Preserving neuromuscular synapses in ALS by stimulating MuSK with a therapeutic agonist antibody
Figures
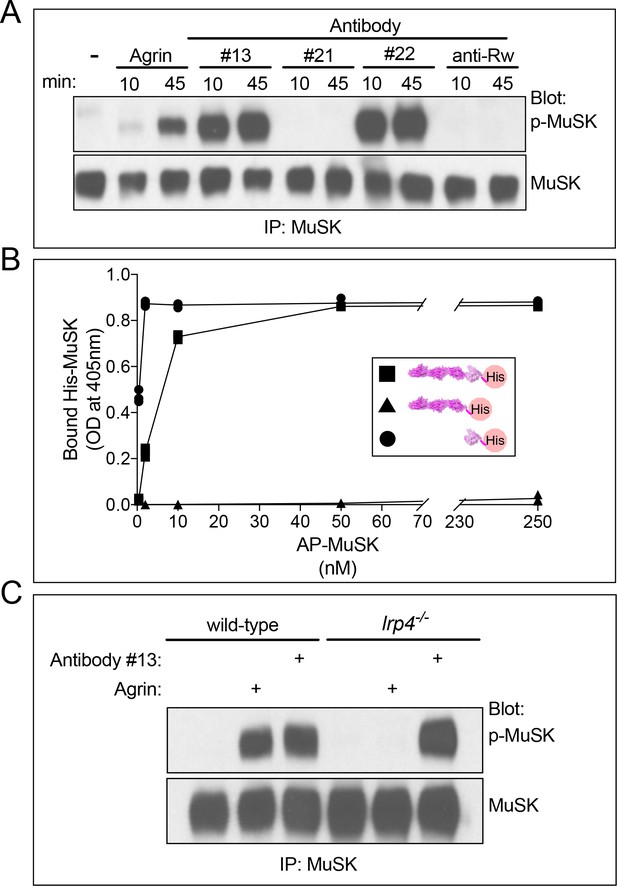
MuSK agonist antibodies activate MuSK, independent of Lrp4, by binding the Fz-like domain in MuSK.
(A) C2 myotubes were treated with neural Agrin or antibodies for the indicated times. MuSK was immunoprecipitated, and Western blots were probed for MuSK or phosphotyrosine. Neural Agrin and MuSK antibodies #13 and #22 stimulate MuSK tyrosine phosphorylation in C2 myotubes, whereas MuSK antibody #21 and a control antibody to Ragweed pollen (Rw) failed to stimulate MuSK phosphorylation. (B) We used a solid-phase binding assay to measure binding of His-tagged MuSK proteins to microtiter wells coated with MuSK agonist antibody #13. The scatter plot shows that full-length ecto-MuSK ( ), as well as the MuSK Fz-like domain alone (
), as well as the MuSK Fz-like domain alone ( ) bind MuSK antibody #13 in a dose-dependent and saturable manner; in contrast, the first three Ig-like domains in MuSK (
) bind MuSK antibody #13 in a dose-dependent and saturable manner; in contrast, the first three Ig-like domains in MuSK ( ) fail to bind the MuSK agonist antibody (n = 3). (C) Wild type and Lrp4 mutant myotubes were treated with neural Agrin or MuSK agonist antibody #13. Agrin stimulates MuSK phosphorylation in wild type but not Lrp4 mutant myotubes, whereas MuSK agonist antibody #13 stimulates MuSK phosphorylation in both wild type and Lrp4 mutant myotubes.
) fail to bind the MuSK agonist antibody (n = 3). (C) Wild type and Lrp4 mutant myotubes were treated with neural Agrin or MuSK agonist antibody #13. Agrin stimulates MuSK phosphorylation in wild type but not Lrp4 mutant myotubes, whereas MuSK agonist antibody #13 stimulates MuSK phosphorylation in both wild type and Lrp4 mutant myotubes.
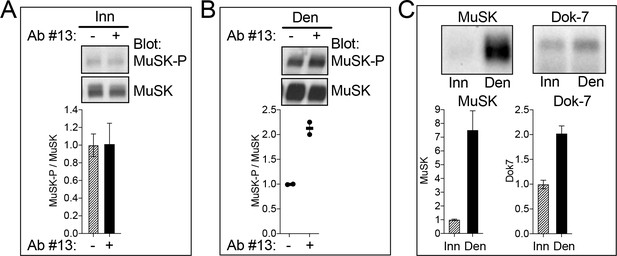
MuSK agonist antibody #13 stimulates MuSK tyrosine phosphorylation in vivo.
