Mechanotransduction: Two views of the same stimulus
The ability of sensory neurons to detect and respond to mechanical force using a process known as mechanotransduction allows us to interpret and navigate the physical world around us. Although a number of different proteins have been linked to mechanotransduction, how they interact with each other or with other signaling pathways in a single sensory neuron is still not well understood.
The study of mechanotransduction has focused primarily upon ion channels that span cell membranes and open in response to mechanical perturbation. In most cases, the channel protein itself is the mechanosensor, responding either to changes in the physical properties of the cell membrane or the tension in a molecular anchor within the cell (Ranade et al., 2015). This promotes the false impression that each type of channel operates independently. However, individual sensory neurons normally express a variety of ion channels and other proteins that allow them to respond to many different signals, such as touch, chemicals, and both hot and cold temperatures (Geffeney and Goodman, 2012; Abraira and Ginty, 2013).
Recent studies have begun to unravel the complex relationship between mechanosensory channels and their environment inside a single neuron. For example, some channels have been shown to interact with members of a superfamily of membrane proteins called the G-protein coupled receptors (GPCRs). The binding of an external signal molecule (ligand) to a GPCR stimulates signaling pathways inside the cell that are involved in a large variety of processes. Furthermore, some of the components in these pathways can interact with mechanosensory channels in response to pain or inflammation (Geppetti et al., 2015; Veldhuis et al., 2015).
All GPCRs contain a seven transmembrane domain embedded within the cell membrane, along with one or more domains inside the cell that trigger the downstream signaling pathways. Members of a subgroup known as the adhesion GPCRs also contain an unusual extracellular domain (ECD) that is thought to interact with components of the extracellular matrix, a scaffold-like structure that surrounds cells to provide structural support (Langenhan et al., 2016)
The ECD is linked to the transmembrane domains by another domain that allows some adhesion GPCRs to cut themselves into two pieces. It has been assumed that this “autoproteolysis” step, which splits the ECD away from the rest of the protein, is essential to activate adhesion GPCRs. Recent studies suggest that some adhesion GPCRs may detect mechanosensory information through the ECD when it is tethered to the extracellular matrix (Petersen et al., 2015; Scholz et al., 2015).
In 2015, a team of researchers led by Robert Kittel and Tobias Langenhan at the University of Würzburg reported that an adhesion GPCR called dCIRL may influence the activity of the NOMPC mechanosensory channel in the chordotonal organ of fruit fly larvae (Scholz et al., 2015). However, it was not clear whether the two proteins directly interact with each other. Now, in eLife, Kittel, Langenhan and co-workers – including Nicole Scholz as first author – report that dCIRL may modulate the activity of NOMPC by stimulating signaling pathways inside the neuron (Scholz et al., 2017).
The chordotonal organ plays crucial roles in a range of mechanosensory processes in fruit fly larvae, and Scholz et al. found that dCIRL (also known as latrophillin) and NOMPC co-localize to the same structures within the neurons in this organ. Mechanical stimuli trigger weaker responses in mutant larvae that are unable to produce dCIRL than they do in normal larvae. In addition, changing the length of the ECD modified the response of dCIRL to mechanical stimuli consistent with an essential role for the ECD in transducing the signal. Together, these results suggest that dCIRL interacts with unidentified ligands outside of the cell to modulate the activity of NOMPC and adjust how strongly a neuron responds to a mechanical stimulus.
Unlike the adhesion GPCRs studied in other animals, dCIRL does not require autoproteolysis to be correctly localized or activated in neurons. Moreover, dCIRL also appears to use different signaling pathways because it decreases the level of a signal molecule called cAMP in cells, whereas the adhesion GPCRs in other animals have the opposite effect (Müller et al., 2015). By using different signaling pathways, the various members of this GPCR subgroup may play different roles in different cell types or species.
The findings of Scholz et al. provide a potential process by which multiple signaling events (such as the complementary mechanical inputs from NOMPC and dCIRL) can interact within a single sensory neuron to precisely modulate the neuron's response to a mechanical perturbation (Figure 1). The diversity of the adhesion GPCR proteins across different cell types and species supports the hypothesis that the way adhesion GPCRs modulate mechanotransduction may also depend upon the type of mechanical signal they receive.
