Sec17/Sec18 act twice, enhancing membrane fusion and then disassembling cis-SNARE complexes
Figures
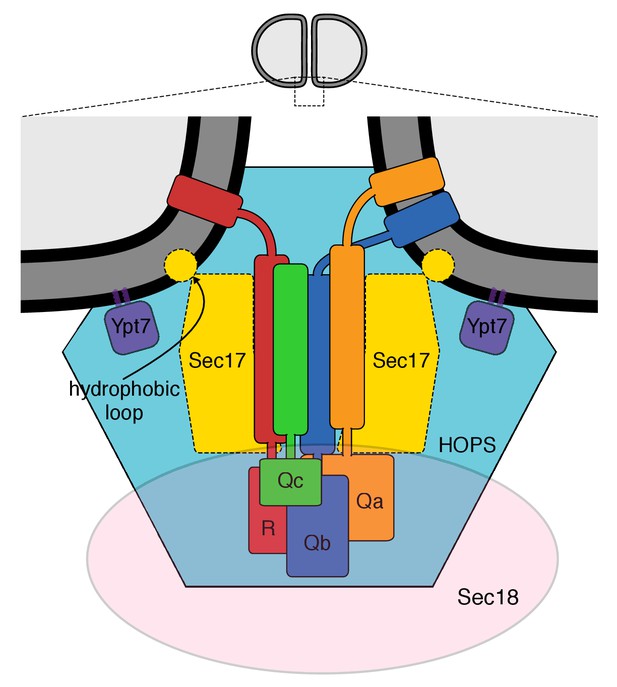
The multiple interactions of proteins and membranes for fusion.
A working model of the proteins and membranes as assembled prior to vacuole or proteoliposome fusion. From Zick et al. (2015).
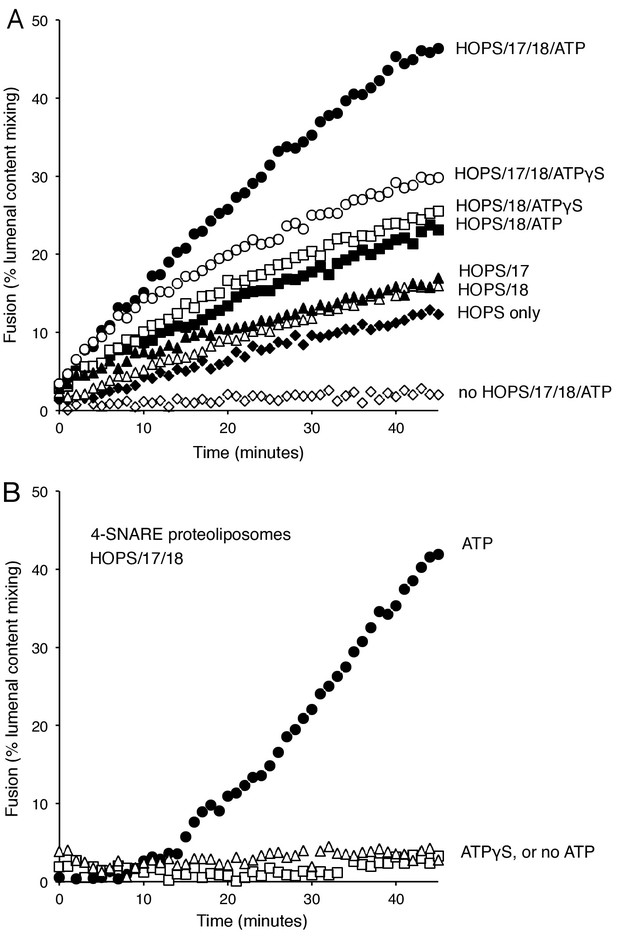
Stimulation of fusion by Sec18 does not require ATP hydrolysis, while Sec18-mediated disassembly of cis-SNARE complexes requires ATP hydrolysis.
(A) The fusion of Ypt7(GTP):R-SNARE proteoliposomes with Ypt7(GTP):QaQb-SNARE proteoliposomes with added Qc-SNARE and HOPS is stimulated by Sec17, Sec18, and ATP or ATPγS. (B) ATP hydrolysis is required for 4-SNARE proteoliposome fusion. Proteoliposomes bearing Ypt7:GTP, each of the four vacuolar SNAREs at a 1:32,000 molar ratio to lipids, and either Cy5-streptavidin or biotinylated-phycoerythrin were prepared, mixed, and assayed for fusion in the presence of HOPS, Sec17, Sec18 and ATP (filled circles), ATPγS (open squares), or without addition of adenine nucleotide (open triangles), as described in Materials and Methods. The data shown in this and the following figures were typical of 3 repeat experiments; means and standard deviations for each experiment are presented in the corresponding Supplementary Figures.
