n = 2, *** Student's T-test, p≤0.001. (G) When added 15 min post-XA activation, 1294 does not inhibit motility of active microgametes, n = 3, *** Student's T-test, p≤0.001.
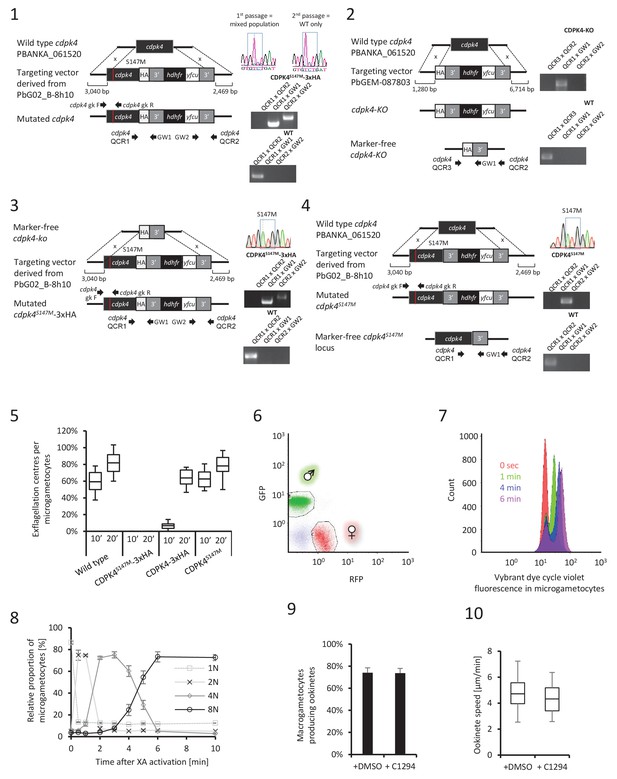
CDPK4 is required to initiate the first round of DNA replication but not for ookinete development or motility.
(1–4) Genetic modification vectors and genotyping data for CDPK4 targeting constructs. Oligonucleotides used for PCR genotyping are indicated and agarose gels for corresponding PCR products from genotyping reactions are shown. (1) We first attempted to generate CDPK4S147M-3xHA parasites but we repeatedly obtained pure parasite populations in which the 3xHA tag and the selection cassette were chromosomally integrated while only a minor population also incorporated the S147M substitution which was rapidly lost after one parasite passage. The resulting CDPK4-3xHA line was further cloned. (2) To select for the S147M substitution, we first generated a marker-free CDPK4-KO clonal line. (3) This CDPK4-KO line was then complemented with a CDPK4S147M-3xHA allele and the line was cloned. (4) To test for the respective fitness cost of S147M substitution and 3xHA tagging we generated a clonal parasite line expressing CDPK4S147M. (5) Effect of S147M substitution or 3xHA tagging on microgametocyte exflagellation 10 or 20 min after stimulation with XA. S147M substitution does not affect exflagellation while C-terminal 3xHA tagging of CDPK4 delays exflagellation. Combination of S147M substitution with a 3xHA tag fusion completely blocks male gametogenesis indicating that both mutations impose a fitness cost on CDPK4 activity but that S147M substitution only imposes a minor cost. The range of whisker plots indicates the 2.5–97.5% percentile, the box includes 50% of all values and the horizontal line shows median values. Data are from two biological replicates. (6) Identification of populations of RFP positive female gametocytes and GFP positive males by FACS in blood infected with parasites of line 820cl1. (7) FACS analysis of Vybrant dye cycle violet fluorescence intensity of micro-gametocytes showing evolution from haploid to octoploid cells in less than 6 min post XA stimulation. (8) Relative proportion of 1N, 2N, 4N and 8N microgametocytes from 0 to 10 min post XA stimulation. Error bars represent standard deviations from two independent biological replicates. (9) Addition of 1 μM 1294 one hour after XA stimulation does not block ookinete development. Error bars are standard deviations from three biological replicates. (10) Addition of 1 μM 1294 on mature ookinetes does not affect their motility as assessed in Matrigel. The range of whisker plots indicates the 2.5–97.5% percentile, the box includes 50% of all values and the horizontal line shows median values. Data are representative of three biological replicates.