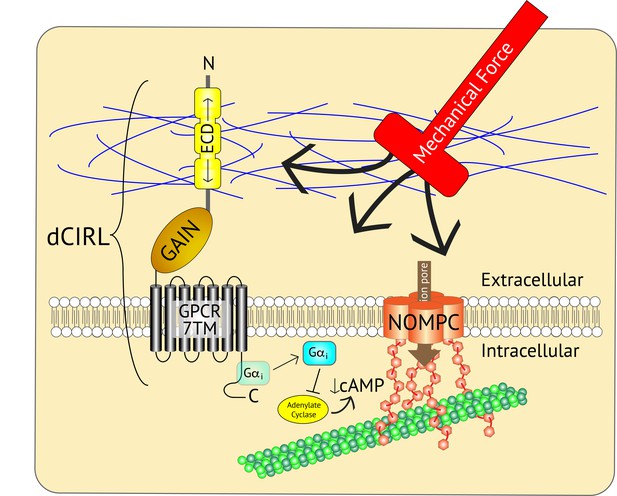
Adjusting the mechanosensory response of sensory neurons.
The ion channel NOMPC (orange) is a transmembrane protein that is anchored to the neuron’s cytoskeleton (green circles), allowing it to detect mechanical force. This stimulates the pore of the channel (brown arrow) to open, leading to the generation of an electrical signal within the neuron. At the same time, the extracellular domain (ECD) of another transmembrane protein, the adhesion GPCR dCIRL, detects the mechanical force differently as a result of being anchored to the extracellular matrix (blue strings). Activation of dCIRL decreases the level of cAMP in the cell, possibly due to the activation of a G protein (Gαi) that inhibits the enzyme that makes cAMP (known as adenylate cyclase). Unidentified signaling components downstream of cAMP alter NOMPC activity to modulate the strength of the overall response. In this manner, both extracellular and intracellular views of the same stimulus are combined to precisely adjust the neuronal response. GAIN, GPCR autoproteolytic domain. 7TM: seven transmembrane domain.
References
-
Adhesion G protein-coupled receptors in nervous system development and diseaseNature Reviews Neuroscience 17:550–561.https://doi.org/10.1038/nrn.2016.86
Article and author information
Author details
Publication history
Copyright
© 2017, Johnson
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,123
- views
-
- 213
- downloads
-
- 5
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Neuroscience
Cancer patients often experience changes in mental health, prompting an exploration into whether nerves infiltrating tumors contribute to these alterations by impacting brain functions. Using a mouse model for head and neck cancer and neuronal tracing, we show that tumor-infiltrating nerves connect to distinct brain areas. The activation of this neuronal circuitry altered behaviors (decreased nest-building, increased latency to eat a cookie, and reduced wheel running). Tumor-infiltrating nociceptor neurons exhibited heightened calcium activity and brain regions receiving these neural projections showed elevated Fos as well as increased calcium responses compared to non-tumor-bearing counterparts. The genetic elimination of nociceptor neurons decreased brain Fos expression and mitigated the behavioral alterations induced by the presence of the tumor. While analgesic treatment restored nesting and cookie test behaviors, it did not fully restore voluntary wheel running indicating that pain is not the exclusive driver of such behavioral shifts. Unraveling the interaction between the tumor, infiltrating nerves, and the brain is pivotal to developing targeted interventions to alleviate the mental health burdens associated with cancer.
-
- Neuroscience
Emotional responsiveness in neonates, particularly their ability to discern vocal emotions, plays an evolutionarily adaptive role in human communication and adaptive behaviors. The developmental trajectory of emotional sensitivity in neonates is crucial for understanding the foundations of early social-emotional functioning. However, the precise onset of this sensitivity and its relationship with gestational age (GA) remain subjects of investigation. In a study involving 120 healthy neonates categorized into six groups based on their GA (ranging from 35 and 40 weeks), we explored their emotional responses to vocal stimuli. These stimuli encompassed disyllables with happy and neutral prosodies, alongside acoustically matched nonvocal control sounds. The assessments occurred during natural sleep states using the odd-ball paradigm and event-related potentials. The results reveal a distinct developmental change at 37 weeks GA, marking the point at which neonates exhibit heightened perceptual acuity for emotional vocal expressions. This newfound ability is substantiated by the presence of the mismatch response, akin to an initial form of adult mismatch negativity, elicited in response to positive emotional vocal prosody. Notably, this perceptual shift’s specificity becomes evident when no such discrimination is observed in acoustically matched control sounds. Neonates born before 37 weeks GA do not display this level of discrimination ability. This developmental change has important implications for our understanding of early social-emotional development, highlighting the role of gestational age in shaping early perceptual abilities. Moreover, while these findings introduce the potential for a valuable screening tool for conditions like autism, characterized by atypical social-emotional functions, it is important to note that the current data are not yet robust enough to fully support this application. This study makes a substantial contribution to the broader field of developmental neuroscience and holds promise for future research on early intervention in neurodevelopmental disorders.






