Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson’s disease
Figures
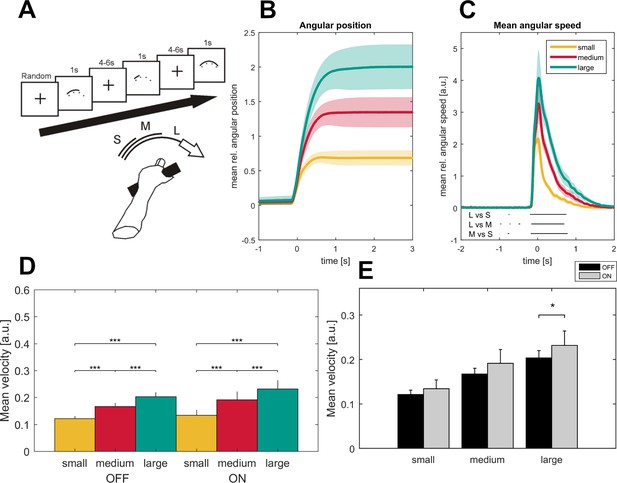
Task description and movement traces.
Patients were asked to perform cued forearm pronation movements of three different sizes (A) after presentation of a baseline fixation cross that resulted in hand movements of three different amplitudes (B; small - yellow, medium - red, large - green) and movement velocities (C) aligned on movement onset. Shaded areas indicate the standard error of the mean. Black lines below the movement velocities indicate statistical significance (p<0.05, FDR corrected). Movement velocity shows a stepwise increase toward the large movement condition in the ON- and OFF- medication state (D). Patients in the dopaminergic OFF state (n = 7, sides averaged for each patient) performed the task significantly slower only in the large movement condition (E).
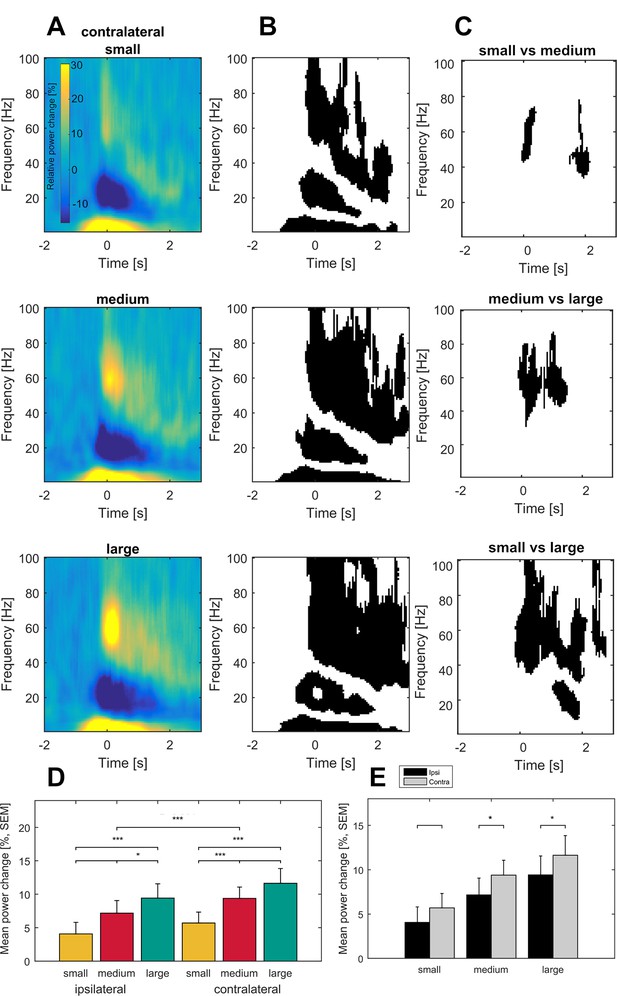
Grand average of subthalamic oscillatory activity aligned to movement onset in the dopaminergic ON state.