(A) The MuSK agonist antibody (Ab #13) failed to stimulate MuSK phosphorylation in innervated (Inn) muscle, suggesting that MuSK may be maximally phosphorylated at synapses by Agrin and Lrp4. (B) Following denervation (Den), non-synaptic regions of muscle express MuSK, but not neural Agrin. Ab #13 stimulated MuSK phosphorylation in denervated muscle by 2.2-fold, demonstrating that Ab #13 activates MuSK in vivo. (C) Following denervation, MuSK expression increases 7.5-fold, but expression of Dok-7, an essential, inside-out activator of MuSK, increases only 2.0-fold. Thus, the activity of Ab #13 in denervated muscle may be limited by low non-synaptic Dok-7 expression. (A, B) The ratio of MuSK-P/MuSK in the absence of Ab #13 was assigned a value of 1.0. (C) The level of MuSK and Dok-7 expression in innervated muscle was assigned a value of 1.0. The SEMs (n = 3) are shown in (A, C); values and averages from two experiments are shown in (B).
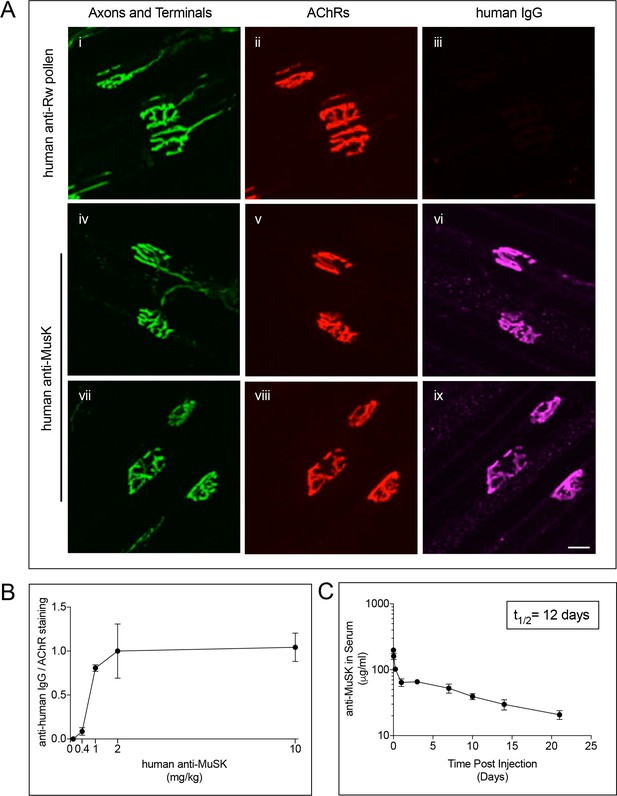
MuSK agonist antibody #13 engages MuSK at the synapse shortly after IP injection.
(A) Staining for the injected MuSK antibody (10 mg/kg) was evident as early as three days (iv-vi) and persisted for at least seven days after a single injection of antibody (vii-ix). A human antibody to Ragweed (Rw) pollen (10 mg/kg) failed to stain synapses (i-iii) (scale bar = 20 μm). (B) 2 mg/kg of the injected MuSK agonist antibody saturated MuSK at the synapse. The ratio of MuSK/AChR staining at 2 mg/kg antibody was assigned a value of 1.0 (±SEM, n = 3), and the values at other doses were expressed relative to this value. (C) Following a single injection of human MuSK antibody #13 (10mg/kg) the level of antibody in serum declines with a half-life of 12.1 days. n = 3.
