Specialized impulse conduction pathway in the alligator heart
Figures
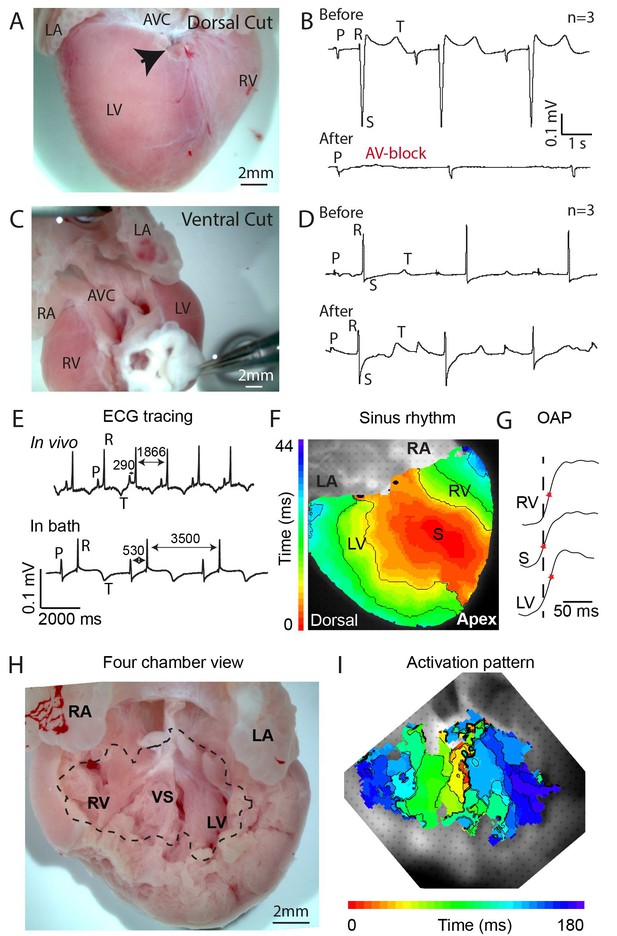
Propagation block by cuts in the dorsal alligator atrioventricular canal reveals a specialized atrioventricular conduction system.
(A-B) Cuts in a small region of the dorsal atrioventricular canal induces atrioventricular block (dorsal cut n = 3 and ventral cut n = 3). (C-D) Extensive cuts to the ventral and lateral atrioventricular canal do not induce atrioventricular block. (E) In vivo and ex vivo (in bath) QRS duration was not different (n = 7). (F) In bath optical mapping of ventricular activation revealed epicardial breakthrough of the impulse deep in the ventricle (n = 6). (G) The maximum rate of depolarization (red star) occurred earlier at position S than at position LV and RV of panel F (n = 6). (H-I) Optical recordings from the inside of the heart (H) show earliest activation in the septum (n = 1) (I). AVC, atrioventricular canal; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; s, ventricular sulcus; VS, ventricular septum.
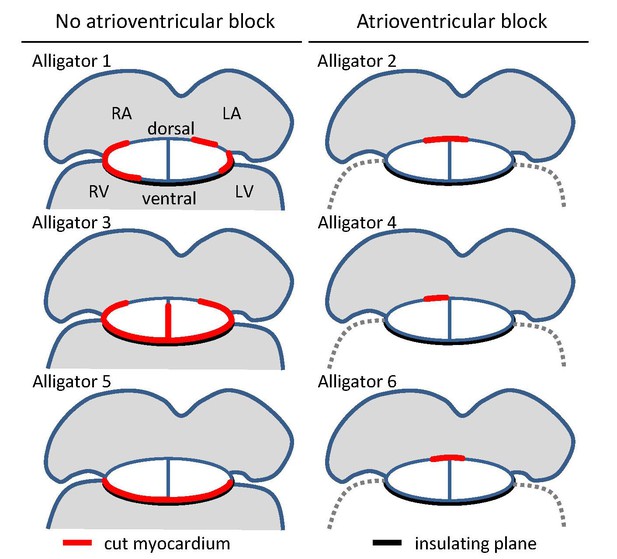
Overview of cuts (red) to the atrioventricular junction and the extent of collagen (black) in the six juvenile American alligators where atrioventricular conduction was assessed and where optical mapping was performed.
