An insect-like mushroom body in a crustacean brain
Figures
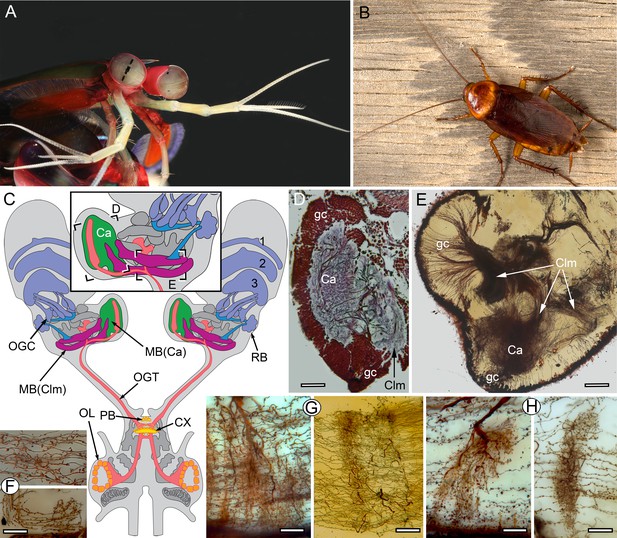
The stomatopod brain and mushroom body organization common to stomatopods and insects.
(A) Eyestalks and antennules of Gonodactylus smithii. With the largest compound eyes of any panarthropod, and long antennules, mantis shrimps are equipped for high-level visual and olfactory perception. Information from both sensory systems reaches the mushroom body calyces and its column-like extensions. (B) Periplaneta americana, the American cockroach provides the hexapod species for comparisons with the stomatopod. (C) The central forebrain of the stomatopod Gonodactylus smithii is defined by the central complex (CX) and associated protocerebral bridge (PB). This contrasts with the forebrain’s enormous lateral protocerebra in which optic neuropils and mushroom bodies (MB) dominate. Bifurcating axon bundles of the main tract from the olfactory lobes (OL) to the protocerebrum, called the olfactory-globular tract [OGT shown orange] supply the mushroom body calyces [MB(Ca), green]. Proximal to the optic lobes, units of the optic glomerular complex (OGC) and the reniform body (RB) send relays to the mushroom body columns [MB(Clm), magenta]. Boxed areas in the inset, show the locations of panels D-F. (D) Silver-stained calyx (Ca) associated with just one of four columns (Clm) is shown with the dense population of globuli cells (gc) that supply all four columns. (E) Golgi impregnation resolving the calyx (Ca) and three of the four mushroom body columns (Clm) originating from the globuli cell layer (gc). (F) Parallel fibers decorated by beaded or spiny arborizations indicative of pre- and postsynaptic sites (Strausfeld and Meinertzhagen, 1998) are a defining feature of insect mushroom bodies. Here parallel fibers in G. smithii (upper panel) are compared to those in the α′-lobe of Drosophila melanogaster (lower panel). (G) Regions along mushroom body columns in G. smithii (left) and the mushroom body lobes of the cockroach P. americana (right) show corresponding orthogonal networks of parallel fibers intercepted by the arborizations of afferent (input) neurons. (H) Parallel fibers intercepted by dendritic trees of output (efferent) neurons in a mushroom body column of G. smithii (left) and a lobe of P. americana (right). Scale bars: b, c, 100 µm; d-f, 20 µm.