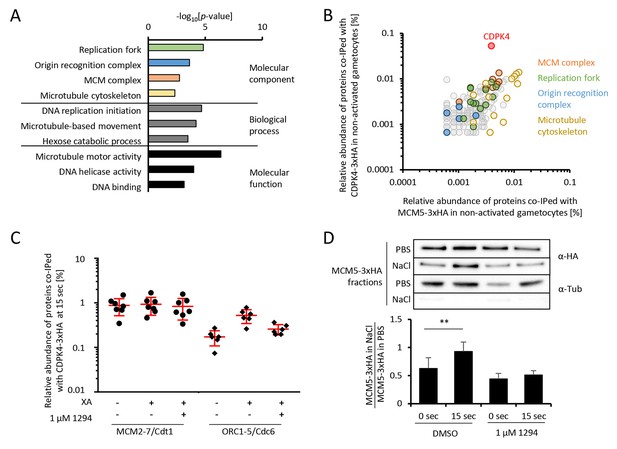
CDPK4 activity facilitates the assembly of the pre-replicative complex during microgametogenesis.
(A) GO term enrichment analysis of proteins co-immunoprecipitated with CDPK4-3xHA in non-activated gametocytes reveals the kinase is interacting with proteins of the pre-replicative complex. Bonferroni corrected p-values are indicated. (B) 178 proteins are immunoprecipitated with both CDPK4-3xHA and MCM5-3xHA including all components of the ORC and the MCM complex. The relative abundance of proteins was determined as the number of spectral counts for each protein divided by the number of total spectral counts in the respective immunoprecipitate. (C) The interaction between CDPK4-3xHA and MCM2-7/Cdt1 proteins is stable during the first 15 s following XA stimulation while interaction with ORC1-5/Cdc6 proteins is increased when the kinase is activated. Data are representative of two independent biological replicates. (D) MCM5-3xHA is enriched in chromatin-enriched NaCl fractions at 15 s post-activation in gametocytes but not when 1294 is added; α-tubulin was used as a soluble control. Error bars show standard deviations, n = 3, ** Student's T-test, p≤0.01.
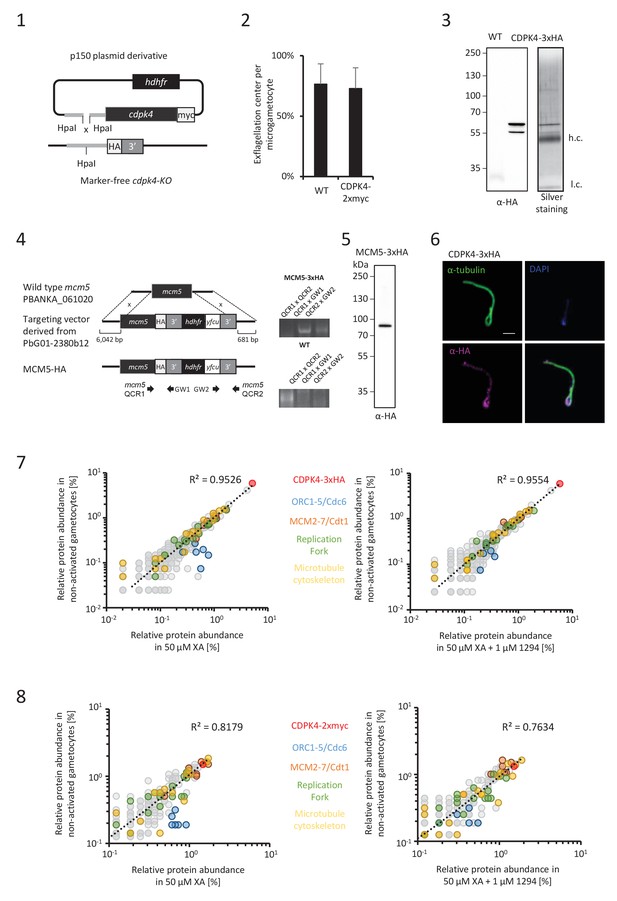
CDPK4 activity is required to assemble the pre-replicative complex.