The positions of the cuts were based on visual inspection of the fixed hearts and confirmed by histology. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
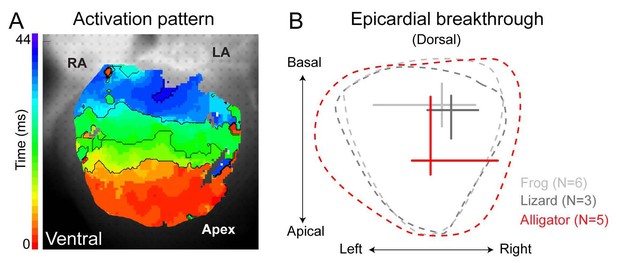
Ventricular activation patterns in reptiles.
(A) Shows the ventricular activation pattern at the ventral side of the alligator heart (n = 6). (B) Summary of the earliest epicardial breakthrough on the dorsal ventricular surface of the American alligator compared to the green anole lizard and the Xenopus frog (lizard and frog modified from [Jensen et al., 2012]). Note that the position of breakthrough in the alligator heart is located closer to the apex compared to the frog and the lizard. RA, right atrium; LA, left atrium.
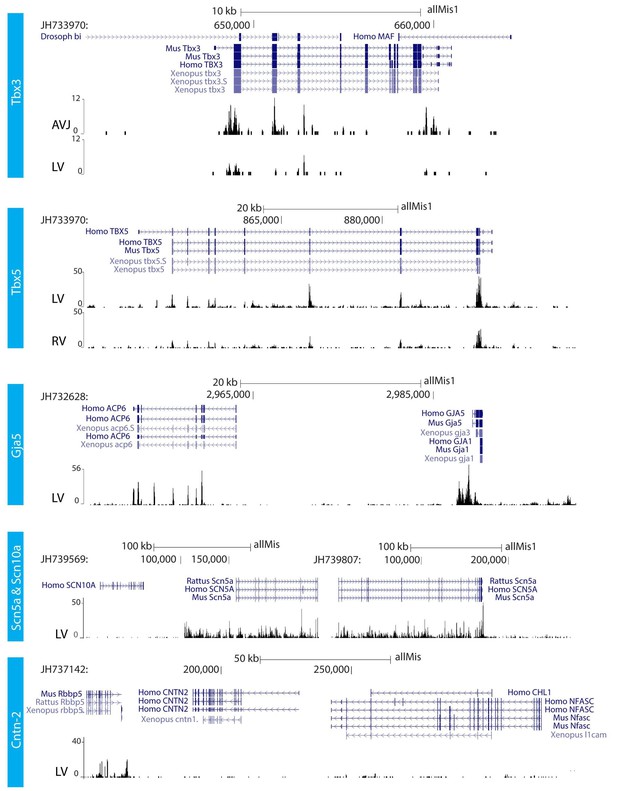
RNA sequencing.
RNA sequencing of atrioventricular junction (AVJ) and ventricular myocardium of the fetal alligator heart (LV, left ventricle; RV, right ventricle). AVJ sample also contained myocardium from the ventricular base. Note that Tbx3 tag count was higher in the AVJ compared to the left ventricle. Tag counts of Tbx5 was higher in the left ventricle than in the right ventricle. Both Gja5 and Scn5a were expressed in the ventricular myocardium of the alligator heart. Cntn2, however, did not show tag counts indicating absence of expression. (The data are deposited as Jensen B. 2018. Alligator mississippiensis Transcriptome or Gene expression. SRA. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA392860).