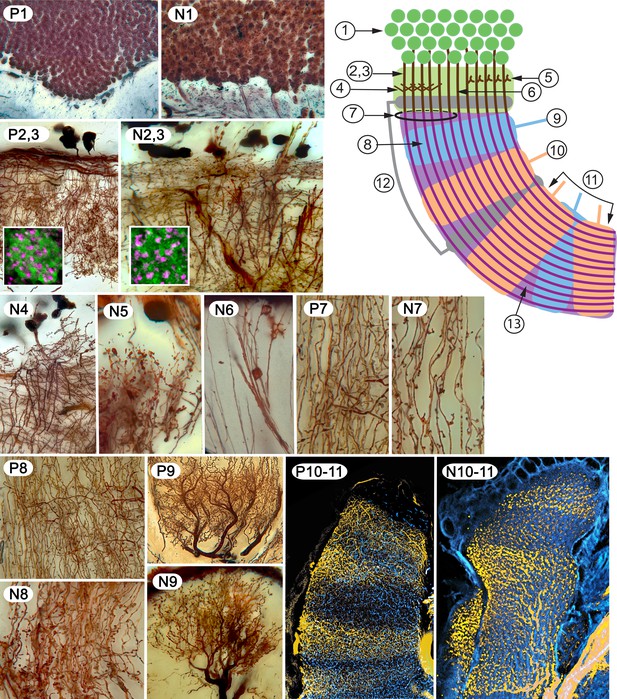
Examples of characters 1–13 shared by insect (Periplaneta americana, (P) and stomatopod (Neogonodactylis oerstedii, (N).
1. Density of dorsal cluster of globuli cells exceeds 3.0 × 106/mm3. 2. Distal calycal/domed (synonymous) neuropil. 3. Distal microglomeruli (in inset to 2,3). 4. Spiny globuli cell dendrites. 5. Clawed globuli cell dendrites. 6. Globuli cells lacking dendrites. 7. Parallel fibers. 8. Orthogonal networks. 9. Efferent dendritic trees intersecting columns/lobes. 10. Aminergic afferents. 11. Afferent/efferent processes partitioned in sequential domains. 12. GAD/GABAergic recurrent pathways. 13. Elevated expression of proteins required for learning and memory.
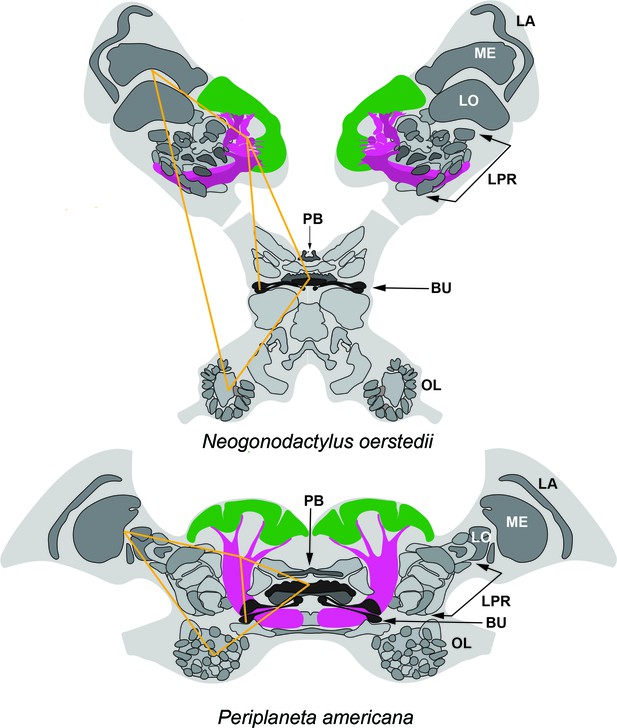
Corresponding topologies of insect and stomatopod cerebral regions.
Globuli cell clusters shown green, lobe/columnar extension shown magenta. Abbreviations: LA, lamina; ME, medulla; LO, Lobula; LPR lateral protocerebrum; PB, protocerebral bridge; BU, lateral bulbs of the central complex; OL, olfactory lobes. Yellow lines connect corresponding locations. The eyestalks of N. oerstedii have been foreshortened.