-
Figure 2—source data 1
Source data file (Excel) for Figure 2A.
- https://doi.org/10.7554/eLife.26646.004
-
Figure 2—source data 2
Source data file (Excel) for Figure 2B.
- https://doi.org/10.7554/eLife.26646.005
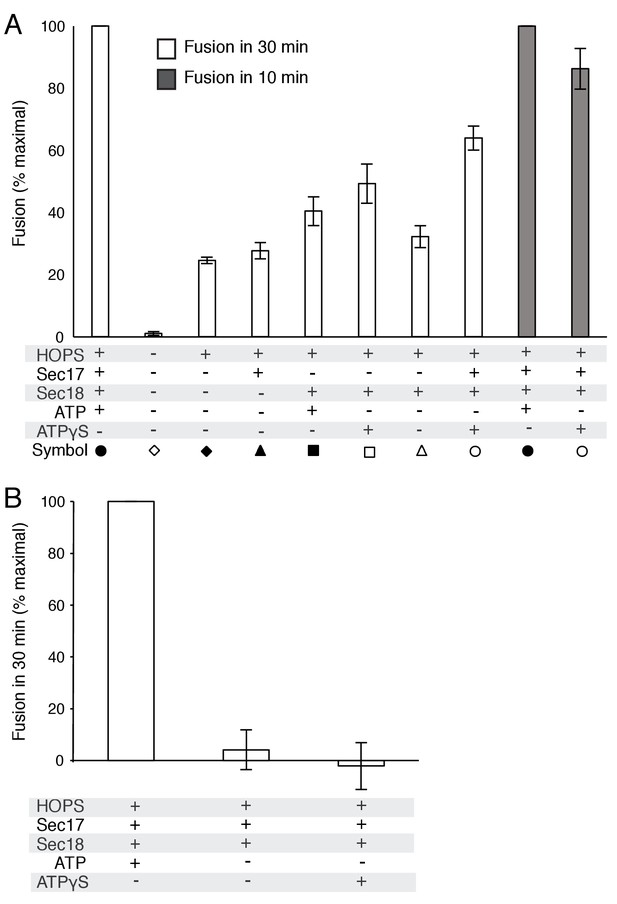
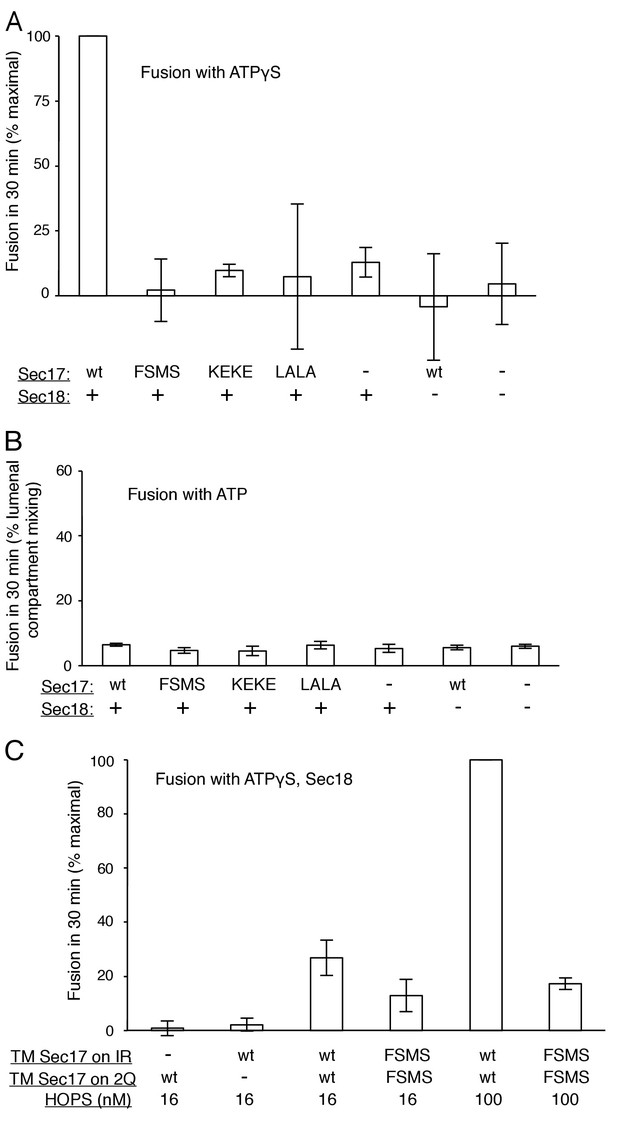
Average and standard deviations of fusion after 30 min for triplicate assays as in Figure 2, relative to the maximal fusion condition.