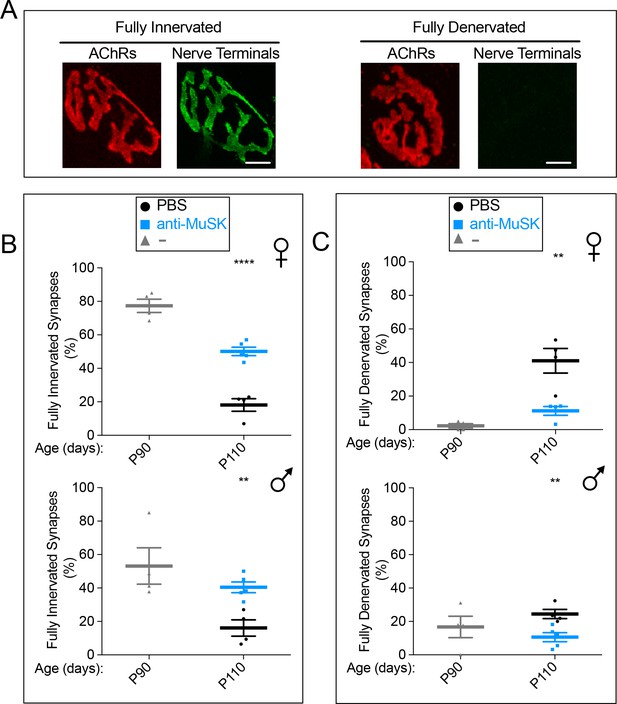
A single injection of the MuSK agonist antibody at P90 reduced synaptic loss for twenty days in SOD1-G93A mice.
(A) AChRs are concentrated in the postsynaptic membrane at innervated and fully denervated synapses (scale bar = 20 μm). (B, C) Denervation is evident in female and extensive in male SOD1-G93A mice at P90 ( ). Over the next twenty days, the extent of full denervation increases and the number of fully innervated synapses decreases (
). Over the next twenty days, the extent of full denervation increases and the number of fully innervated synapses decreases ( ). A single injection of agonist antibody #13 (
). A single injection of agonist antibody #13 ( ) reduces the extent of denervation and the loss of innervation. The scatter plot shows the values for individual mice (n = 4 or 5), as well as the mean values and SEM; **p<0.01, ****p<0.0001.
) reduces the extent of denervation and the loss of innervation. The scatter plot shows the values for individual mice (n = 4 or 5), as well as the mean values and SEM; **p<0.01, ****p<0.0001.
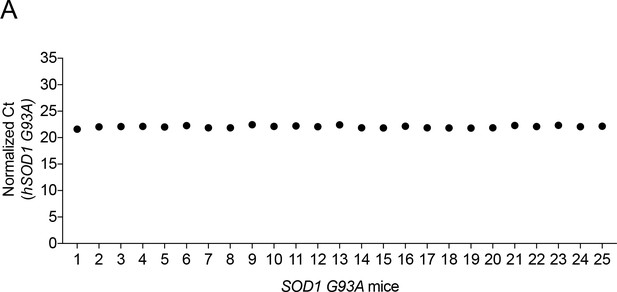
hSOD1-G93A copy number remained unchanged over generations and throughout the experiments.
(A) The copy number of the human SOD1-G93A gene was quantified by real-time PCR and normalized to GAPDH. All mice included in this study had 21–26 copies of hSOD1-G93A. The normalized hSOD1-G93A Ct values are shown for 25 samples (filled circles).
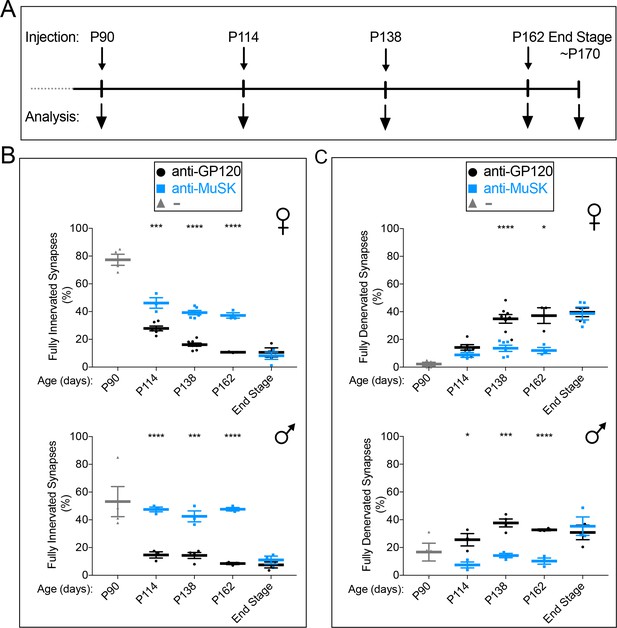
Chronic dosing with the MuSK agonist antibody prevents further denervation in SOD1-G93A mice.