Baseline corrected time-frequency representations were averaged across contact pairs of one electrode, hemisphere in relation to the moved hands of each patient and across patients (A). Note the scaling of gamma amplitude across movement conditions (small – top, medium – middle, large - bottom). Statistical analysis of time-frequency representations across patients revealed significant movement-related modulation from baseline in the theta, beta and gamma band in all conditions (B, p<0.05; FDR corrected). (C) Significantly different time-frequency bins were predominantly in the gamma band and highest in the comparison of small and large movements (lower row) in the contralateral hemisphere. All p-values<0.05 after FDR correction for multiple comparisons. (D) Across patient averages (n = 16 ON medication) revealed a stepwise increase in gamma power (40–90 Hz) toward the large movement condition (small – yellow, medium – red, large – green) both ipsi- and contralateral to the moved side (ipsilateral – left, contralateral – right). In the contralateral hemisphere, stronger gamma synchronization was seen in the medium and large movement conditions when compared to the ipsilateral hemisphere (E; grey– contralateral, black – ipsilateral). No significant modulation by movement condition and hemisphere was seen in the theta (2–8 Hz) and beta bands (13–30 Hz) (not shown).
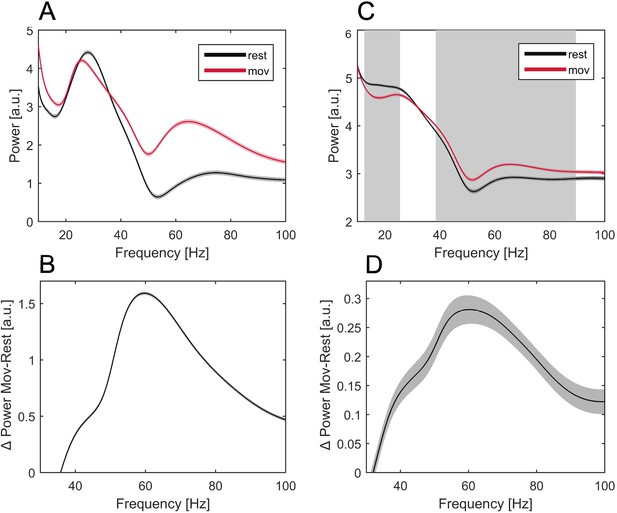
Averaged power spectra at rest and movement in an exemplary patient and across all patients.
Power spectra averaged across all trials over rest (−2 to −1.5 s before MO, black line) and movement time (0–0.5 s after MO, red line) of a single exemplary patient (A) and across all patients (C). Frequencies that show significant power change during movement across patients are labeled (C, grey background). The absolute difference of power in each frequency bin between rest and movement time is shown for the single patient (B) and across patients (D, grey shading = SEM). Note the narrow peak in gamma power increase during movement (A–D).
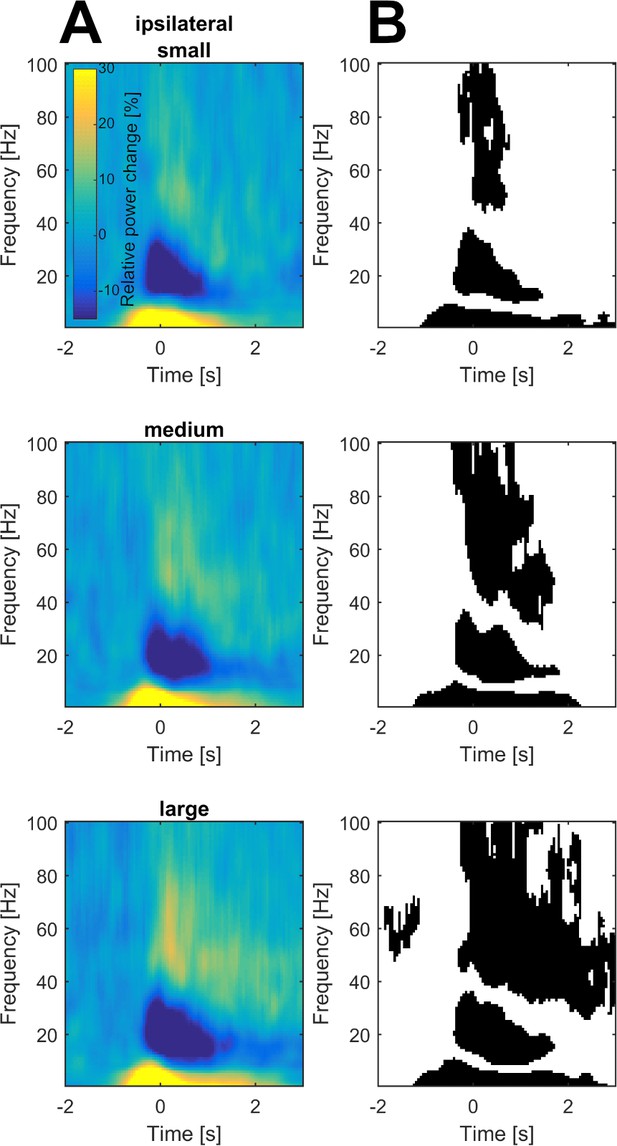
Grand average of subthalamic oscillatory activity in the ipsilateral hemisphere aligned to movement onset in the dopaminergic ON state.
