Tumor-derived CSF-1 induces the NKG2D ligand RAE-1δ on tumor-infiltrating macrophages
Figures
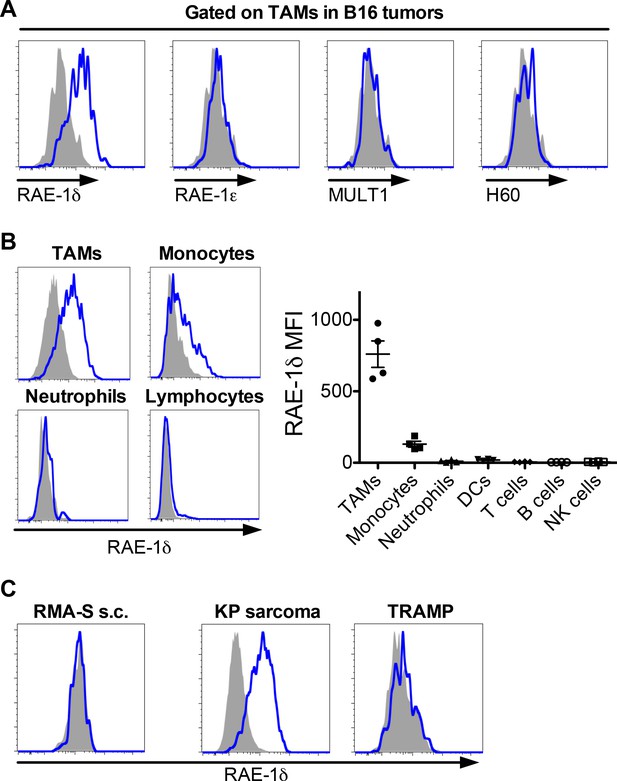
RAE-1δ is induced on tumor-associated macrophages in subcutaneously transferred and spontaneous tumors.
(A) Established B16 S.C. tumors were dissociated and analyzed for NKG2D ligand expression on tumor-associated macrophages. (B) RAE-1δ expression (left) and MFI quantification (right) on the indicated cell types in B16 tumors. (C) RAE-1δ expression on TAMs in spontaneous KP sarcoma, but not in spontaneous TRAMP prostate adenocarcinoma or transferred RMA-S lymphoma. Data are representative of >3 independent experiments.
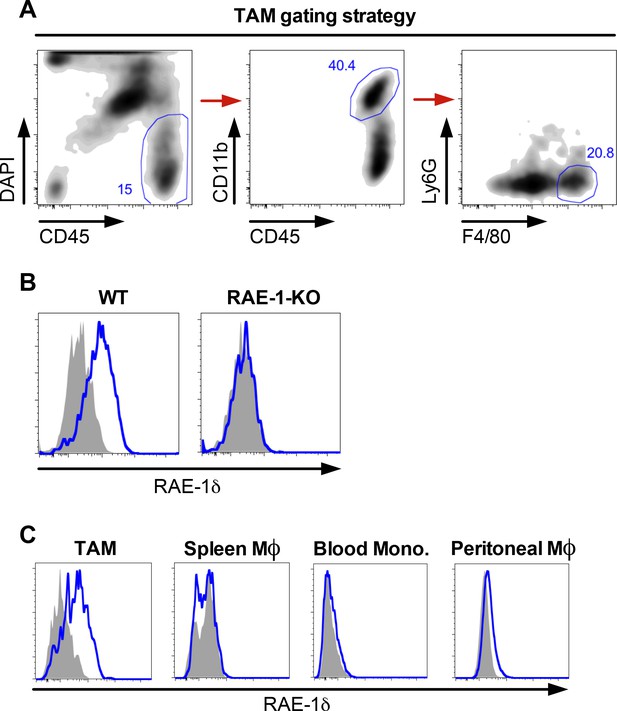
Gating strategy and RAE1δ expression on tumor-associated macrophages and monocytes in mice with B16 tumors.
(A) Gating strategy to identify tumor-associated macrophages in B16 tumors. (B) Expression of RAE-1δ on TAMs in B16 S.C. tumors in WT and RAE-1-KO mice. (C) RAE-1δ expression on TAMs, splenic macrophages, blood monocytes, or peritoneal macrophages in WT mice with established B16 S.C. tumors. Data are representative of >3 independent experiments.