(A) The reverse chimera MuSK agonist antibody #13 was injected at P90 ( ) (B) The extent of full innervation decreases progressively from P90 to P162 in female and male SOD1-G93A injected with a control antibody to GP120 (
) (B) The extent of full innervation decreases progressively from P90 to P162 in female and male SOD1-G93A injected with a control antibody to GP120 ( ). The reverse chimera MuSK agonist antibody #13 (
). The reverse chimera MuSK agonist antibody #13 ( ) halts this progressive loss, as the number of fully innervated synapses is unchanged between P114 and P162. (C) Full denervation increases progressively from P90 to P162 in female and male SOD1-G93A injected with a control antibody to GP120 (
) halts this progressive loss, as the number of fully innervated synapses is unchanged between P114 and P162. (C) Full denervation increases progressively from P90 to P162 in female and male SOD1-G93A injected with a control antibody to GP120 ( ). The reverse chimera MuSK agonist antibody #13 prevents this progressive increase in denervation, as the number of fully denervated synapses is unchanged between P114 and P162 (
). The reverse chimera MuSK agonist antibody #13 prevents this progressive increase in denervation, as the number of fully denervated synapses is unchanged between P114 and P162 ( ). At disease end-stage, the number of fully innervated and denervated synapses was identical in SOD1-G93A mice injected with the MuSK agonist or control antibody. The scatter plot shows the values for individual mice (n = 3 to 8), as well as the mean values and SEM; *p<0.05, ***p<0.001, ****p<0.0001.
). At disease end-stage, the number of fully innervated and denervated synapses was identical in SOD1-G93A mice injected with the MuSK agonist or control antibody. The scatter plot shows the values for individual mice (n = 3 to 8), as well as the mean values and SEM; *p<0.05, ***p<0.001, ****p<0.0001.
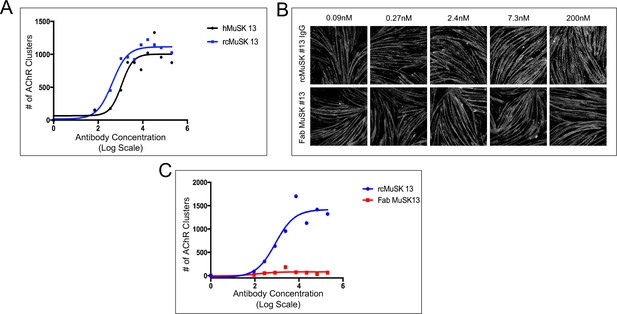
The human and reverse chimera versions of MuSK agonist antibody #13 induce acetylcholine receptor (AChR) clustering in C2C12 myotubes whereas a Fab from MuSK antibody #13 fails to stimulate AChR clustering.
(A) Human (h) and reverse chimera (rc) MuSK #13 antibodies are similarly effective in stimulating AChR clustering in C2C12 myotubes (n = 3). (B,C) C2C12 myotubes were treated with rc MuSK #13 or a Fab from MuSK #13 for 16 hr at the indicated concentrations and stained with α-BGT. We found that the Fab fragment from antibody #13, unlike the intact IgG or the scFv, was unable to stimulate clustering of AChRs, indicating that antibodies must be dimeric and force-dimerize MuSK and promote an orientation that is favorable for trans-phosphorylation. (C) The rc antibody #13 stimulates AChR clustering in a dose-dependent and saturable manner, whereas Fab #13 fails to stimulate AChR clustering (n = 3).
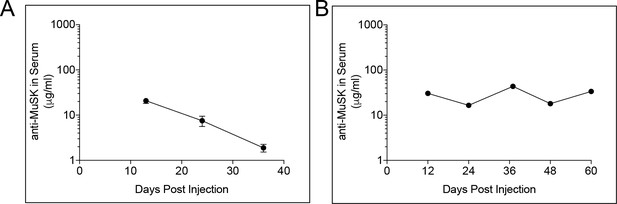
The reverse chimera MuSK agonist antibody #13 has a half-life of 11 days and chronic dosing with this antibody maintains the agonist antibody at levels that are sufficient to saturate MuSK at the synapse.