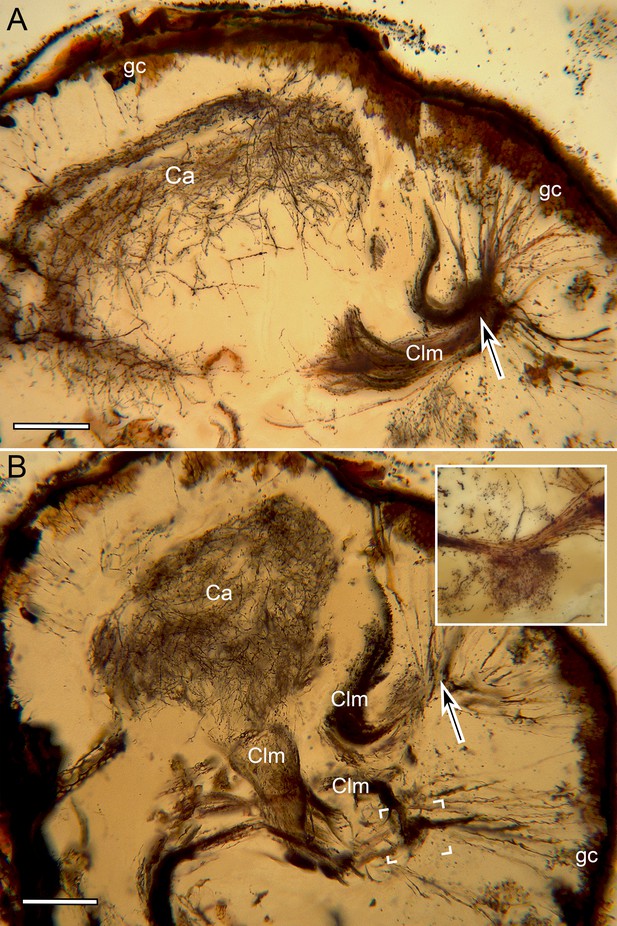
Mushroom body calyces in Neogonodactylus oerstedii.
(A, B). Two successive Golgi-impregnated sections of the lateral protocerebrum show impregnated globuli cells (gc) with their converging neurites (at arrows) denoting the origin of columns (Clm). Calyces (Ca) are associated with just two of the four columns. One calyx is large, bilayered (A) with the deeper inner volume (B) giving rise to its column. The second calyx is very small (boxed area, panel B enlarged in inset upper right) and entirely separate from the larger calyx. Scale bar A, B: 100 µm.
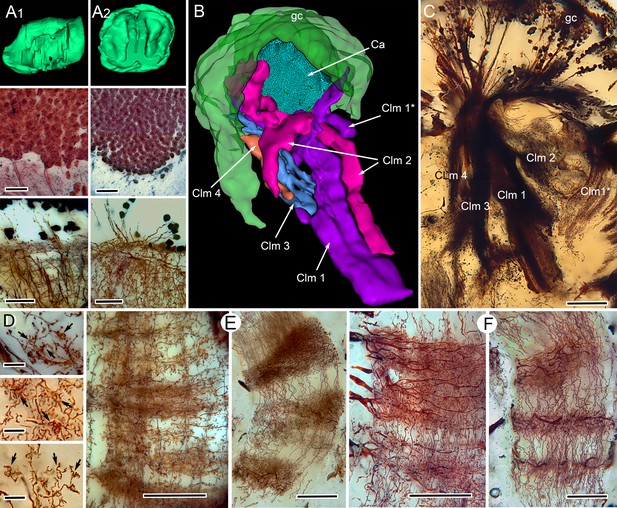
Correspondences of stomatopod and insect mushroom bodies.
(A1, A2 upper panels) Globuli cell domains reconstructed from serial sections from Neogonodactylus oerstedii (A1) and Periplaneta americana (A2). (A1, A2 middle panels): Globuli cell packing (reduced-silver-stained sections). (A1, A2 lower panels) Golgi impregnations showing corresponding neuronal organization in calyces. (B) Three-dimensional reconstruction from serial sections of N. oerstedii, stained by reduced silver, demonstrates a mushroom body consisting of a globuli cell layer (gc, green), a calyx (Ca, cyan) and four distinct columns (Clm 1–4, purple, magenta, blue, orange), with Clm 1 accompanied by distal branch (Clm 1*). (C) Golgi impregnation of the columns, each defined by its characteristic arrangement of parallel fibers. (D) Clawed specializations (arrowed) are mushroom body identifiers found on dendrites arising distally from parallel fibers. These are shown here in the stomatopod (upper panel), cockroach (middle panel) and honeybee (lower panel). (E, F) The dense spiny processes of efferent (output) neurons form discrete domains along the length of the columns. Paired corresponding arrangements are here demonstrated from the stomatopod N. oerstedii (left in each pair) and P. americana. Scale bars: A, panels, second row, 25 µm; lower panels, 20 µm; C, 100 µm; D, scales in all panels, 10 µm; E-F, all scales = 50 µm.