Baseline corrected time-frequency representations were averaged across contact pairs and hemisphere in relation to the moved hands (A). Statistical analysis of time-frequency representations across patients revealed significant movement-related modulation from baseline in the theta, beta and gamma band in all conditions (B, p<0.05; FDR corrected).
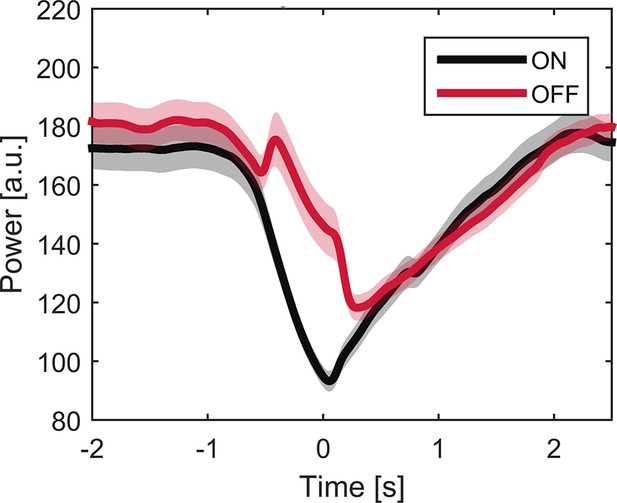
Averaged beta power (13–30 Hz) over trials ON and OFF medication.
Time course of averaged beta power (13–30 Hz) across trials ON (black line) and OFF medication (red line). Shaded areas indicate SEM. Note that dopamine-dependent difference in beta band modulation varies at time points. Difference is largest at time point of movement onset but does not reach significance when averaged around maximal velocity (p=0.063).
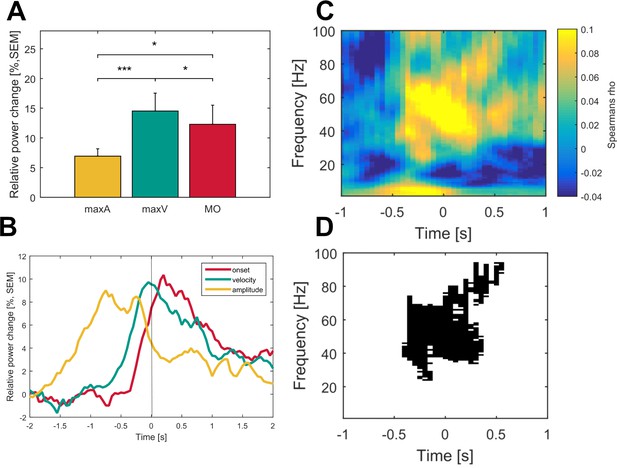
Gamma activity is strongest during maximum movement velocity and directly correlates with movement velocity on a single-trial level across patients.