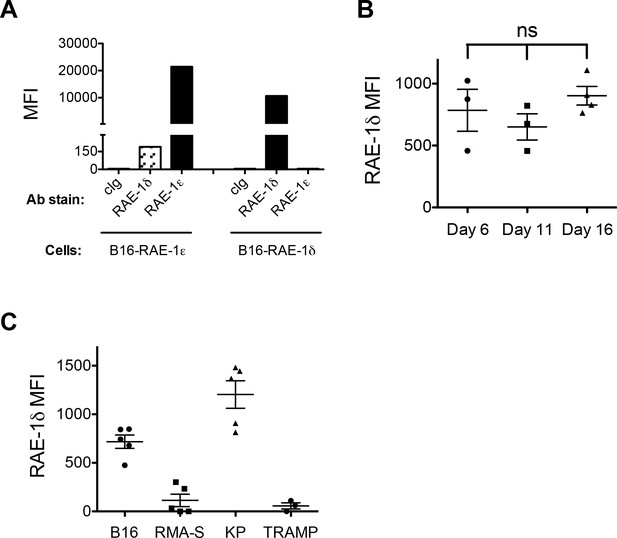
RAE-1 antibody validation and RAE-1δ staining on TAMs in different tumors.
(A) B16 cells transduced to stably express RAE-1δ or RAE-1ε were stained with biotinylated antibodies against RAE-1δ or RAE-1ε, followed by fluorophore-conjugated streptavidin. (B) Expression of RAE-1δ on TAMs in B16 S.C. tumors in WT mice at the indicated time after injection of 1 × 106 tumor cells. (C) Expression of RAE-1δ on TAMs in S.C. B16 and RMA-S tumors and autochthonous KP and TRAMP tumors. Data are compiled from several independent experiments.
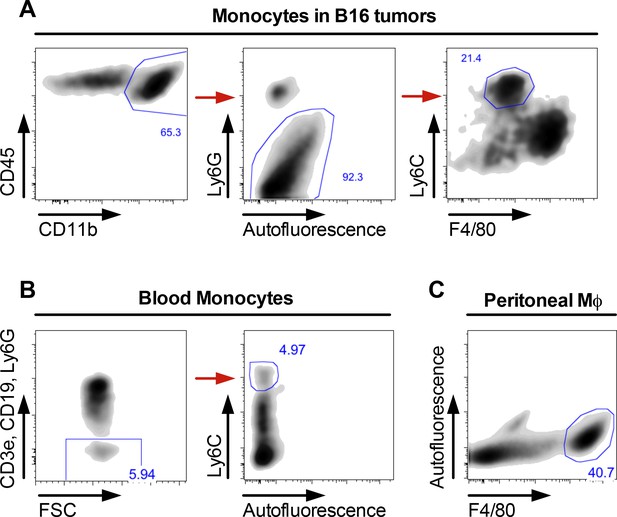
Gating strategies for blood and tumor-associated monocytes and peritoneal macrophages.
Gating strategies for (A) monocytes in B16 tumors, (B) blood monocytes, and (C) peritoneal macrophages.
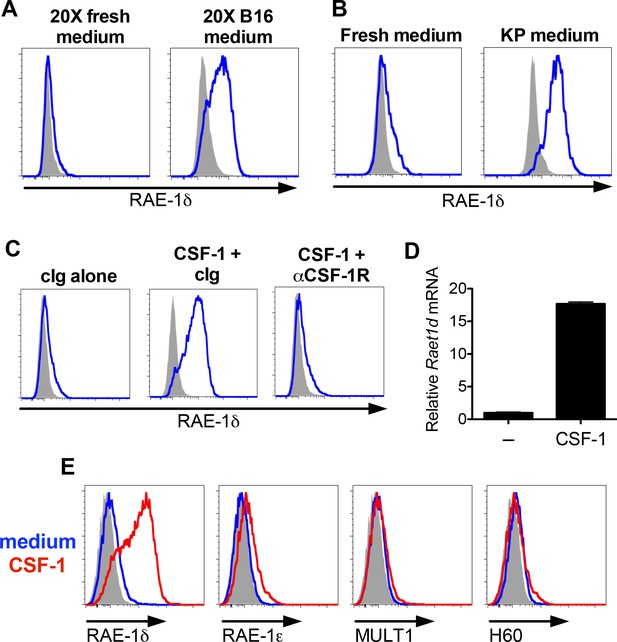
B16 and KP cell line conditioned medium and CSF-1 induces RAE-1δ on macrophages.