Three dimensional reconstructions of the stomatopod mushroom body.
Serial section reconstructions demonstrate the dispositions and relative sizes of mushroom body columns originating from the dorsal globuli cell cluster.
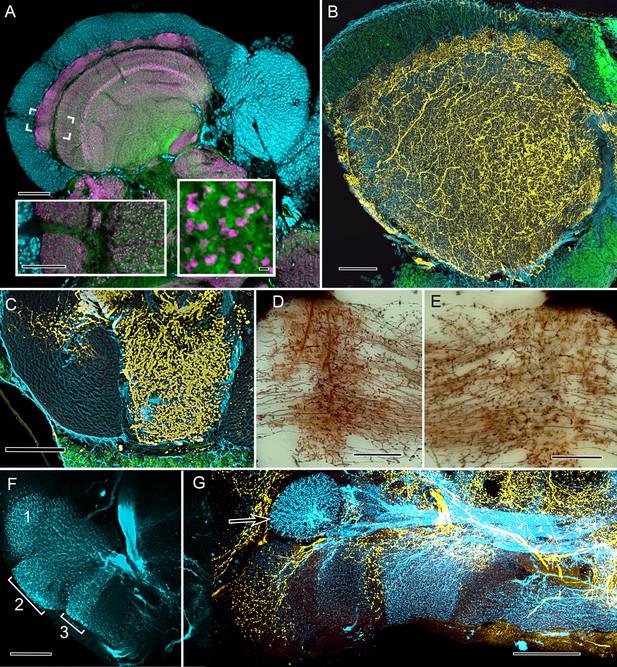
Cardinal features of the stomatopod calyx and neuromodulatory neurons in MB columns.
(A) Double Immunolabeling with actin-phalloidin (green), anti-synapsin (magenta), and the fluorescent nuclear stain Syto-13 (blue) resolves globuli cells and the concentric synaptic layers in the calyx of Neogonodactylus oerstedii. Brackets indicate the location of the left inset, an enlargement of the outer two layers, the deeper of which is characterized by synaptic microglomeruli (enlarged in right inset), which correspond to those in insect calyces. (B) Top-down view of a 60 µm section cut tangential to the stomatopod calyx labeled with anti-GAD (yellow), anti-α-tubulin (blue) and Syto-13 (green). (C) Part of mushroom body column 2 showing a dense domain of GAD-immunoreactive processes (yellow) with a smaller arborization upper left. (D) Afferent terminal in column 2 with profusely decorated varicosities possibly corresponding to those of the anti-GAD labeled arborization in (C). (E) An intrinsic orthogonal network provided by branches from parallel fibers. (F) Anti-TH whole-mount labeling shows "segmentation" into three afferent domains . (G) Double-labeling with anti-5HT (yellow) and anti-TH (blue) shows segmentation of column 1 by domains occupied by serotonin and tyrosine hydroxylase. A single-TH afferent, shown as a transverse profile, reveals column 3 in cross section (arrow, left). Scale bars A and loer left inset 100 µm, inset lower right 1 µm. C, 100 µm; C-F, 50 µm; G, 100.
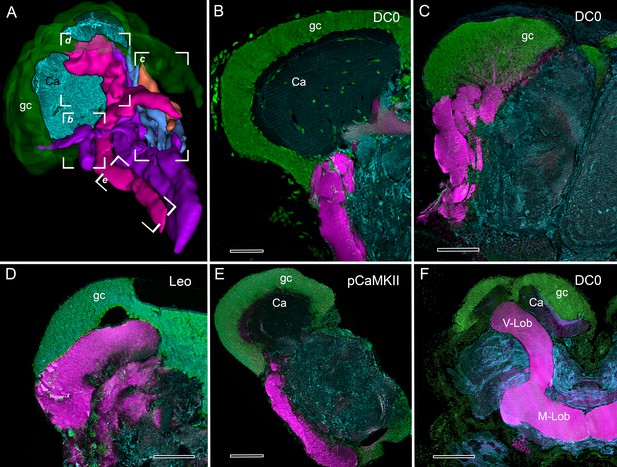
Selective affinity of stomatopod and insect mushroom bodies to antisera against proteins involved in Drosophila learning and memory.