Rc MuSK antibody #13 was produced to minimize the occurrence of an immune response to a human antibody, as well as to eliminate the danger of eliciting an immune response at the neuromuscular synapse. (A) Following a single 10 mg/kg injection of reverse chimera MuSK agonist antibody #13 in wild type mice, the amount of antibody in the blood decreased over time as a single exponential with a half-life of 11 days. (B) Repeated 10 mg/kg injections of reverse chimera MuSK agonist antibody #13, every 24 days, in SOD1-G93A mice restored antibody levels and maintained the antibody at levels that were sufficient to saturate MuSK at the synapse. The mean values for individual mice (n = 5) and the SEM are shown.
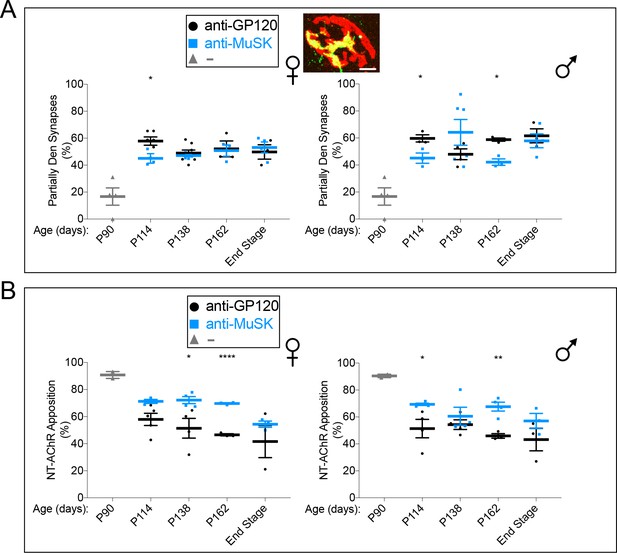
Chronic dosing with the MuSK agonist antibody increases the extent of nerve terminal coverage at partially innervated synapses in SOD1-G93A mice.
(A) The number (~50%) of partially innervated synapses in SOD1-G93A mice is not altered by chronic injection of reverse chimera MuSK agonist antibody #13 ( ). The inset shows a representative partially innervated synapse (scale bar = 20 μm). (B) The extent of the AChR-rich postsynaptic membrane that is apposed by nerve terminals (NT) is greater in SOD1-G93A mice injected with the MuSK agonist antibody (
). The inset shows a representative partially innervated synapse (scale bar = 20 μm). (B) The extent of the AChR-rich postsynaptic membrane that is apposed by nerve terminals (NT) is greater in SOD1-G93A mice injected with the MuSK agonist antibody ( ) than with a control antibody to GP120 (
) than with a control antibody to GP120 ( ). The scatter plot shows the values for individual mice, as well as the mean values and SEM; *p<0.05, **p<0.01, ****p<0.0001.
). The scatter plot shows the values for individual mice, as well as the mean values and SEM; *p<0.05, **p<0.01, ****p<0.0001.
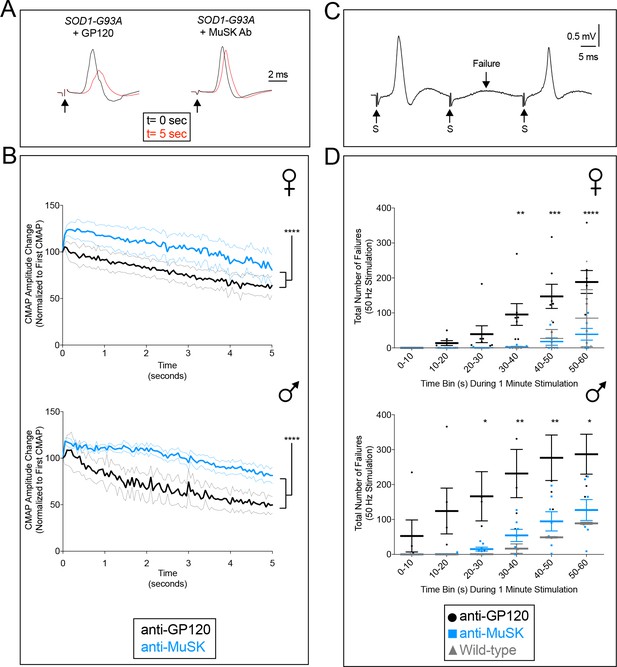
The MuSK agonist antibody improves motor system output in the diaphragm muscle.