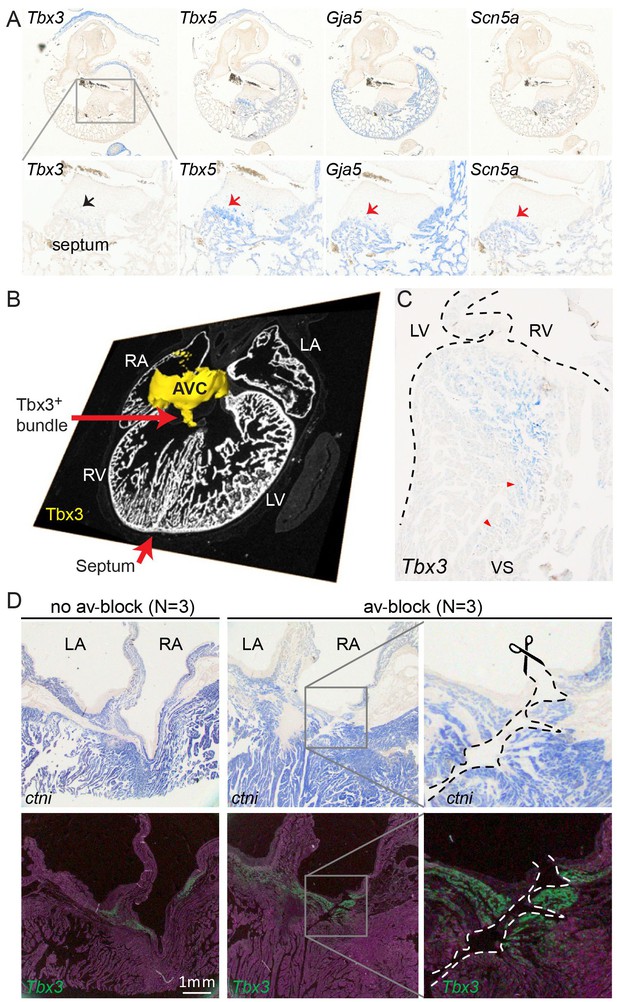
Molecular identification of the alligator atrioventricular conduction axis.
(A) mRNA detection by in situ hybridization in the Ferguson stage 16 embryo, showing a mammal and avian like phenotype of the septal crest and trabeculated myocardium. (B) Reconstruction of Tbx3 expression in the Ferguson stage 18 embryo reveals a dorsal bundle connecting the atrioventricular junction to the ventricular septum. (C) Tbx3 is expressed in the crest of the ventricular septum, which showed early activation (same heart as in Figure 1H–I). (D) Tbx3 expressing myocardium of the dorsal atrioventricular canal was damaged when atrioventricular block was induced (dorsal cut n = 3 and ventral cut n = 3).
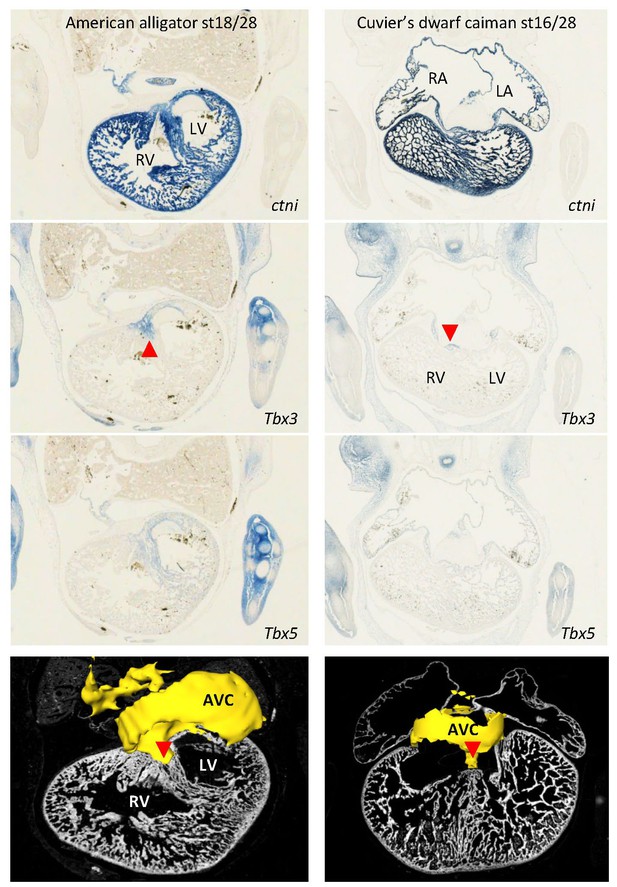
Expression of Tbx3 and Tbx5 were similar in embryos of American alligator and Cuvier’s dwarf caiman, suggesting evolutionary conservation of expression between crocodilians.