(A) Peritoneal wash cells were cultured with a 1:1 mixture of fresh medium plus 20X concentrated fresh medium or 20X concentrated B16 cell culture supernatants, and macrophage RAE-1δ was analyzed by flow cytometry 48 hr later. (B) Peritoneal wash cells were stimulated 48 hr ex vivo with a 1:1 mixture of fresh medium supplemented with fresh medium or conditioned medium from cultures of a KP sarcoma cell line generated from a primary KP sarcoma, and macrophage RAE-1δ was analyzed 48 hr later by flow cytometry. (C) Peritoneal wash cells were cultured with or without 10 ng/ml CSF-1, with the addition of control Ig or CSF-1R antibody (1 μg/ml), and macrophage RAE-1δ was analyzed 48 hr later by flow cytometry. (D) Peritoneal macrophage Raet1d mRNA 48 hr after stimulation with or without the addition of CSF-1 (10 ng/ml). (E) Peritoneal macrophage expression of the indicated NKG2D ligands 48 hr after stimulation with CSF-1 or control medium. Data are representative of >3 independent experiments.
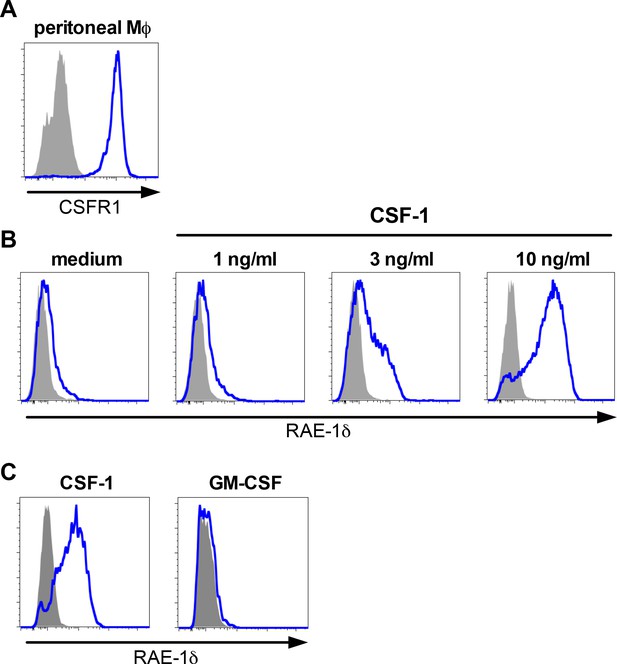
Peritoneal macrophage CSFR1 expression and dose-dependent RAE-1δ induction by CSF-1, and bone marrow macrophage stimulation with CSF-1 or GM-CSF.
(A) CSF-R1 expression on peritoneal macrophages ex vivo. (B) RAE-1δ expression on peritoneal macrophages cultured 48 hr with the indicated concentration of CSF-1. (C) RAE-1δ expression on bone-marrow-derived-macrophages generated using 10 ng/ml CSF-1 or GMCSF. Data are representative of 2–3 independent experiments.
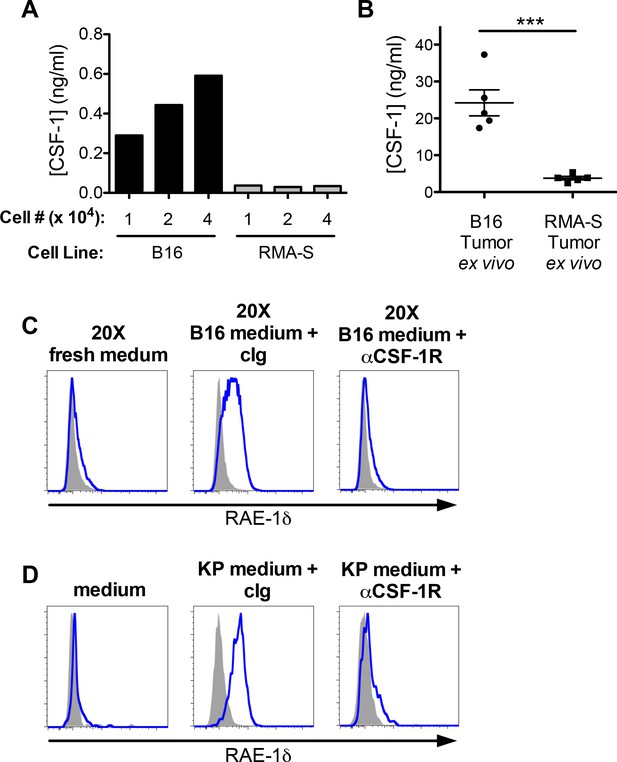
CSF-1 is necessary for macrophage RAE-1δ induction by tumor conditioned media.