(1) Complementation of a CDPK4-KO line with a CDPK4-2xmyc allele. (2) A transgenic line expressing CDPK4-2xmyc does not show exflagellation defects. Error bars are standard deviations from two biological replicates. (3) Western blot and silver staining analysis of CDPK4-3xHA immunoprecipitation. Eluates show specific isolation of the 3xHA fusion protein. Molecular weights are indicated in kDa. hc = heavy chain, lc = light chain. (4) Generation and genotyping of a non-clonal line expressing MCM5-3xHA. (5) Western blot analysis of MCM5-3xHA lysates produced from non-activated gametocytes. (6) CDPK4-3xHA is incorporated in the exflagellated male gamete. Scale bar is 1 μm. (7) Relative abundance of proteins co-immunoprecipitated with CDPK4-3xHA in non-activated gametocytes compared with 15 second-activated gametocytes in presence or absence of 1 µM 1294. The six components of the ORC/cdc6 complex show a significant increase in abundance when CDPK4 is active. (8) Relative abundance of proteins co-immunoprecipitated with CDPK4-2xmyc in non-activated gametocytes compared with 15 second-activated gametocytes in presence or absence of 1 µM 1294.
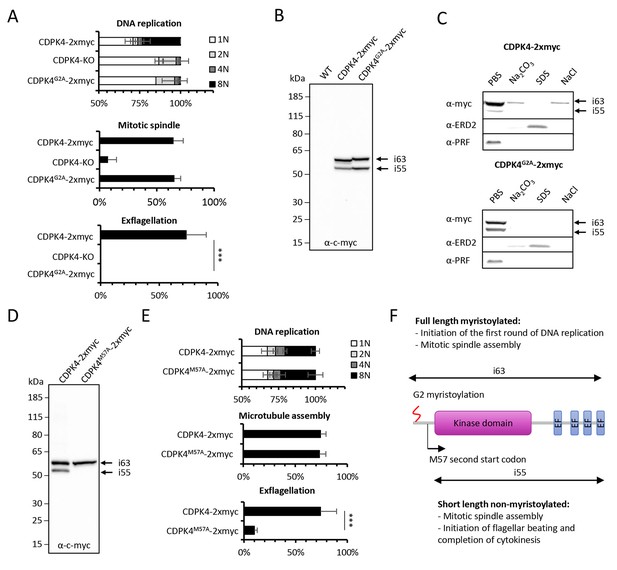
Myristoylation of CDPK4 is required to initiate the first round of DNA replication while non-myristoylated CDPK4 is important to complete gametogenesis.
(A) Effect of cdpk4 deletion or G2A substitution on microgametocyte DNA replication, mitotic spindle assembly, and exflagellation. G2A mutation mimics cdpk4 deletion with ca. 70% of microgametocytes remaining at the 1N state and another 20% blocked at the diploid stage (n = 3). Consistently G2A substitution completely blocks exflagellation (n = 3) but does not prevent formation of mitotic spindles (n = 2). Error bars show standard deviations, *** Student's T-test, p≤0.001. (B) Western blot analysis showing expression of CDPK4-2xmyc and CDPK4G2A-2xmyc proteins in complemented CDPK4-KO lines. Migration of the CDPK4G2A-2xmyc isoform around 63 kDa (i63) is affected, suggesting it is myristoylated while the 55 kDa isoform (i55) migration is not affected suggesting it is not myristoylated. (C) CDPK4-2xmyc and CDPK4G2A-2xmyc are both mainly detected in the PBS fraction. A minor population of CDPK4G2A-2xmyc i63 is partly recovered in membrane-associated or NaCl fractions while the CDPK4G2A-2xmyc i55 is fully solubilised in PBS. Antibodies against the ERD2 integral membrane protein and the soluble PRF protein were used as controls. (D) M57A substitution leads to expression of only i63, indicating that i55 originates from a second translation start at methionine 57. (E) A line expressing CDPK4M57A-2xmyc shows a strong defect in exflagellation but is not affected in DNA replication and axoneme assembly indicating that the short non-myristoylable CDPK4 isoform is mainly required to control late gametogenesis but not to initiate the first round of DNA replication. Error bars show standard deviations, n = 3, *** Student's T-test, p≤0.001. (F) The two major CDPK4 isoforms and their identified roles during gametogenesis.