In both species, Tbx3 is expressed in the atrioventricular canal (AVC) and in the dorsal crest of the ventricular septum (red arrowhead). In the American alligator, note the left ventricle (LV) has greater expression of Tbx5 than the right ventricle, as also found with RNA-sequencing (Figure 2).
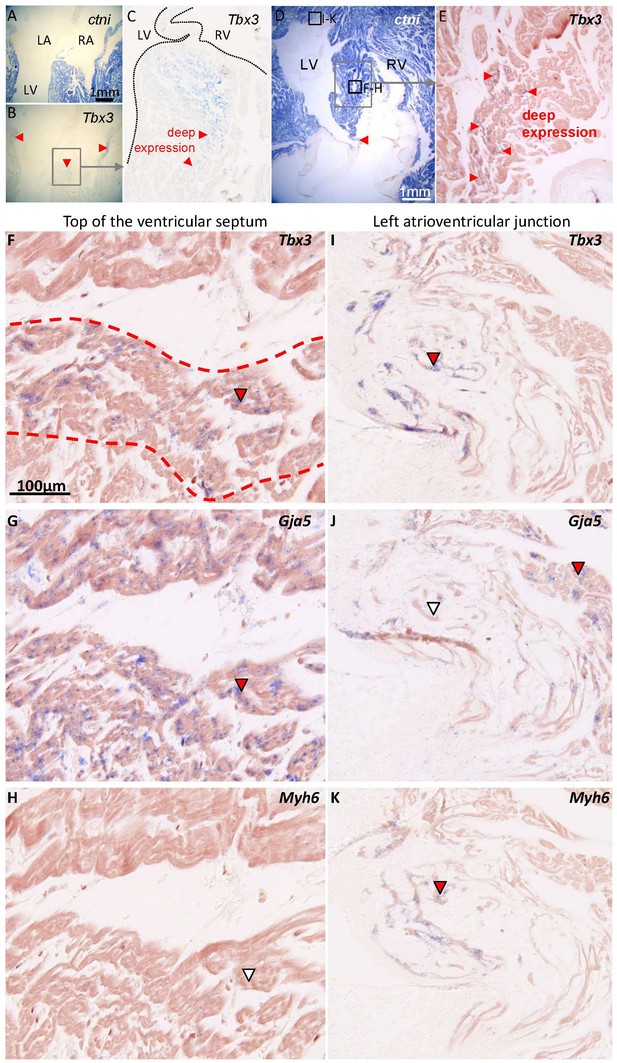
Characterization of the ventricular septum in 1-year-old alligators.
(A) Four-chamber view of the ventricular base, shows that the membranous septum is rich in myocardium. (B) Tbx3 is expressed in the atrioventricular canal myocardium and in the crest of the ventricular septum (red arrowheads). (C) Expression of Tbx3 is deep in the ventricular septum. (D) Transverse section, showing the ventricular septum (framed) and the membranous septum, again rich in myocardium (red arrowhead). (E) Tbx3 is, like in B-C, expressed deep in the ventricular septum. (F-H) Shows a higher magnification of the area shown in E, in neighboring sections, to show an overlap of expression of Tbx3 (F) and Gja5 (G). This part of the Tbx3 expressing domain did not express atrial myosin (Myh6, white arrowhead) (H). (I-K) In contrast, the Tbx3 expressing myocardium of the left atrioventricular junction (I), did express atrial myosin (K) and showed little if any expression of Gja5 (J). This suggests that the Tbx3 identified bundle in the alligator, is ventricular as in mammals (Aanhaanen et al., 2010).