(A) The indicated numbers of B16 or RMA-S cells were seeded in 12-well plates, and CSF-1 levels in the supernatants were measured by ELISA 48 hr later. (B) Established B16 or RMA-S tumors were dissociated, and CSF-1 levels in dissociation supernatants were measured by ELISA; intra-tumoral concentrations were calculated using tumor volume measurements (total ng of CSF-1 divided by the tumor volume at time of harvest). (C) Peritoneal macrophage RAE-1δ expression 48 hr after culture with concentrated fresh medium, concentrated B16 conditioned medium plus control Ig (1 ug/ml), or concentrated B16 conditioned medium plus anti-CSF-1R (1 ug/ml). (D) Peritoneal macrophage RAE-1δ 48 hr after culture with fresh medium, KP conditioned medium plus control Ig, or KP conditioned medium plus anti-CSF-1R (1 ug/ml). Data are representative of >3 independent experiments.
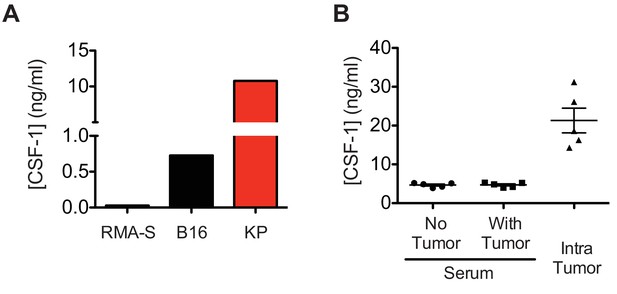
In vitro and in vivo CSF-1 production in tumors.
(A) CSF-1 concentrations in the indicated cell line culture supernatants. (B) CSF-1 concentrations in B16 tumors or serum of naïve or tumor-bearing mice. Data are representative of 2–3 independent experiments. Concentrations in tumors are depicted as total ng of CSF-1 per tumor dissociate divided by volume of tumor at time of harvesting.
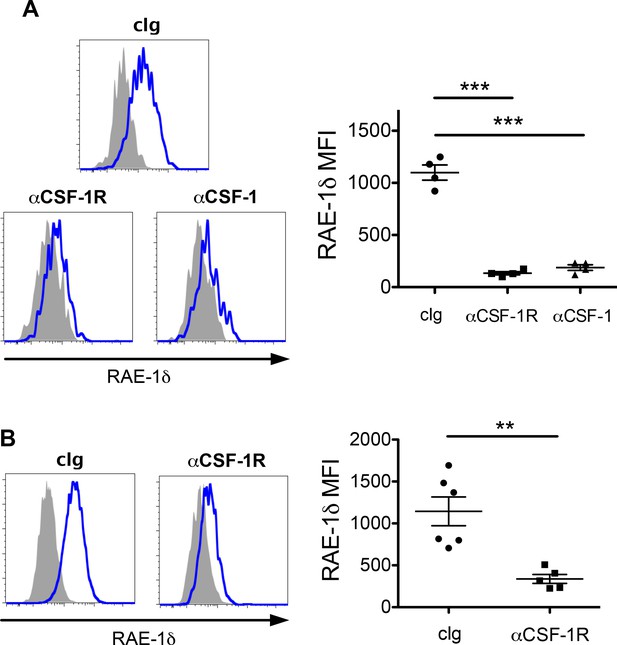
Blockade of CSF-1 or CSF-1R abrogates RAE-1δ expression by TAMs in vivo.
(A) Mice with established B16 tumors were injected i.p. with 200 ug of the indicated antibody, and RAE-1δ on TAMs was analyzed 48 hr later. (B) KP mice with established sarcomas were injected i.p. with 200 ug of the indicated antibody, and RAE-1δ on TAMs was analyzed 48 hr later. Statistical significance was determined using one-way ANOVA with Bonferroni post-tests (A) or a two-tailed unpaired Student’s t test (B). Data represent means ±SEM. Data are representative of >3 independent experiments.