(A) Reconstruction from reduced-silver serial sections of the mushroom body of Neogonodactylus oerstedii (globuli cell layer, green; calyx, cyan; columns are shown purple, magenta, blue, orange). Brackets indicate panels B-E showing corresponding regions of the columns. (B–E) Mushroom body columns in N. oerstedii labeled by anti-DC0 in magenta (B, C). (D) Labeling for Leo (magenta). (E) Labeling for pCaMKII (magenta). (F) Mushroom body lobes of Periplaneta americana labeled with antibodies against DC0 in magenta and showing globuli cells (gc), the underlying calyx (Ca) and vertical and medial lobes (V-Lob, M-Lob). In panels B-F, globuli cells are labeled green with the nucleic acid stain, Syto-13; antibodies against α-tubulin (cyan) reveal background structure. Scale bars: 100 µm.
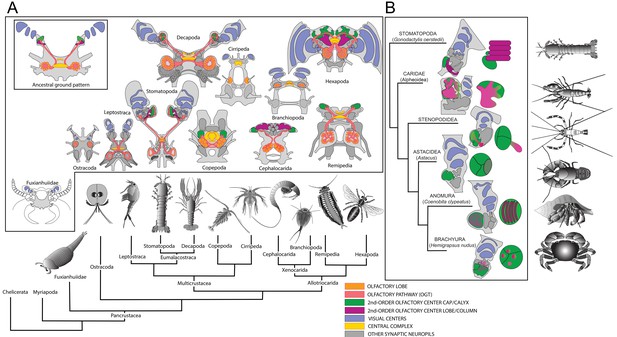
Molecular phylogeny (from Oakley et al., 2013) showing the pattern of derived cerebral arrangements across Pancrustacea (Crustacea+Hexapoda) with corresponding organization in Hexapoda and Stomatopoda.
(A) Molecular phylogenetics resolves Hexapoda more closely related to Remipedia than either is to Malacostraca (see Oakley et al., 2013). In insects and stomatopods mushroom bodies share all the key characters identified in this study, and the brains of these two groups possess other common organization: three nested optic neuropils, fusion of three brain neuromeres – all identified as a ground pattern in the lower Cambrian euarthropod Fuxianhuia protensa (Ma et al., 2012) – as well as corresponding central complexes. These features define the ancestral ground pattern of the pancrustacean brain. In contrast, all other brains have evolved divergent modifications including reduction and loss of some or all of these ancestral components. (B) Phyletic positions of five eumalacostracan sister groups to Stomatopoda showing modifications of the ancestral columnar mushroom body present in Stomatopoda. In representative species belonging to these lineages, column-like extensions from globuli cells have become enormously enlarged (Alpheoidea), greatly reduced but still indicated by DC0 immunoreactivity (Stenopodidea), subsumed as discrete layers within the hemiellipsoid body (Anomura), or lost entirely (Brachyura and Astacidea). Confocal images of each example, comparing these with the lobed organization of the Drosophila mushroom body, are shown in Figure 5—figure supplements 1 and 2. In Stenopodidea and crownward, the calyx observed in Stomatopoda assumes the morphology of a hemiellipsoid body.
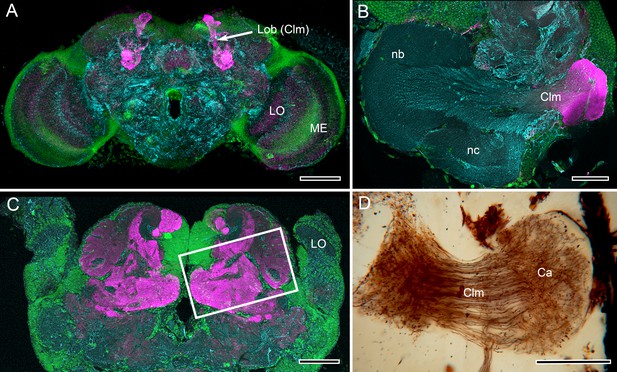
Mushroom body lobes are recognized by their DC0 immunoreactivity across pancrustaceans.