(A,B) 20 Hz stimulation (arrow) of the phrenic nerve from SOD1-G93A mice injected with the control antibody to GP120 led to a rapid and severe decline in the CMAP amplitude. In contrast, the CMAP amplitude decreased gradually and modestly in SOD1-G93A mice injected with the MuSK agonist antibody. After 5 s, the MuSK agonist improved CMAP amplitude by 13.6% in females and by 31.7% in males (n = 6–7; p<0.0001). The faint grey and blue lines indicate the SEMs. (C,D) 50 Hz stimulation (S, arrow) of the phrenic nerve in SOD1-G93A mice injected with the control antibody to GP120 led to frequent failures (F, arrow) to elicit a CMAP, whereas CMAPs were reliably elicited in SOD1-G93A mice injected with the MuSK agonist antibody, similar to the number of failures seen in wild type mice. The MuSK agonist antibody reduced the number of failures by 88% in females and 70% in males during 1 min of stimulation. The scatter plot shows the values for individual mice, as well as the mean values and SEM; *p<0.05, **p<0.01, ***p<0.001. The baseline CMAP amplitude data are as follows: anti-GP120-treated males, 5.95 ± 1.14 mV; MuSK agonist antibody-treated males, 5.93 ± 0.52 mV; anti-GP120-treated females, 4.95 ± 0.54 mV; MuSK agonist antibody-treated females, 5.81 ± 0.63 mV.
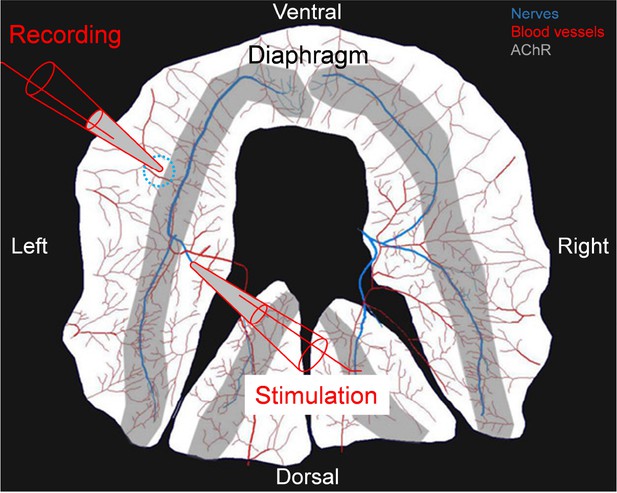
Drawing of experimental protocol to stimulate the phrenic nerve and record CMAPs in the diaphragm muscle.
The phrenic nerve was placed in a bipolar suction electrode for stimulation. CMAPs were recorded from a suction recording electrode placed in the same place (dotted circle) across the different animals used in this study. The phrenic nerve is shown in blue, the blood vessels in red and the area with acetylcholine receptors in grey.
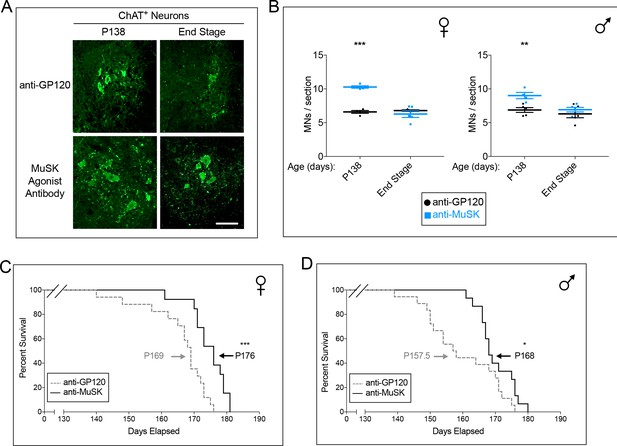
Chronic dosing with the MuSK agonist antibody increases motor neuron survival and extends the lifespan of SOD1-G93A mice.