Averaged gamma band activity was aligned to different time points of the movement execution (A; onset – red, velocity – green, amplitude – yellow) to allow comparison of a temporal focus in gamma synchronization. (B) Gamma activity was significantly higher aligned to the time point of maximum velocity (green) when compared to an alignment on maximum amplitude (yellow, p<0.001) and movement onset (red, p=0.02). (C) Single-trial Spearman’s correlations with movement velocity across all time frequency bins aligned to maximum movement velocity (time 0 s) were conducted in all patients. (D) Robust positive correlations were spectrally and spatially distinct to the gamma band (p<0.05, FDR corrected) of the contralateral hemisphere. No significant time frequency bins were revealed for the ipsilateral hemisphere (data not shown).
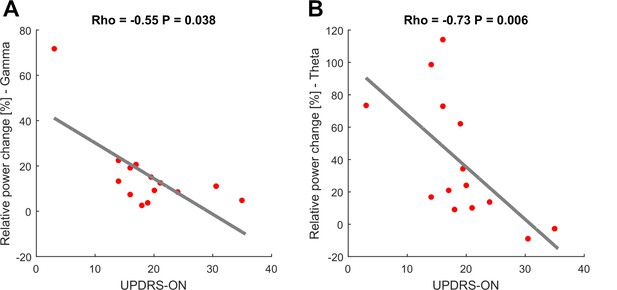
Movement-related theta and gamma oscillations are correlated with parkinsonian symptom severity in the dopaminergic ON state.
To investigate a potential association of movement-related activity, Spearman’s correlations were conducted between averaged theta (2–8 Hz), beta (13–30 Hz) and gamma (40–90 Hz) amplitudes with concurrent motor impairment as assessed by UPDRS-III (available in 14/16 patients). Significant negative correlations were found for the theta (A; Spearman’s ρ = −0.073, p<0.01) and gamma (C; Spearman’s ρ = −0.55, p=0.038) amplitudes.
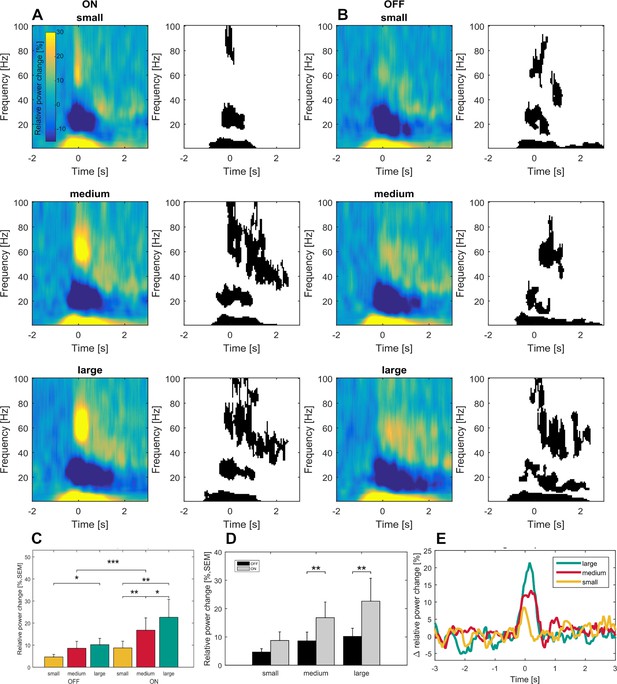
Time frequency representations and dopamine-dependent-scaling of gamma activity in the subgroup of seven patients ON and OFF medication.
Significant movement-related modulation of the contralateral baseline corrected time-frequency representation was found for theta, beta and gamma frequency bands (ON: A- right panel, OFF: D – right panel; p<0.05, FDR corrected) in the dopaminergic ON (A) and OFF (B) state in a subgroup of seven patients. Scaling of movement to gamma oscillations was present only in the dopaminergic ON, but not in the OFF state (C). Gamma band activity difference between ON and OFF states were scaled to movement velocity (D) and significantly different only in the medium and large movement conditions in the subgroup of seven patients with OFF recordings. No significant difference was found for the theta and beta bands (not shown). An increasing difference in gamma power between ON and OFF state toward the large movement condition is shown in (E). Across conditions, grand average gamma activity was significantly higher in the dopaminergic ON state, when compared to the OFF state (C; p<0.001).