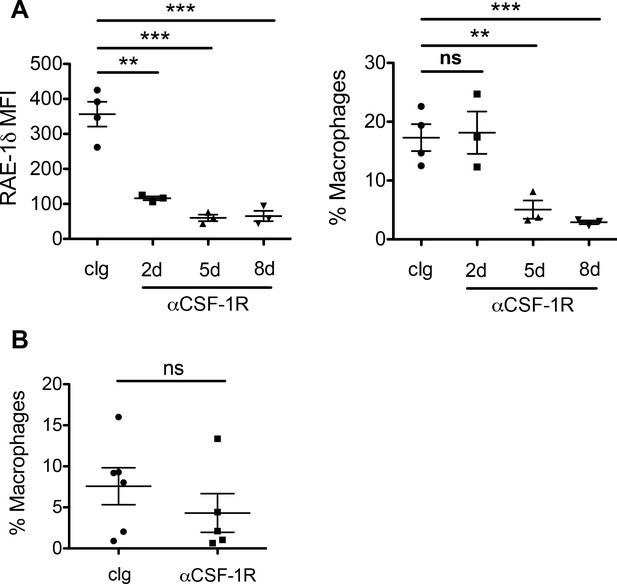
Tumor associated macrophage numbers and RAE-1δ expression after treatments with anti-CSF-1R.
(A) RAE-1δ on TAMs in B16 tumors from mice given control Ig or anti-CSF-1R at days −1, 2, and 5 and harvested at the indicated time point. (B) TAMs as a percent of total live cells in B16 tumors from mice given control Ig or anti-CSF-1R for 48 hrs. Statistical significance was determined using one-way ANOVA with Bonferroni post-tests. Data are representative of 2 independent experiments.
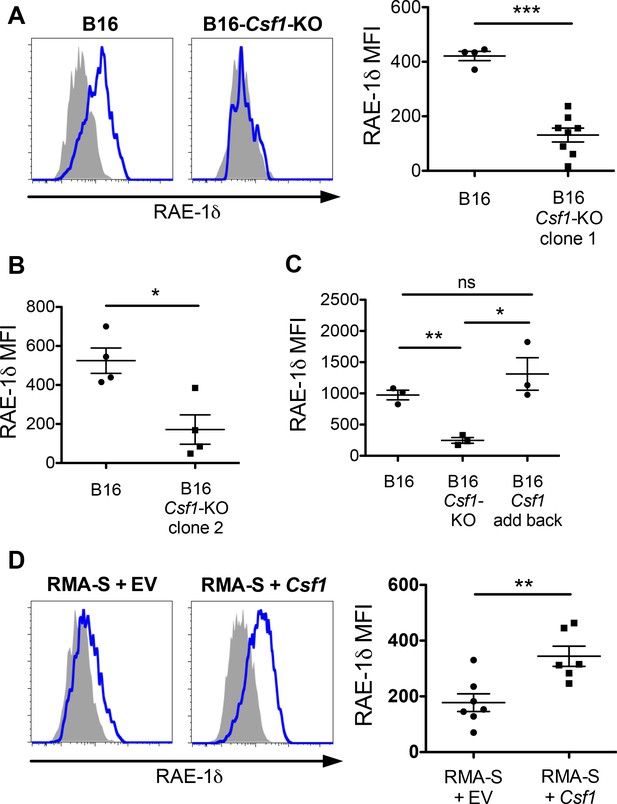
Tumor-derived CSF-1 is required for TAM RAE-1δ expression in vivo.
(A) RAE-1δ expression on TAMs in established B16 or B16-Csf1-KO tumors. (B) RAE-1δ on TAMs in mice with established B16 tumors or tumors of a second clone of B16-Csf1-KO cells. (C) RAE-1δ on TAMs in mice with established B16, B16 Csf1-KO, or B16 Csf1-KO tumors in which CSF-1 expression had been restored by transduction (add-back tumors). (D) RAE-1δ on TAMs in mice with established RMA-S or RMA-S-Csf1-overexpressing tumors. Statistical significance was determined using one-way ANOVA with Bonferroni post-tests (C) or a two-tailed unpaired Student t test (A, B, D). Data represent means ±SEM, and are representative of 2–4 independent experiments.