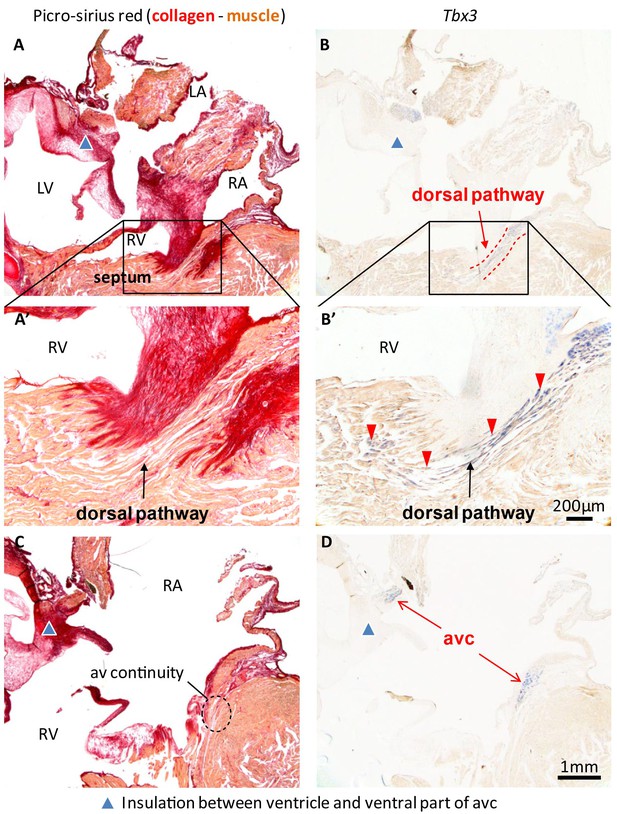
Insulation by collagen in the atrioventricular junction and near the atrioventricular bundle.
(A) Sagittal section of the ventricular base of a 1-year alligator, showing complete insulation by connective tissue ventrally (blue arrowhead) and myocardial atrioventricular continuity dorsally (insert, enlarged in A’). (B-B’) The dorsal pathway expresses Tbx3. (C) Dorsally, there is broad atrioventricular continuity, this section is 800 µm to the right of the section of A and there is still atrioventricular continuity (dashed circle). (D) In this region of the heart, Tbx3 is confined to the atrioventricular canal (avc) and is not expressed in the ventricle as in B.
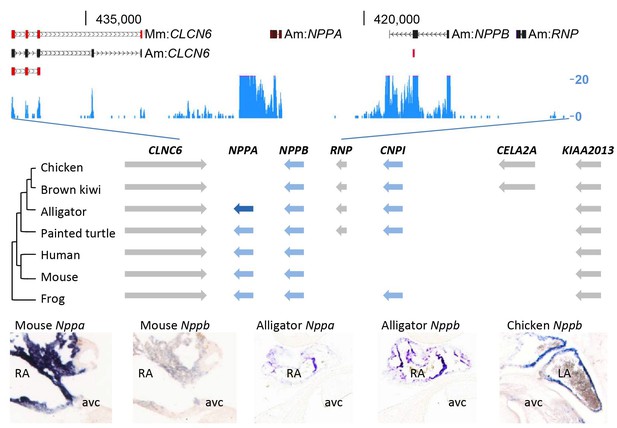
Evolution of the natriuretic peptide gene locus.
RNA-sequencing tag counts aligned to BAC JH731559 revealed the unexpected presence of transcripts of orthologues of both Nppa and Nppb. The alligator cardiac natriuretic peptide gene locus is homologous to that of amphibians (frog) and reptiles (turtle). Birds have lost Nppa, and CELA2A and surrounding sequences have been inserted. Mammals have replaced the region in between Nppb and KIAA2013. The presence of Nppa and Nppb transcripts was validated by in situ hybridization using specific probes. Am, Alligator mississippiensis; avc, atrioventricular canal; Hs, Homo sapiens; LA, left atrium; Mm, Mus musculus; RA, right atrium.