(A) Vertical lobes (Lob) of the Drosophila mushroom bodies. (B) The single DC0 immunoreactive lobe originating from the modified calyx of the cleaner shrimp Stenopus hispidus. The calyx is synonymous with the hemiellipsoid body described by Sullivan and Beltz (Montgomery et al., 2016), here shown at a level that depicts their ‘b’ and ‘c’ neuropils (nb, nc). The lobe corresponds to their ‘lateral protocerebral complex’ (ref 17, Figure 3D). (C) Large convoluted lobes of the mushroom body of the pistol shrimp Alpheus bellulus. As in other pistol shrimps, the visual system is greatly reduced and the lateral protocerebrum is situated entirely within the head capsule. (D) Alpheus bellulus. Golgi-impregnation of some of the calyx and parallel fibers of the mushroom body lobes. Anti-DC0 is labeled in magenta, cell bodies are labeled in green with the nucleic acid stain Syto-13, and anti-α-tubulin is labeled in cyan. Abbreviations as for Figure 1. Scale bars: A, 50 µm; B, C, D. 100 µm.
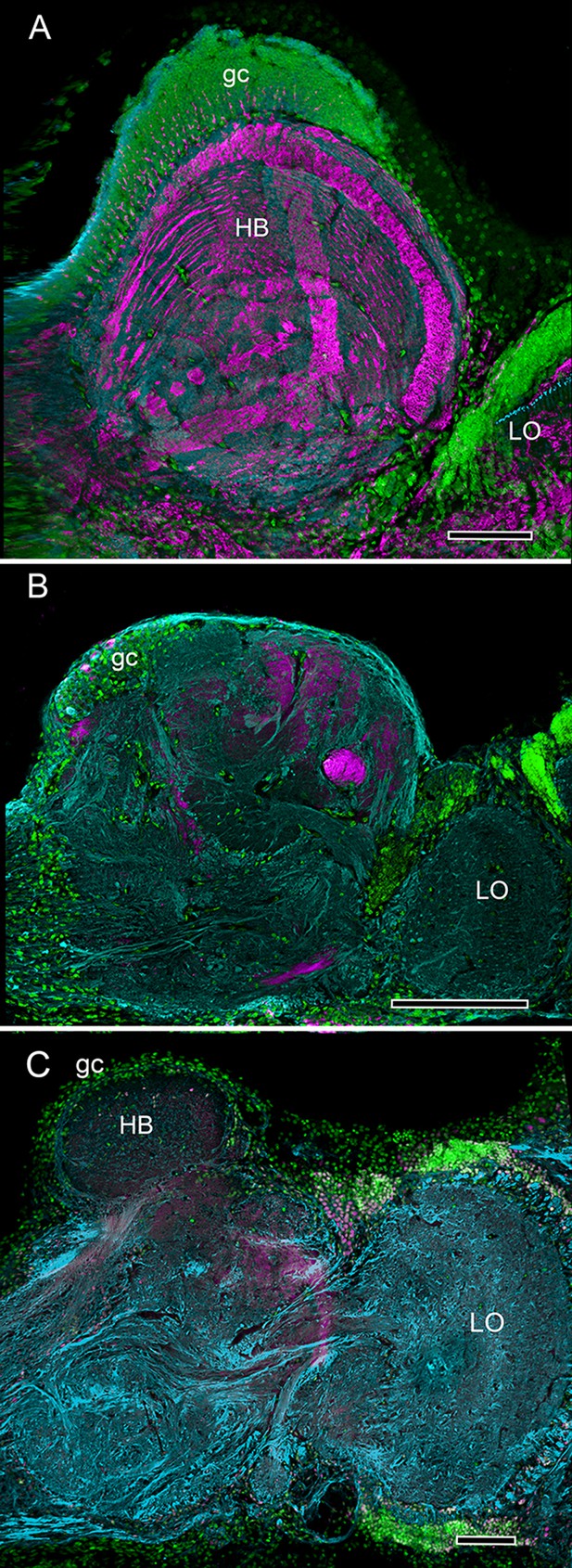
Divergence of decapod hemiellipsoid bodies.