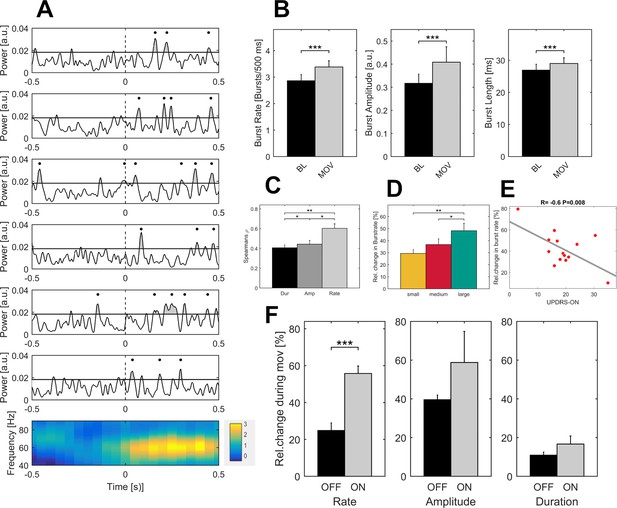
Properties of gamma bursts scale during movement and indicate both the dopaminergic and the clinical state.
(A) The raw LFP signal was filtered around the gamma range (40–90 Hz) and for all contact pairs of one electrode, the same threshold was set at the 75th percentile of the gamma amplitude of the entire recording. A burst was considered when the duration of threshold crossing (black line) was at least one gamma cycle long (black dot). Shown are six trials of the large movement condition from the same patient (Subject 2, right hemisphere, contact pair 1–2) and the averaged time-frequency plot across large condition trials for this patient (bottom row). After movement onset (dashed line), the rate of transient synchrony in gamma bursts increases while the overall level of gamma power appears unchanged. Gamma bursts occur at different time points within each trial leading to a seemingly continuous gamma synchronization when averaging across trials (bottom row). (B) When averaged across patients (n = 16, ON-state), gamma bursts show increased rate, amplitude and duration during movement (grey bar – MOV), compared to baseline (black bar – BL). (C) Averaged correlations of changes in gamma-power and –burst properties for each patient showed significantly higher correlation with increases in burst rate (light grey - Rate) than burst amplitude (grey - Amp) or duration (black – Dur). (D) Relative changes of burst rate show a stepwise increase toward the large movement condition. (E) Burst rate increase correlates with clinical state. UPDRS-ON scores were available in 14 patients. Across these patients, a significant negative correlation (Pearson’s r = −0.6, p=0.008) was seen between increases in burst rate during movement and the UPDRS-III motor score. (F) When comparing averaged increases in burst rate, amplitude and duration ON and OFF medication (n = 7), only burst rate showed a significant decrease, while movement-related increases in burst duration and amplitude seemed not associated with the dopaminergic state. * indicate p-values<0.05; **p<0.01; ***p<0.001.
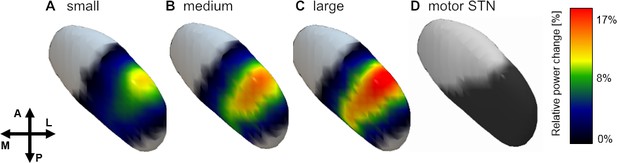
Spatial distribution of gamma power across conditions.
Averaged gamma power for small (A), medium (B) and large (C) conditions was spatially interpolated between all contact pairs (left electrodes flipped to right hemisphere) and mapped to the subthalamic surface in standard MNI space. The resulting map revealed a clear peak in the dorsolateral portion of the subthalamic nucleus, which overlapped with the subthalamic motor segment (Ewert et al., 2017) shown in (D). Note the parametric peak size increase with larger movement amplitude.
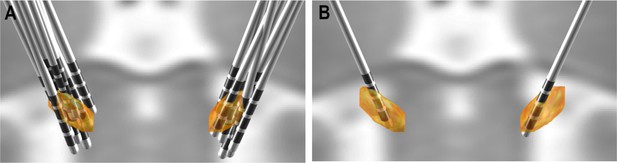
Three-dimensional electrode reconstruction.