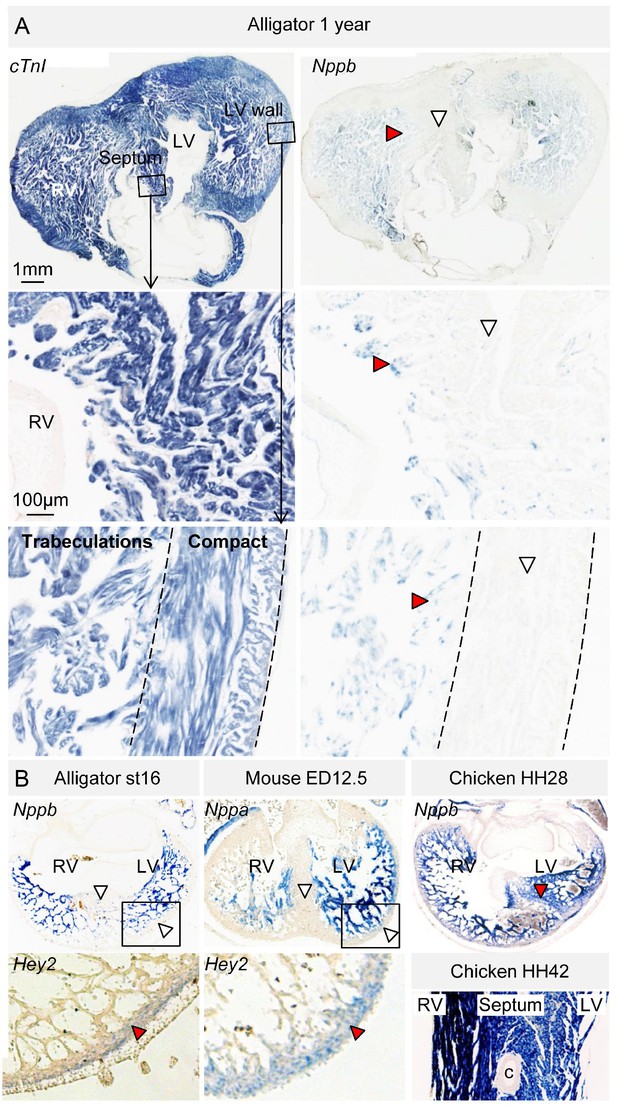
Evolutionary conservation of the trabeculated and compact ventricular wall.
(A) The ventricular septum of the alligator is highly trabeculated (also Figure 6—figure supplement 1). Yet the alligator septum does not express the trabecular marker Nppb (n = 5). (B) The developing septum of alligators and mouse is empty for Nppb, in contrast to chicken (HH28 and HH42), whereas the developing septum of alligators and chicken is highly trabeculated, in contrast to the septum in mouse which is compact. The compact wall of both alligator (n = 1), caiman (n = 1), and mouse expresses Hey2. Arrowheads (white, no stain; red, stain); c, coronary artery; LV, left ventricle; RV, right ventricle.