(A) In the land hermit crab Coenobita clypeatus (Anomura) elevated expression of DC0 resolves strata in the hemiellipsoid body (HB) containing orthogonal networks corresponding to those of insect mushroom body lobes (Brown and Wolff, 2012; Wolff et al., 2012). (B) In the shore crab Hemigrapsus nudus the hemiellipsoid body lacks elevated DC0 although an axon tract from the lobula (LO) shows clear DC0 immunoreactivity. (C) In the freshwater crayfish Procambarus clarkii the subdivided hemiellipsoid body shows no elevated DC0 although structures distal to it, corresponding to the reniform body show moderate expression. Scale bars: A, C 100 µm; B 50 µm.
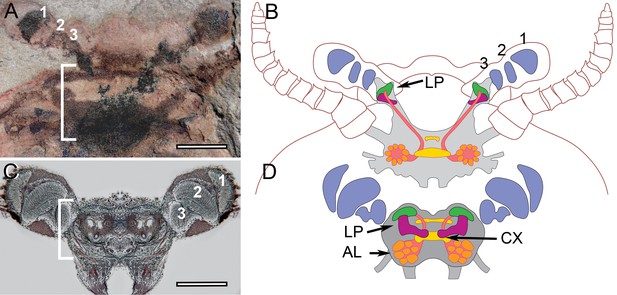
Stability of brain organization over time.
(A) Fossil brain of the lower Cambrian stem arthropod Fuxianhuia protensa. (B) Centers common to Stomatopoda and Hexapoda superimposed onto the brain profile of F. protensa. (C) Silver-stained brain of the extant coleopteran Chauliognathus profundus, shown at the same magnification as that of panel A. (D) Schematic of the brain of C. profundus. The brain of F. protensa preserved as a carbon film (Ma et al., 2015) corresponds to that of C. profundus in comprising 3 fused brain segments, which are indicated by, (1) an enlarged forebrain attached to 3 nested optic neuropils, and two peripheral sensory nerve trunks, (2) to the antennae and (3) post-antennal appendage (not shown at this level). The lateral protocerebrum (LP) in the stem arthropod extends into the eyestalk, suggesting that is the plesiomorphic condition, whereas the insect protocerebrum’s location in the head capsule is the derived condition.
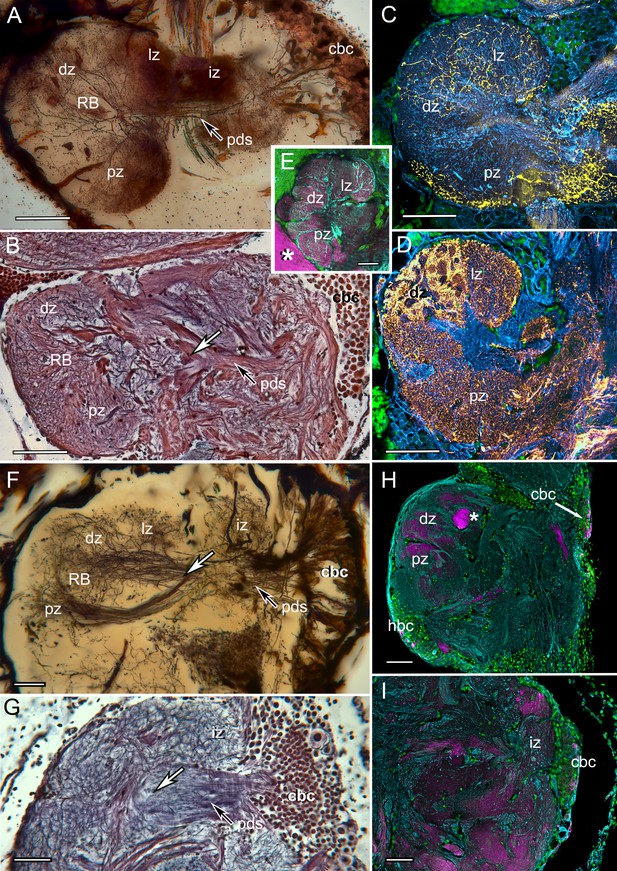
The reniform body: A multicomponent center possibly unique to Eumalacostraca.