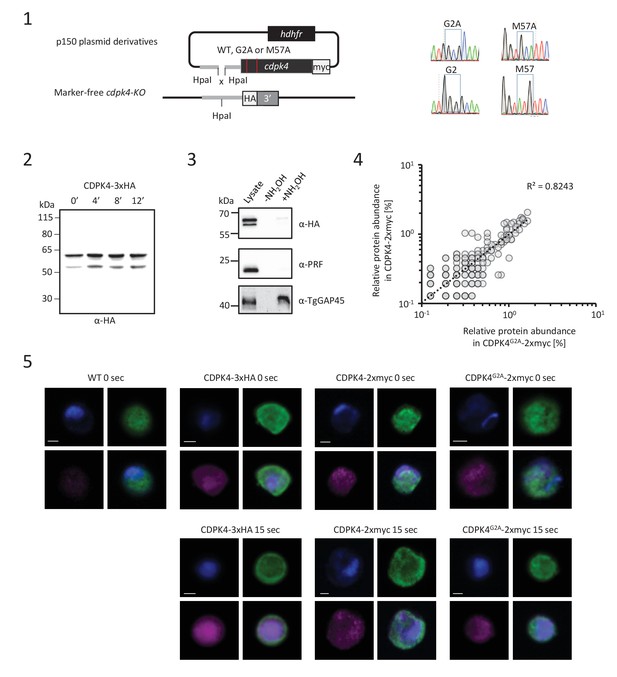
CDPK4 myristoylation does not affect the kinase localisation and related protein-protein interactions.
(1) Complementation of a CDPK4-KO line with CDPK4G2A-2xmyc or CDPK4M57A-2xmyc alleles and genotyping. (2) Western blot analysis of CDPK4-3xHA during gametogenesis indicates that the same migration pattern is observed at 0, 4, 8 and 10 min after stimulation with XA. (3) Acyl-RAC assay suggesting an absence of CDPK4 palmitoylation. Western blot analysis of CDPK4-3xHA gametocytes after acyl-biotin exchange in the absence (−NH2OH) or presence (+NH2OH) of hydroxylamine. Cleavage of the thioester bond, linking palmitate to a putative cognate cysteine residues, by hydroxylamine should create reactive cysteine residues that can be detected by the thio-reactive resin. No signal is observed for CDPK4-3xHA or profilin. Toxoplasma gondii tachyzoites lysates processed in parallel together with an α-TgGAP45 against the palmitoylated protein TgGAP45 were used as a positive technical control for S-acylated protein enrichment. (4) Immunofluorescence assays did not allow the detection of any obvious differences in CDPK4G2A-2xmyc localisation at 0 or 15 s post activation suggesting that CDPK4 myristoylation is not a major determinant for the cellular localisation of CDPK4. Scale bars = 1 μm. (5) No difference in relative abundance of protein co-immunoprecipitated with CDPK4-2xmyc and CDPK4G2A-2xmyc could be detected suggesting myristoylation does not affect CDPK4 interaction with its partners. Data is from one biological replicate.
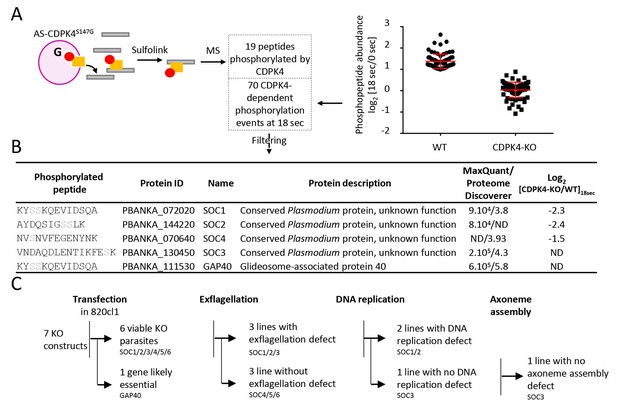
Identification of CDPK4-dependent phosphorylation during the first seconds of gametocyte activation.