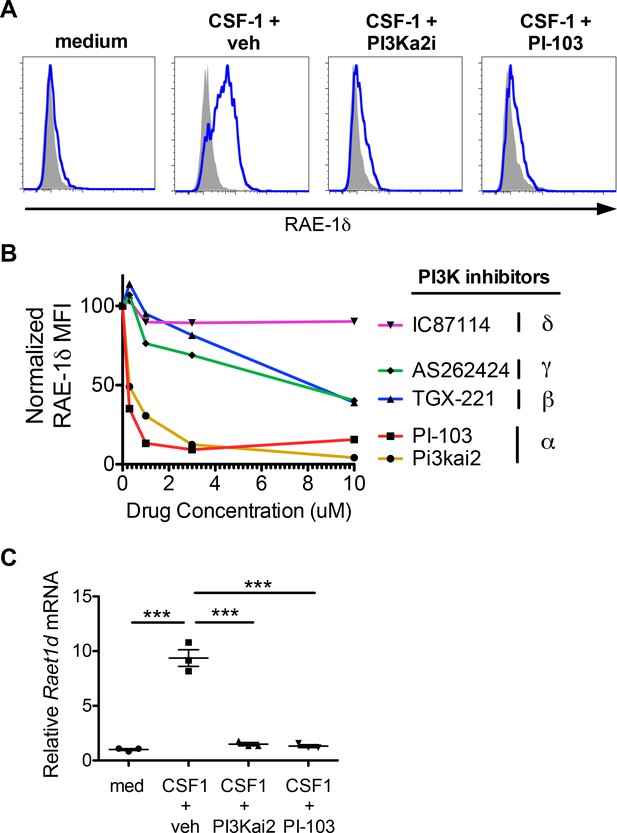
PI3Kα signals are required for macrophage RAE-1δ induction by CSF-1.
(A) Peritoneal wash cells were stimulated with CSF-1 plus vehicle control or PI3Kα inhibitors at 3 μM, and macrophage RAE-1δ was analyzed at 24 hr. (B). Relative macrophage RAE-1δ MFI 24 hr after stimulation with CSF-1 plus the indicated concentrations of the indicated PI3K inhibitors. (C) Relative Raet1d mRNA levels 24 hr after macrophage stimulation with CSF-1 plus vehicle control or PI3Kα inhibitors at 3 μM. Statistical significance was determined using one-way ANOVA with Bonferroni post-tests. Data are representative of 3–4 independent experiments.
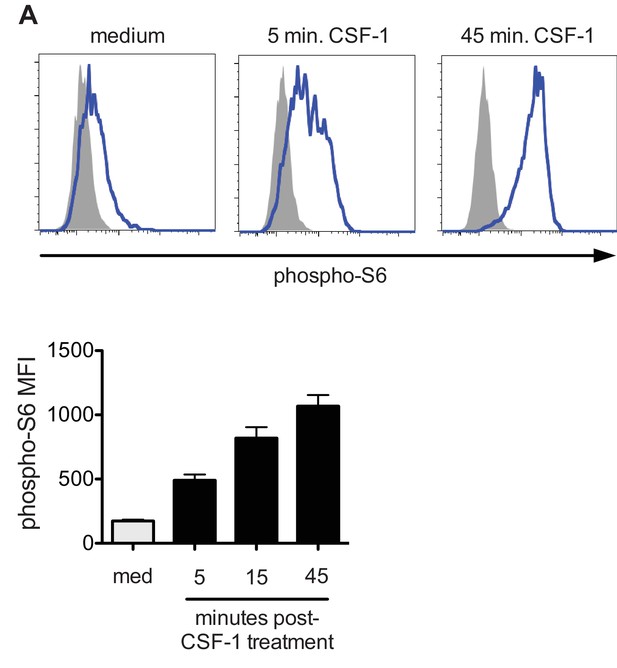
Induction of phospho-S6 by CSF-1.
(A) Phospho-S6 staining (top) and MFI quantification (bottom) in macrophages stimulated with control medium or 10 ng/ml CSF-1 for the indicated times. Data are representative of 3 independent experiments.