In panel A, the relative fusion after 10 min is also shown for reactions with HOPS, Sec17, Sec18, and ATP or ATPγS.
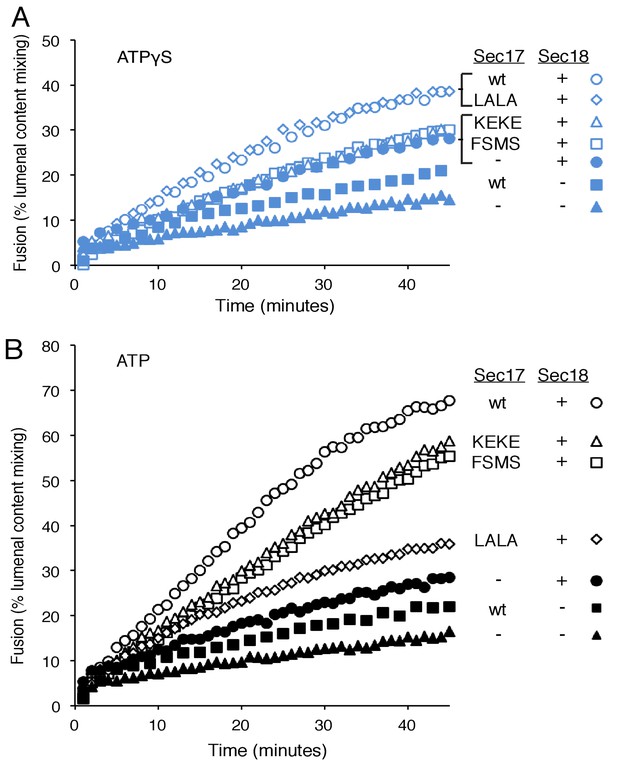
Fusion of Ypt7(GTP):R-SNARE and Ypt7(GTP):QaQb-SNARE proteoliposomes in the presence of HOPS, Qc, ATP or ATPγS, and Sec17 (wild type or mutants) and/or Sec18, as indicated.
Fusion conditions were as described in Materials and methods. (A) Fusion with ATPγS. (B) Fusion with ATP. Sec17 was wild type (wt) or had the mutations F21S,M22S (FSMS), K159E,K163E (KEKE), or L291A,L292A (LALA). These data are from the same experiment.
-
Figure 3—source data 1
Source data file (Excel) for Figure 3 Parts A, B, and C.
- https://doi.org/10.7554/eLife.26646.008
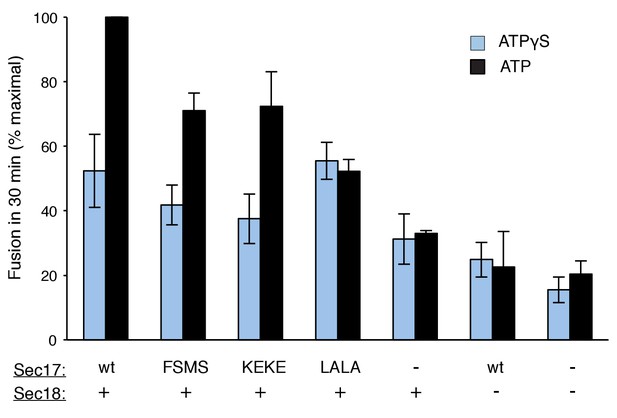
Average and standard deviations of fusion after 30 min for triplicate assays as in Figure 3, relative to the maximal fusion condition.
Blue bars are for incubations from part A, with ATPγS, and black bars from part B, with ATP.