(A) Analogue sensitive-CDPK4S147G uses N6-phenylethyl ATP-γS (Red) to thiophosphorylate its substrates while the CDPK4S147M allele that cannot accommodate N6-phenylethyl ATP-γS is used as a control. Thiophospho-tryptic peptides are captured by SulfoLink Coupling Resin and submitted to LC-MS/MS analysis. Nineteen phosphopeptides were found to be thiophosphorylated by AS-CDPK4S147G. Phosphoproteomic analysis revealed that 70 phosphopeptides on 61 proteins showed a 2-fold increase at 18 s post XA activation in the WT but not in the CDPK4-KO line. Data are from two biological replicates. (B) Among these latter, SOC1, SOC2 and SOC4 are phosphorylated by AS-CDPK4S147G and could represent early effectors of CDPK4. Conversely, GAP40 and SOC3 were not differentially phosphorylated in the CDPK4-KO line but were robustly identified as phosphorylated by AS-CDPK4S147G and could represent CDPK4 effector to control late gametogenesis. List of corresponding peptides and description of their proteins, MaxQuant intensity/Proteome Discoverer Xcorr, and log2[CDPK4-KO/WT]18sec phosphorylation ratio; sites assigned as phosphorylated by the two search engines are highlighted in grey. (C) GAP40 could not be deleted in asexual stages, suggesting an essential role in erythrocytic asexual multiplication. Knocking-out SOC4 or the SOC5 and SOC6 controls did not produce any defect in exflagellation nor DNA replication while SOC1, 2 and 3 KO lines showed a strong reduction in exflagellation. For SOC1-KO and SOC2-KO lines a defect in DNA replication was observed while SOC3-KO was not impaired in DNA replication and axoneme assembly.
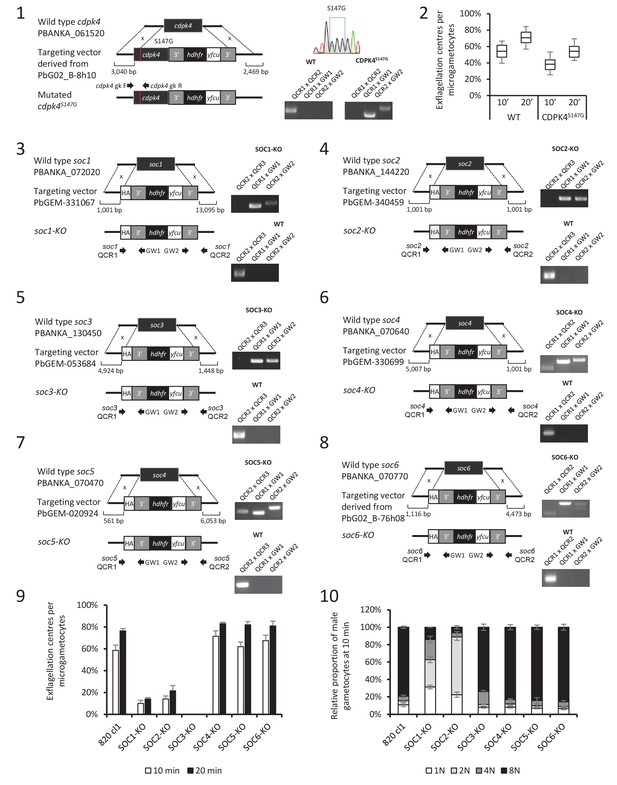
Identification and preliminary characterisation of CDPK4 substrates.
(1) Generation of a CDPK4S147G clonal line and genotyping. (2) A CDPK4S147G clonal line shows a slight decrease in exflagellation at both 10 and 20 min after stimulation by XA. The range of whisker plots indicates the 2.5–97.5% percentile, the box includes 50% of all values and the horizontal line shows median values. Data are representative of two biological replicates. (3–8) Generation and genotyping of SOC4/5/6-KO non-clonal lines and of SOC1/2/3-KO clonal lines and their phenotyping for exflagellation (9) and DNA replication during male gametogenesis (10).