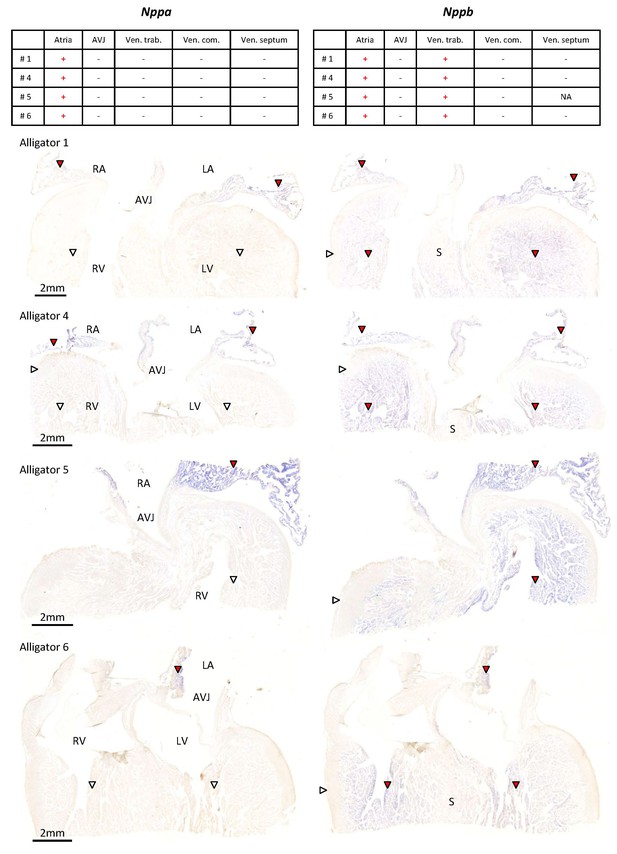
Expression of Nppa and Nppb in the ventricular base of 1 year old alligators.
The tables summarize the pattern of expression in the images below. Nppa was only found in atrial myocardium (red arrowheads) and was not detected in the atrioventricular junction (AVJ). Nppb was expressed in the atria and the trabeculated myocardium of the ventricle (red arrowheads) but was not detected from the compact wall of the ventricle (white arrowheads) and the core of the ventricular septum (S).
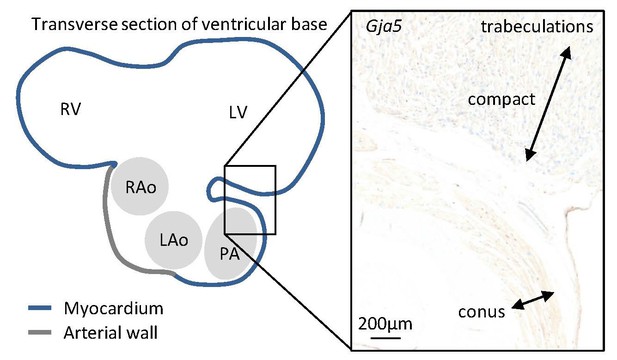
In the juvenile alligators, Gja5 expression was found throughout the trabecular ventricle and in the substantial compact myocardium (more than 0.5 mm thick).
Only the myocardial outflow tract (the conus arteriosus) expressed very little Gja5.
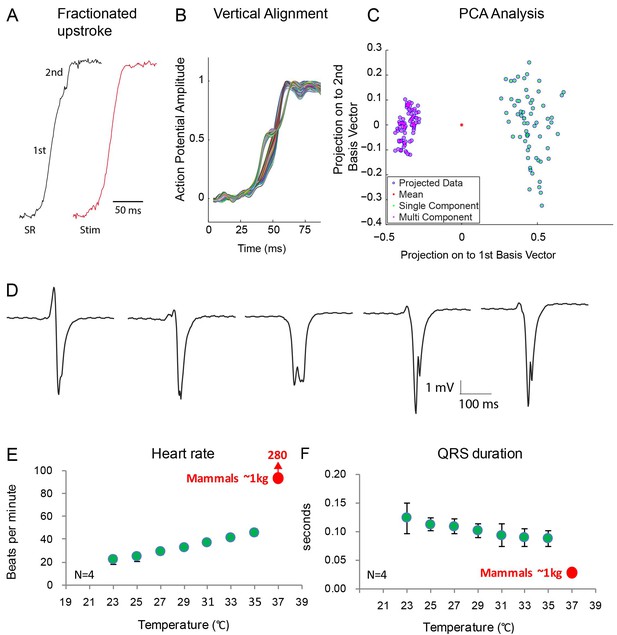
Fractionated upstroke of the optical action potentials indicated two distinct tissue layers.