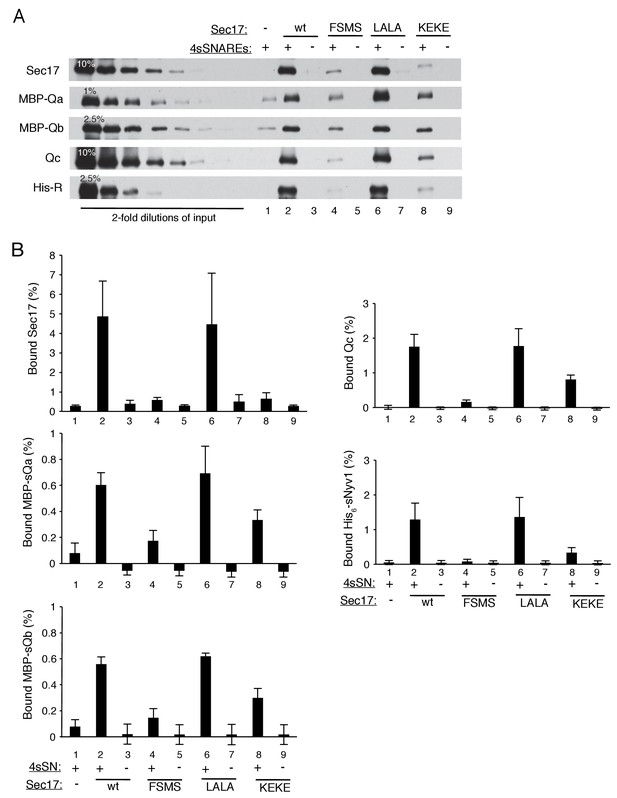
The assembly of a complex of soluble vacuolar SNAREs with Sec17 allows both the sSNAREs and the Sec17 to bind to liposomes (Zick et al., 2015).
(A) The sSNAREs (deprived of their trans-membrane anchor domains) were mixed with Sec17 and protein-free liposomes, incubated, and the liposomes and bound proteins were isolated by floatation, as described (Zick et al., 2015) with modifications. Liposomes were prepared with Rhodamine-DHPE (Invitrogen Eugene, OR) in place of NBD-DHPE, and gradients comprised of 80 µl sample overlaid with 50 µl of each density step were centrifuged in a TLA100 rotor (Beckman Coulter) for 3 hr at 100,000 rpm. Liposome-bound proteins were assayed by immunoblot and analyzed with UN-SCAN-IT software (Silk Scientific, Orem, UT, RRID:SCR_013725). Sec17 was wild-type (wt) or had the mutations F21S,M22S (FSMS), K159E,K163E (KEKE), or L291A,L292A (LALA). (B) Average and standard deviations of the per cent of each protein which bound to liposomes in triplicate repeats of the experiment in part A.
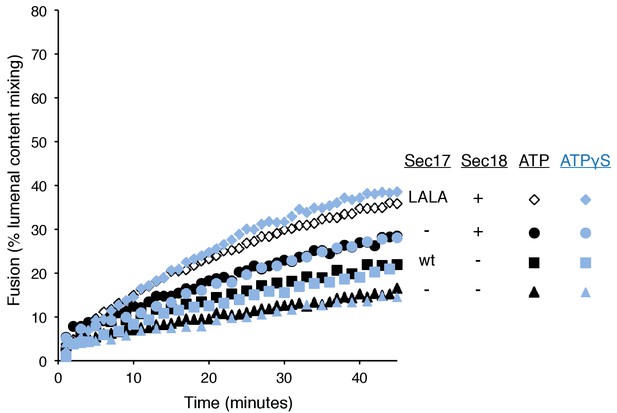
Without productive interaction between Sec17 and Sec18, there are equivalent fusion kinetics in the presence of ATP or ATPγS.
Data are from Figure 3A and B, which were part of the same experiment.
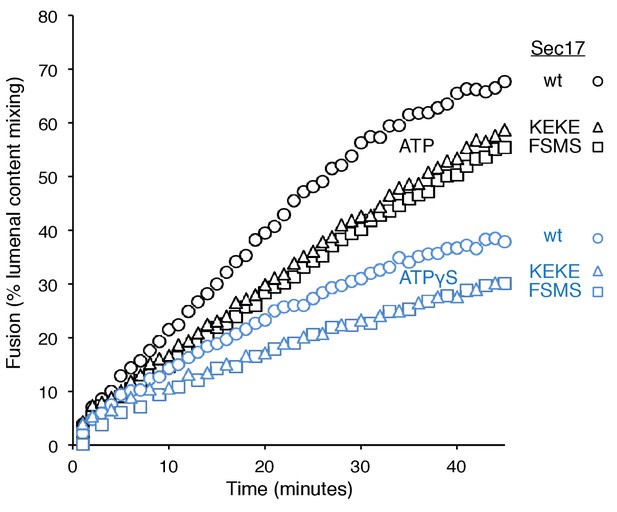
The Sec17 K159E,K163E or F21S,M22S mutations diminish fusion to the same extent in incubations with ATP or ATPγS, and there is similar enhancement of fusion by hydrolysable ATP for each Sec17, wild-type or mutants.
Data are from Figure 3A and B, which were part of the same experiment.