Preoperative and postoperative MR and CT imaging were utilized to reconstruct electrode positions with Lead-DBS in relation to the subthalamic nucleus in all patients (A). Contact pairs that did not have at least one contact in the subthalamic nucleus were excluded from the analysis. Representative electrode localization with all contact pairs included is shown in (B).
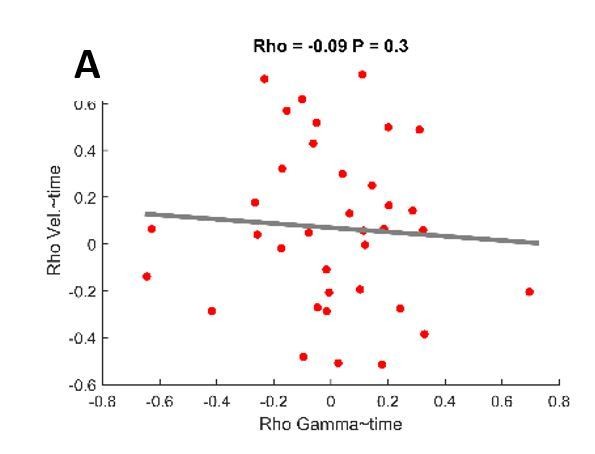
Changes in maximal velocity and gamma synchronization over trials do not correlate.
Spearman’s correlation was conducted for both maximal velocity and averaged gamma power over trials. No robust correlation was seen between the correlation coefficients of both variables (Rho=-0.09, P=0.3).
Tables
Clinical details
https://doi.org/10.7554/eLife.31895.014| Case | Age/Gender | Surgical center | Disease duration (Years) | Predominant symptoms | UPDRS med ON/OFF | Δ UPDRS [%] Stim ON/OFF* | Medication decrease* |
|---|---|---|---|---|---|---|---|
| 1 | 59/M | Berlin | 10 | Bradykinesia, Rigidity | --/26 | 42% | NA |
| 2 | 44/M | Berlin | 12 | Bradykinesia, Rigidity, Resting Tremor | 31/41 | 25% | NA |
| 3 | 42/M | Berlin | 4 | Action Tremor, Resting Tremor, Gait Disturbance | 16/42 | 60% | 100% |
| 4 | 53/M | Berlin | 8 | Bradykinesia, Rigidity, Resting Tremor | 3/20 | 53% | 68% |
| 5 | 70/M | Berlin | 19 | Bradykinesia, Rigidity, Resting Tremor | 38/48 | NA | NA |
| 6 | 55/M | Berlin | 15 | Peak-Dose Dyskinesia, Wearing-Off | 18/32 | 75% | 50% |
| 7 | 71/F | Berlin | 18 | Bradykinesia | 17/34 | 39% | 45.5% |
| 8 | 68/M | Berlin | 20 | Speech Difficulties, Hypokinesia | 16/20 | 40% | 61.3% |
| 9 | 67/M | Berlin | 14 | Bradykinesia | 14/24 | 74% | 50% |
| 10 | 49/M | Hannover | 7 | Rigidity | 19/40 | 50% | 14.3% |
| 11 | 39/F | Berlin | 6 | Resting Tremor, Action Tremor | 21/-- | NA | 68.4% |
| 12 | 71/M | Berlin | 4 | Resting Tremor | 20/23 | NA | 55.2% |
| 13 | 68/F | Berlin | 5 | Resting Tremor | 14/20 | NA | 74.3% |
| 14 | 58/M | Berlin | 12 | Bradykinesia, Fluctuations, Freezing | --/35 | 69% | 36.3% |
| 15 | 75/M | Berlin | 15 | Action Tremor, Resting Tremor | 20/38 | 69% | 50% |
| 16 | 53/M | Hannover | 10 | Bradykinesia, Rigidity | 24/42 | 53% | 33.3%% |
-
*after 3 months post-operatively ** NA = not available archive data.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.31895.015