(A) Upstroke during sinus rhythm (SR) and induced by stimulation (Stim). (B) Vertical alignment of optical action potentials on the dorsal side of the alligator heart. (C) Principle component analysis (PCA) illustrating the different morphology of the upstroke with and without fractionation. (D) shows local electrograms recorded from the dorsal and ventral side of the heart shown in Figure 7—figure supplement 1. (E-F) In vivo heating of anaesthetized 2-year-old alligators leads to an increase in heart rate and a decrease in ventricular activation time as assessed by QRS duration (points are averages, error bars are standard deviation). The QRS duration of warm alligators never approach the much shorter QRS duration of eutherian mammals (Detweiler, 2010).
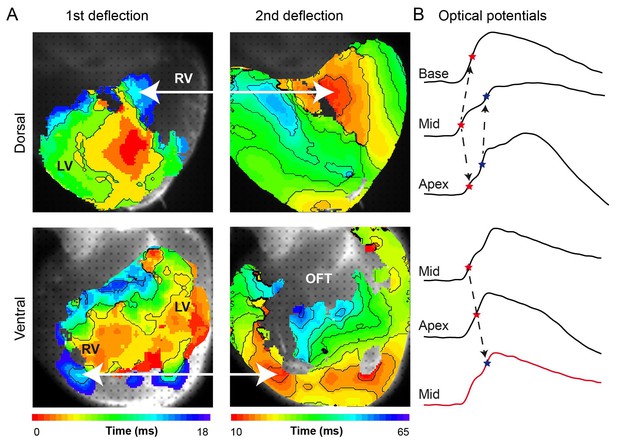
(A) Activation maps from the dorsal and ventral surfaces of the ventricle based on the first and second deflection of the upstroke. (B) Representative optical action potentials used in A. This figure is based on a typical example. LV, left ventricle; RV, right ventricle; OFT, outflow tract.
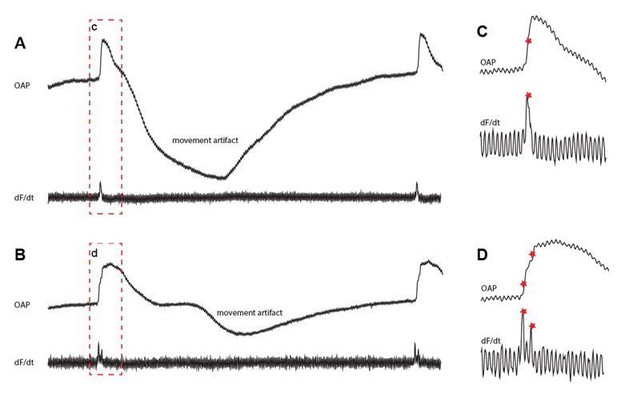
Fractionated upstrokes measured from the epicardium.
The traces in A and B shows the optically recorded action potential (upper) and its the derivative (lower). Panel C and D show magnifications of the red box from panel A and B respectively.
Additional files
-
Source data 1
Contains three sheets.
Sheet ‘Figure 1E' gives the parameters of the ECGs of the hearts used for optical mapping and surgical cuts. Sheet ‘In situ hybridization’ provides an overview of all in situ hybridization and in which figures the data is shown. Sheet ‘Figure 7E, F' gives the parameters of the ECGs of the animals that were heated to mammalian body temperatures, summarized in Figure 7E, F.
- https://doi.org/10.7554/eLife.32120.017
-
Supplementary file 1
Supplementary Table 1: Overview of the literature.
The presence of a specialized conduction system has been investigated in many reptile species and no consensus has emerged. The lack of consensus reflects the very heterogeneous quality of the previous studies and different definitions of ‘specialization’. To claim specialization, anatomists placed much emphasis on how pale cells were, that is how Purkinje cell-like they were, whereas many electrophysiologists placed emphasis on function, for instance whether there was an atrioventricular delay under the influence of nervous activity. Currently, ‘specialization’ is much informed by molecular biological data in the setting of the mammal heart.
- https://doi.org/10.7554/eLife.32120.018
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32120.019