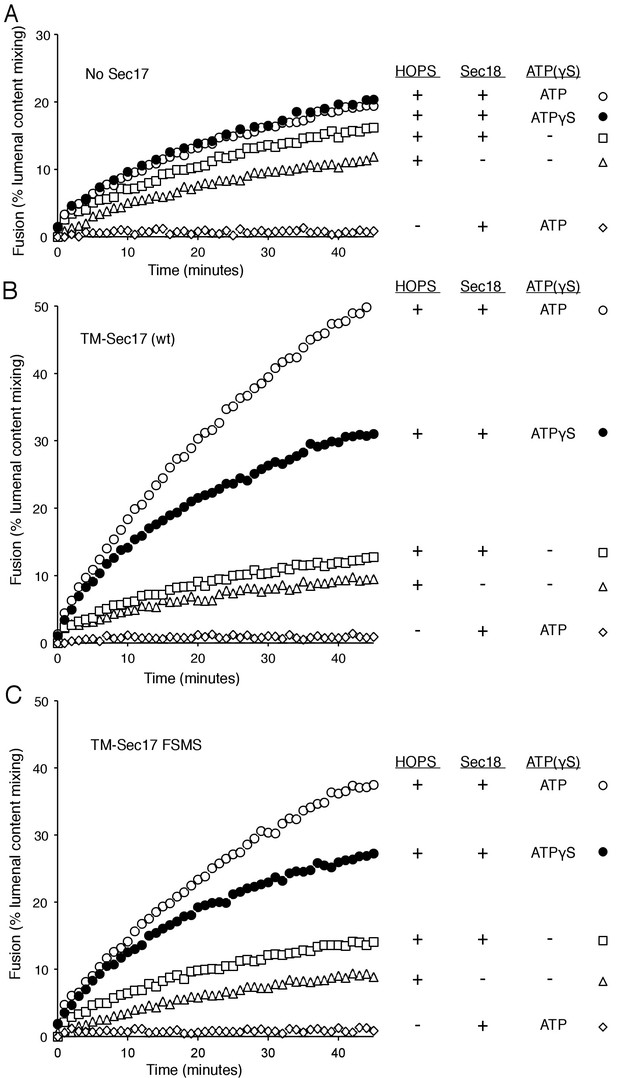
Membrane-anchoring Sec17 bypasses the requirement for the apolarity of its N-domain loop.
The transmembrane domain from the Qb-SNARE Vti1 was joined to the N-terminus of Sec17 (wt) or Sec17 F21S,M22S (FSMS) to give TM-Sec17 (wt) and TM-Sec17 FSMS. (A) No Sec17, (B) TM-Sec17 (wt), or C). TM-Sec17 FSMS were included at a 1:8000 molar ratio to lipids in the reconstitution of Ypt7(GTP):R-SNARE and Ypt7(GTP):QaQb-SNARE proteoliposomes (Ypt7 and each SNARE were added at 1:8000 and 1:40,000 molar ratios to lipids, respectively). Fusion incubations with these proteoliposomes had HOPS, Qc, Sec18, and no ATP, ATP, or ATPγS as indicated.
-
Figure 4—source data 1
Source data file (Excel) for Figure 4 Parts A, B, and C.
- https://doi.org/10.7554/eLife.26646.014
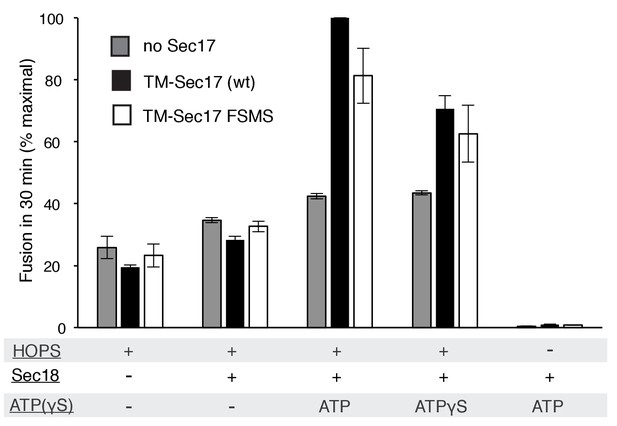
Average and standard deviations of fusion after 30 min for triplicate assays as in Figure 4, relative to the maximal fusion condition.
Proteoliposomes bore TM-Sec17 wild-type or TM-Sec17 F21S,M22S as indicated, and reactions had HOPS, Sec18, Sec18 with ATP, or Sec18 with ATPγS as indicated.