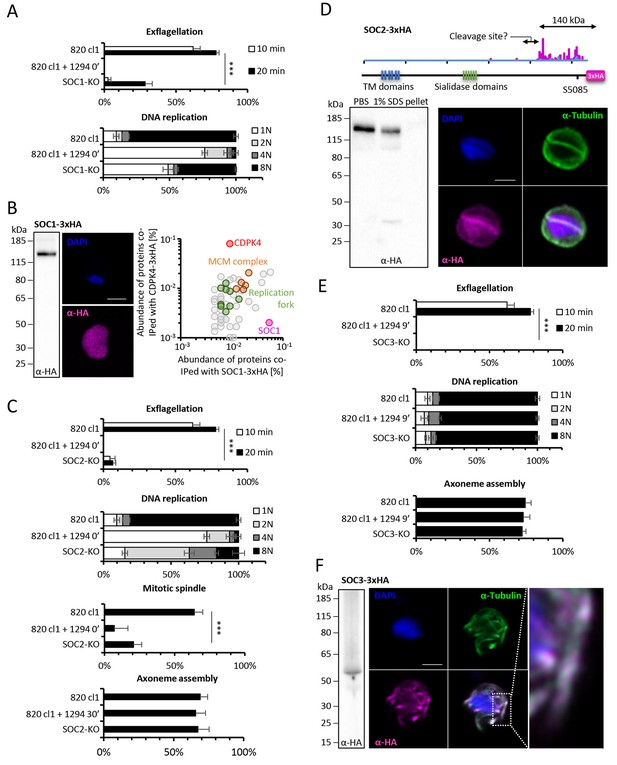
Functional characterisation of three identified substrates of CDPK4.
(A) A SOC1-KO line shows a strong reduction in exflagellation (n = 3). SOC1 is required only for the 1N/2N transition as parasites reaching the 2N level were able to further progress to the octoploid state (n = 2). Error bars show standard deviations, *** Student's T-test, p≤0.001. (B) SOC1-3xHA shows a diffuse cytoplasmic distribution (scale bar = 2 µm) but interacts with CDPK4 and proteins of the MCM complex. (C) SOC2-KO line is strongly impaired for exflagellation (n = 3). SOC2 is not required for the 1N/2N transition but for each following transition indicating it is not involved in the initiation of DNA replication nor for DNA replication per se but most likely during the three successive endomitoses (n = 2). Consistently, a strong reduction in parasites exhibiting mitotic spindles at 1 min post-activation is observed in the SOC2-KO line (n = 2). However no defect in axoneme assembly could be detected (n = 2). Error bars show standard deviations, *** Student's T-test, p≤0.001. (D) SOC2 is predicted to be a 645 kDa polytopic protein. Immunoprecipitation of SOC2-3xHA recovers peptides covering the last 1707 a.a. only; corresponding unique spectral counts are indicated in pink. Similarly co-immunoprecipitation of CDPK4 only recovered peptides covering the last 1137 a.a. of SOC2; corresponding unique spectral counts are indicated in blue. The C-terminal fragment colocalises with mitotic spindle α-tubulin but not with peripheral α-tubulin. Scale bar = 2 µm. (E) SOC3 is not required for DNA replication (n = 2) and axoneme assembly (n = 2) but is essential for exflagellation (n = 3). Error bars show standard deviations, *** Student's T-test, p≤0.001. (F) SOC3 colocalises with axonemal α-tubulin in exflagellating gametes. Scale bar = 2 µm.