Neurohistological techniques confirm the presence of reniform bodies in the stomatopod Neogonodactylus oerstedii and the varunid shore crab Hemigrapsus nudus. (A) Golgi impregnation through a depth of 250 µm of the lateral protocerebrum of N. oerstedii showing that the reniform neuropils originate from a dense cluster of cell bodies (cbc) that provide a pedestal-like arrangement (pds) of smooth parallel neurites supplying four domains: an initial zone (iz), lateral zone (lz), distal zone (dz) and proximal zone (pz), which are also resolved in silver-stained thin sections (B). Antisera against serotonin (yellow) (C) and GAD (yellow) (D) further confirm distinctive zones in the stomatopod reniform body, the dz comprising discrete GAD-delineated glomerular divisions. (E) Anti-DC0 expressed in the lz, dz, and pz domains. Asterisk indicates part of a mushroom body column. (F) Golgi impregnation in H. nudus, showing three domains, lz, dz, and pz, corresponding to those in N. oerstedii. The cbc provides a pedestal-like domain comprising numerous smooth undecorated parallel neurites that bifurcate (at arrow) to provide the pz and dz+lz neuropils. This bifurcating trajectory is also resolved in silver-stained sections of N. oerstedii (arrow in B) and H. nudus (arrow in G). (H, I) DC0 (magenta) in H. nudus is absent or at very low levels except for a bundle of axons (asterisk in H) leading centrally from the optic lobes. Scattered neuron cell bodies in the cell body cluster (cbc) show elevated DC0 immunoreactivity (H, I), as do some cell bodies of the hemiellipsoid body (hbc). Scale bars: A-E, 100 µm; F-I, 50 µm.
Tables
Matrix of characters 1–13 defining insect-type mushroom bodies.
‘1’ denotes character presence, ‘0’ denotes character absence, and “– “denotes lack of data. Character list. 1. Density of dorsal cluster of globuli cells exceeds 3.0 × 106/mm3. 2. Distal calycal/domed (synonymous) neuropil. 3. Distal microglomeruli. 4. Spiny globuli cell dendrites. 5. Clawed globuli cell dendrites. 6. Globuli cells lacking dendrites. 7. Parallel fibers. 8. Orthogonal networks. 9. Efferent dendritic trees intersecting columns/lobes. 10. Aminergic afferents. 11. Afferent/efferent processes partitioned into domains. 12. GAD/GABAergic recurrent pathways. 13. Elevated expression of proteins required for learning and memory. Examples of characters. For character 1–11, see Figure 1—figure supplement 1; for example of character 12 see Figure 3B,C; for examples of character 13 see Figure 4 and Figure 5—figure supplements 1 and 2. Species list. Neopteran insects: Drosophila melanogaster, Periplaneta americana, Odonata (dragonflies, darters): Perithemis tenera, Libellula depressa, Aeschna sp.). Stomatopod crustaceans: Neogonodactylus oerstedii, Gonodactylus smithii. Decapod crustaceans: Alpheus bellulus, Stenopus hispidus, Procambarus clarkii, Coenobita clypeatus, Hemigrapsus (H. nudus and H. oregonensis).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. melanogaster | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P. americana | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Odonata | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | 1 |
| Stomatopoda | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| A. bellulus | 1 | 1 | – | 1 | 1 | 0 | 1 | 1 | – | – | – | – | 1 |
| S. hispidus | 1 | 1 | – | – | – | – | 1 | 1 | 0 | – | – | – | 1 |
| C. clypeatus | 1 | 1 | – | 0 | 1 | 0 | 0 | 1 | 0 | – | 1 | – | 1 |
| P. clarkii | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 |
| Hemigrapsus | 0 | 1 | – | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29889.018