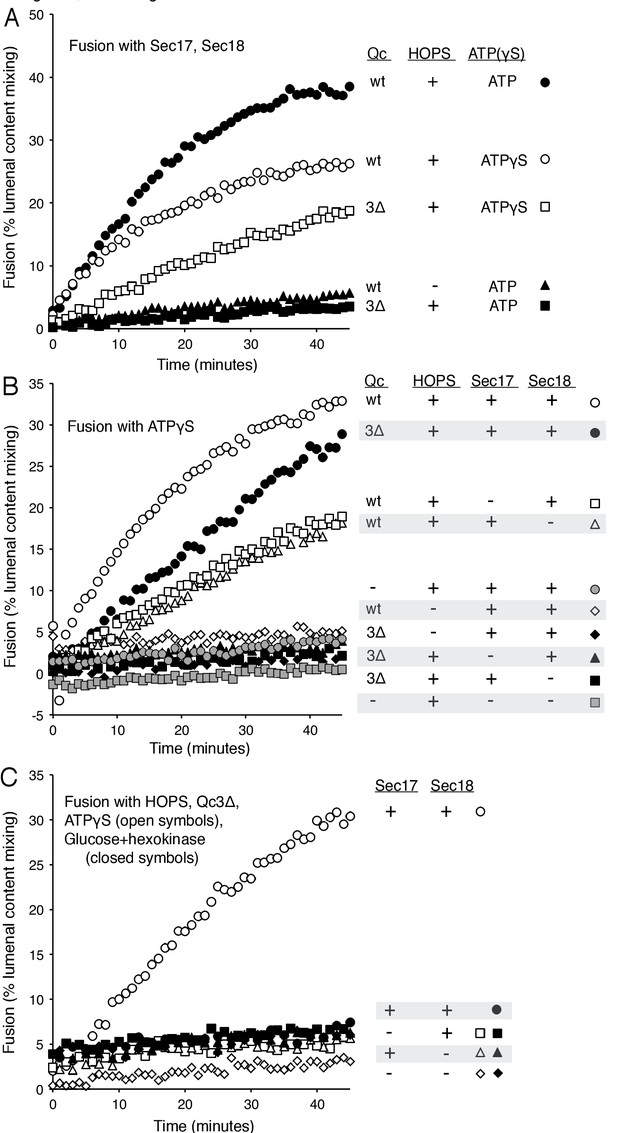
Requirements for fusion in the absence of C-terminal SNARE domain zippering.
(A) Fusion is lost without SNARE zippering unless ATP is replaced by ATPγS. The fusion of proteoliposomes with Ypt7:GTP and either the R-SNARE or QaQb-SNAREs in the presence of Sec17 and Sec18 was assayed with ATP or ATPγS, with wild-type Vam7 or Vam7-3Δ and with or without HOPS, as indicated, as described in Materials and Methods. The final concentration of MgCl2 was 0.69 mM. (B) With incomplete SNARE zippering, fusion requires HOPS, Sec17, and Sec18. Fusion reactions had ATPγS, and either wild-type Qc or Qc-3Δ as well as Sec17, Sec18, and HOPS. Fusion reactions with wild-type Qc are depicted with open symbols, those with Qc-3Δ are filled symbols. Fusion with Vam7-3Δ was lost upon omission of HOPS (filled black diamonds), Sec17 (solid triangles), or Sec18 (solid squares). Fusion reactions without any Qc are in gray symbols, in the presence of HOPS, Sec17 and Sec18 (circles) or without Sec17/Sec18 (squares), as described in Materials and methods. (C) Arrested SNARE zippering requires Sec17, Sec18, and ATPγS for fusion. Fusion of Ypt7(GTP):R-SNARE proteoliposomes with Ypt7(GTP):QaQb-SNARE proteoliposomes was performed as described in Materials and Methods, but with HOPS and the C-terminally truncated Qc SNARE Vam7-3Δ and with either ATPγS (open symbols) or glucose/hexokinase (filled symbols). Incubations had Sec17 and Sec18 (circles), Sec18 (squares), Sec17 (triangles), or neither (diamonds). The final concentration of MgCl2 was 0.69 mM.
-
Figure 5—source data 1
Source data file (Excel) for Figure 5A.
- https://doi.org/10.7554/eLife.26646.017
-
Figure 5—source data 2
Source data file (Excel) for Figure 5B.
- https://doi.org/10.7554/eLife.26646.018
-
Figure 5—source data 3
Source data file (Excel) for Figure 5C.