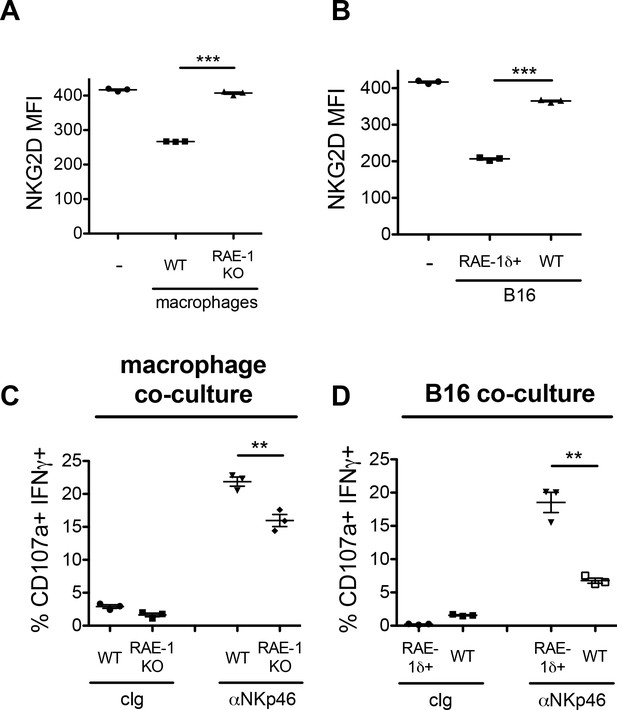
Co-culture of NK cells with RAE-1δ-expressing macrophages and tumor cells.
(A) Peritoneal macrophages from WT or RAE-1-KO mice or were stimulated with 10 ng/ml CSF-1 for 48 hr and then co-cultured with WT splenocytes for 18 hr, and NKG2D levels were analyzed by flow cytometry. (B) B16 or B16-RAE-1δ cells were co-cultured with WT splenocytes for 18 hr, and NKG2D levels on NK cells were analyzed by flow cytometry. (C) WT splenocytes were co-cultured with CSF-1-stimulated WT or RAE-1-KO macrophages for 18 hr, followed by 5 hr stimulation with plate-bound antibody against the NK cell activating receptor NKp46, or control Ig, and NK cell IFNγ and degranulation were analyzed by flow cytometry. (D) WT splenocytes were co-cultured with B16 or B16-RAE-1δ cells for 18 hr, followed by 5 hr stimulation with plate-bound antibody against the NK cell activating receptor NKp46, and NK cell IFNγ and degranulation were analyzed by flow cytometry.
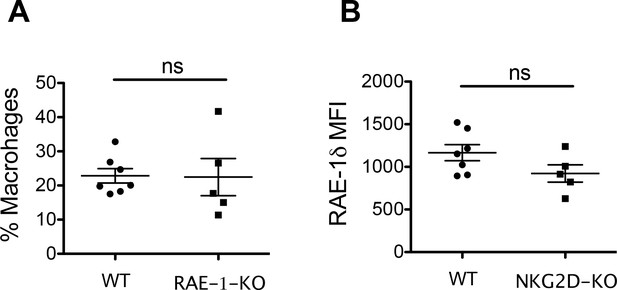
Tumor associated macrophage numbers and RAE-1δ expression in RAE-1-KO and NKG2D-KO mice.
(A) TAMs as a percentage of CD45 + cells in established B16 tumors in WT and RAE-1-KO mice. (B) RAE-1δ on TAMs in B16 tumors in WT and NKG2D-KO mice.
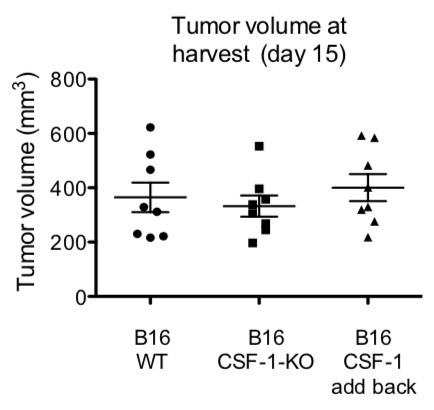
WT mice were injected with 1 x 106 of the indicated tumors.
Volumes were analyzed at day of harvest (day 15).
Tables
Cytokine stimulation of macrophages for RAE-1δ induction.
https://doi.org/10.7554/eLife.32919.008| Treatment | Macrophage RAE-1δ induction? |
|---|---|
| IL-1α | |
| IL-1β | |
| IL-4 | |
| IL-6 | |
| IL-12 | |
| IFNβ | |
| IFNγ | |
| TNFα | |
| CSF1 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32919.018