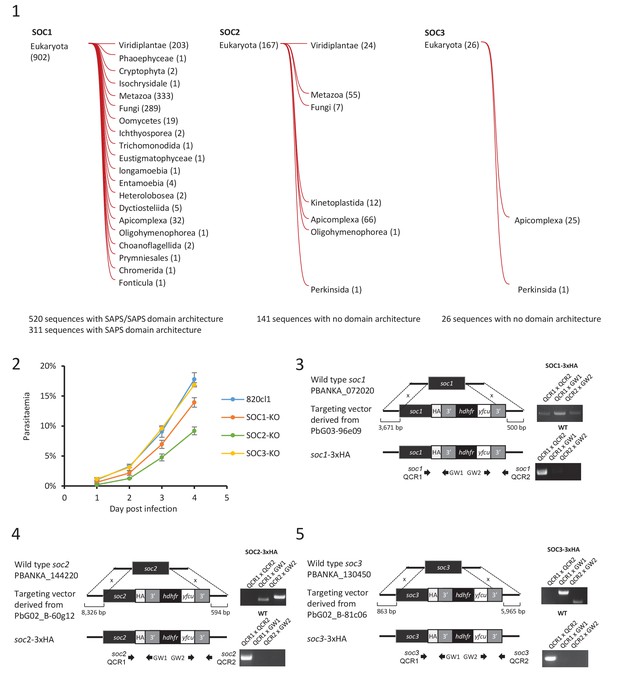
Characterisation of three identified substrates of CDPK4.
(1) Taxonomic distribution of homologues of each characterised substrate of CDPK4 determined by HMMER (http://hmmer.org/). Numbers represent the number of homologues in each clade. (2) Effect of gene knock-out of soc1/2/3 on asexual blood stages growth showing that deletion of SOC1 and SOC2 are associated with different fitness costs. Error bars show standard deviations from three infections. (3–5) Generation and genotyping of SOC1/2/3-3xHA non-clonal lines.
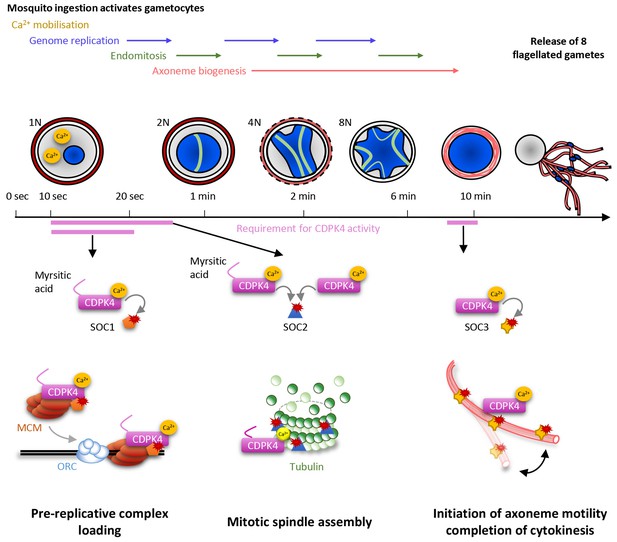
Model showing the roles of CDPK4 during Plasmodium male gametogenesis.
Activation of male gametocytes by a drop in temperature and xanthurenic acid leads to mobilisation of intracellular calcium within a lag phase of ten seconds. Calcium activates a myristoylated isoform of CDPK4 which activity is required during the next ten seconds to facilitate the loading of the MCM2-7/Cdt1 complex onto ORC1-5/Cdc6 complex requiring SOC1. The subsequent assembly of mitotic spindles also requires (i) CDPK4 activity within the 30 fist second of gametogenesis and (ii) SOC2 which is a microtubule-associated protein phosphorylated by CDPK4 within the twenty fist seconds of gametogenesis. Between 30 s and 9 min, CDPK4 activity is neither required for the three successive rounds of genome replication and endomitosis nor for the assembly of the eight axonemes. Finally, activity of a non-myristoylable isoform of CDPK4 is required for the initiation of axoneme motility via the CDPK4 substrate SOC3.
Additional files
-
Supplementary file 1
Number of unique spectral counts detected for CDPK4, MCM5 and SOC1 interactors.
- https://doi.org/10.7554/eLife.26524.014
-
Supplementary file 2
Peptides thiophosphorylated by AS-CDPK4S147G.
- https://doi.org/10.7554/eLife.26524.015
-
Supplementary file 3
Phosphoproteome profiling of 2.34 and CDPK4-KO lines at 0 and 18 s post-activation.
- https://doi.org/10.7554/eLife.26524.016
-
Supplementary file 4
Parasite lines generated in this study.
- https://doi.org/10.7554/eLife.26524.017
-
Supplementary file 5
Oligonucleotides used in this study.
- https://doi.org/10.7554/eLife.26524.018