- https://doi.org/10.7554/eLife.26646.019
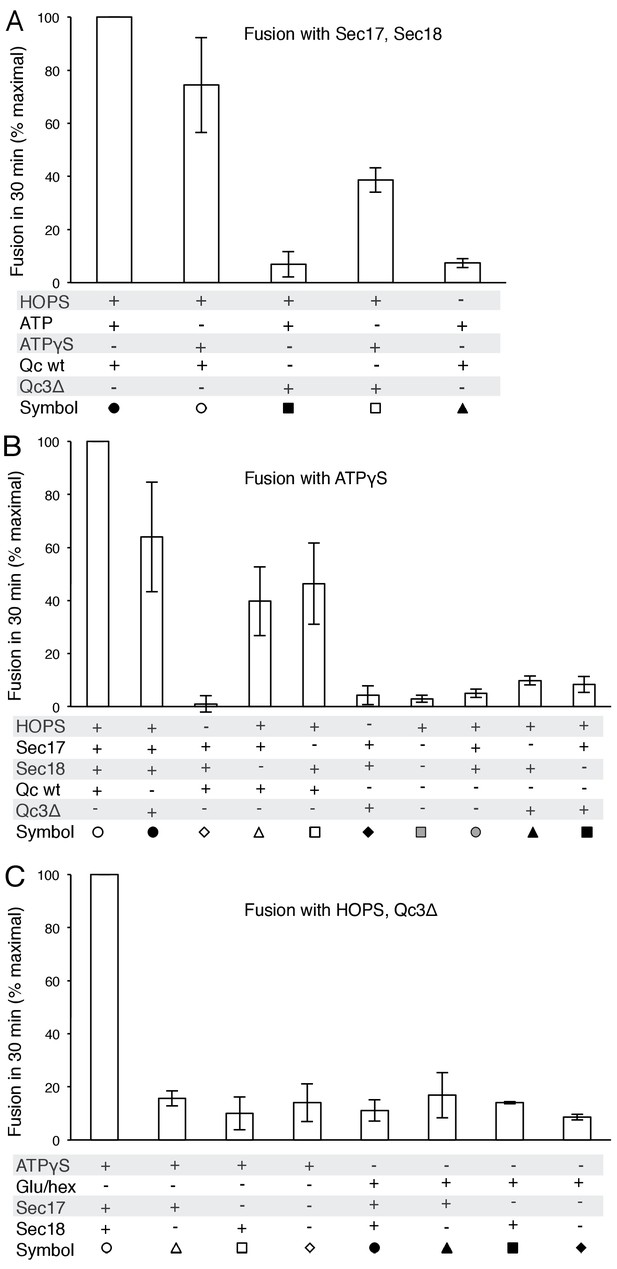
Average and standard deviations of fusion after 30 min for triplicate assays as in Figure 5, relative to the maximal fusion condition.
https://doi.org/10.7554/eLife.26646.020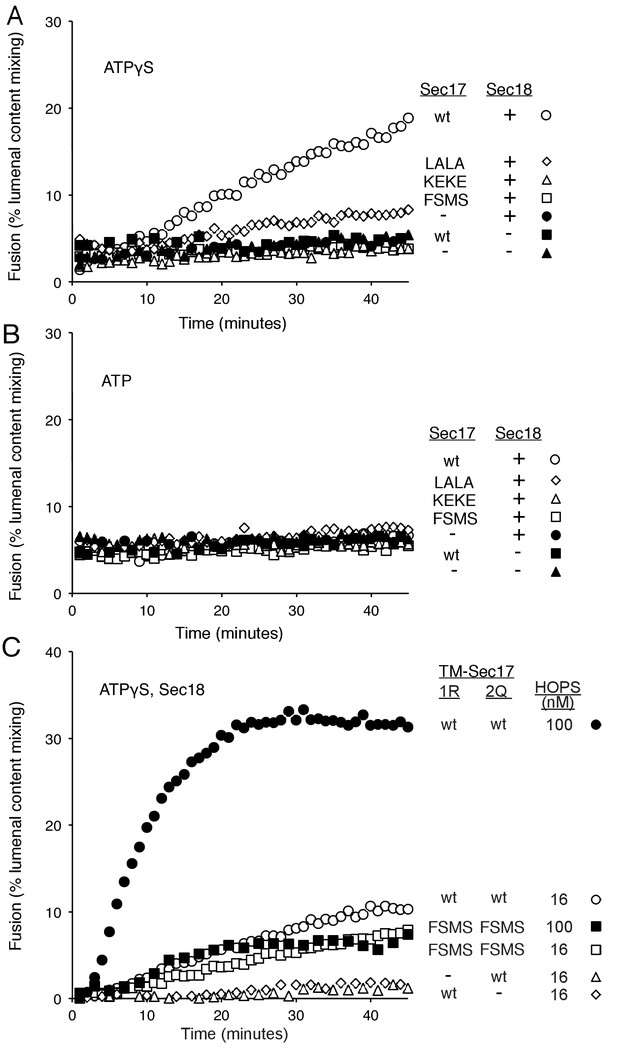
Fusion of proteoliposomes with Qc-3Δ.
(A) Fusion reactions were performed with ATPγS as in Figure 3A, but with Qc-3Δ instead of Qc. (B) Fusion reactions were performed as in part A, but with ATP instead of ATPγS. Sec17 was wild type (wt) or had the mutations F21S,M22S (FSMS), K159E,K163E (KEKE), or L291A,L292A (LALA). (C) Fusion with Qc-3Δ needs the Sec17 apolar loop for more than membrane anchoring. Fusion with ATPγS and Qc-3Δ was with proteoliposomes bearing TM-Sec17 wild-type or TM-Sec17 F21S,M22S, and with 16 nM HOPS (open symbols) or 100 nM HOPS (filled symbols), as indicated.
-
Figure 6—source data 1
Source data file (Excel) for Figure 6 Parts A and B.
- https://doi.org/10.7554/eLife.26646.022
-
Figure 6—source data 2
Source data file (Excel) for Figure 6C.
- https://doi.org/10.7554/eLife.26646.023
Average and standard deviations of fusion after 30 min for triplicate assays as in Figure 6C, relative to the maximal fusion condition in panels A and C, and as % lumenal compartment mixing in panel B.
https://doi.org/10.7554/eLife.26646.024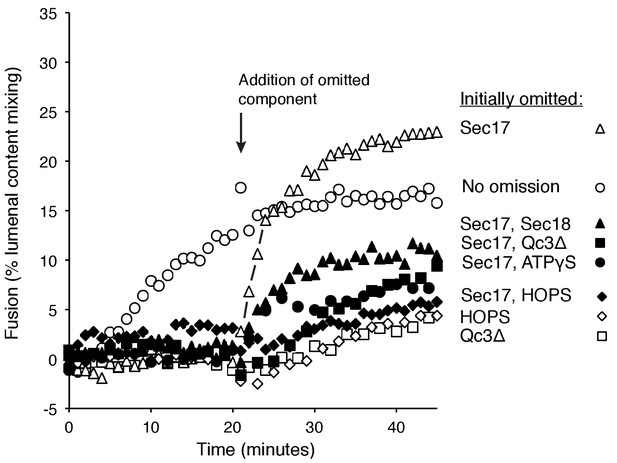
Assembly of an intermediate for rapid, Sec17-triggered fusion.
Fusion incubations were performed with Ypt7:GTP, 1R-SNARE proteoliposomes and Ypt7:GTP, QaQb-SNARE proteoliposomes in the presence of HOPS, Sec17, Sec18, ATPγS, and Qc-3Δ as described in Materials and methods, with all components (open circles) or with omission of HOPS (open diamonds), Qc-3Δ (open squares), Sec17 (open triangles), Sec17 and Sec18 (filled triangles), Sec17 and Qc-3Δ (filled squares), or Sec17 and ATPγS (filled circles). After 20 min, the initial incubations (16 μl) were supplemented with 4 μl of Rb150 or of this buffer with the previously omitted components, so that each reaction then contained all the components at the same final concentrations: HOPS (31 nM), Sec17 (1.2 μM), Sec18 (72 nM), ATPγS (0.7 mM), and Qc-3Δ (16 nM).
-
Figure 7—source data 1
Source data file (Excel) for Figure 7.
- https://doi.org/10.7554/eLife.26646.026
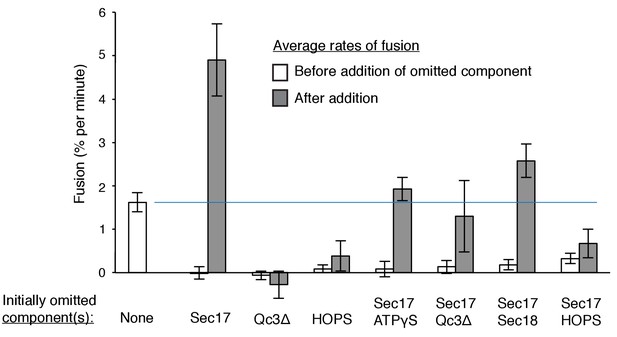
Average and standard deviations of the fusion rate for triplicate assays as in Figure 7.
Open bars are the average rate for the first 20 min, filled bars are the average rates for the first 6 min after the restoration of the missing component(s) at 20 min. One outlier data point was omitted in this analysis.