(A) Representative images of lumbar spinal cords stained with antibodies to ChAT (scale bar = 100 μm). (B) At P138, during the peak period of motor neuron cell death, the number of spinal motor neurons in the lumbar enlargement is greater in SOD1-G93A mice treated with the agonist antibody to MuSK ( ) than in mice treated with the control antibody to GP120 (
) than in mice treated with the control antibody to GP120 ( ). The scatter plot shows the values for individual mice (n = 3 to 5), as well as the mean values and SEM; *p<0.05, **p<0.01, ***p<0.001. (C, D) Female and male SOD1-G93A mice chronically injected with the control antibody to GP120 have a life span of 169 and 157.5 days, respectively (dotted line). Chronic injection of the reverse chimera MuSK agonist antibody prolongs longevity by 7 and 10 days in female and male SOD1-G93A mice, respectively (solid line). n ≥ 13; *p<0.05, **p<0.01, ***p<0.001.
). The scatter plot shows the values for individual mice (n = 3 to 5), as well as the mean values and SEM; *p<0.05, **p<0.01, ***p<0.001. (C, D) Female and male SOD1-G93A mice chronically injected with the control antibody to GP120 have a life span of 169 and 157.5 days, respectively (dotted line). Chronic injection of the reverse chimera MuSK agonist antibody prolongs longevity by 7 and 10 days in female and male SOD1-G93A mice, respectively (solid line). n ≥ 13; *p<0.05, **p<0.01, ***p<0.001.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| strain, strain background (mouse) | Mouse: B6.Cg-Tg(SOD1*G93A)1Gur/J | The Jackson Laboratory | RRID:IMSR_JAX:004435 | |
| strain, strain background (mouse) | Mouse: C57BL/6J | The Jackson Laboratory | RRID:IMSR_JAX000664 | |
| cell line (mouse) | Mouse C2C12 skeletal muscle cells | Burden lab | ATCC Cat# CRL-1772, RRID:CVCL_0188 | |
| cell line (mouse) | Mouse: Immortalized wild type muscle cells | Burden lab | PMID: 18848351 | |
| cell line (mouse) | Mouse: Immortalized Lrp4 mutant muscle cells | Burden lab | PMID: 18848351 | |
| antibody | Rabbit anti-Neurofilament-L | SYnaptic SYstems | Cat# 171 002, RRID:AB_887743 | |
| antibody | Rabbit anti-Synapsin 1/2 | SYnaptic SYstems | Cat# 106 002, RRID:AB_887804 | |
| antibody | Alexa 647-anti-human IgG | Life Technologies | Cat# A-21445, RRID: AB_2535862 | |
| antibody | anti-Choline Acetyltransferase | Millipore | Cat# AB144P-200UL, RRID:AB_90661 | |
| antibody | anti-NeuN | Millipore | Cat# MAB377, RRID: AB_2298772 | |
| antibody | Rabbit anti-MuSK | Burden lab | PMID: 10781064 | |
| antibody | Goat anti-MuSK | R and D Systems | Cat# AF3904, RRID:AB_2147242 | |
| antibody | Rabbit anti-Dok-7 | Burden lab | PMID: 18848351 | |
| antibody | Mouse anti-phosphotyrosine 4G10 | Millipore | ||
| peptide, recombinant protein | Alexa 594-alpha-bungarotoxin | Life Technologies | Cat#B13423 | |
| peptide, recombinant protein | Alexa 488-alpha-bungarotoxin | Life Technologies | Cat#B13422 | |
| peptide, recombinant protein | Hoechst 33342 | Thermo Fisher | Cat# 62249 | |
| peptide, recombinant protein | Recombinant Rat Agrin Protein | R and D Systems | Cat#550-AG-100 | |
| commercial assay or kit | GAPDH TaqMan Assay Mm00186822_cn | Thermo Fisher | Cat# 4400291 | |
| software, algorithm | Prism 7.0 | http://www.graphpad.com/scientific-software/prism/ | RRID:SCR_002798 | |
| software, algorithm | Volocity 3D Image Analysis Software | http://www.perkinelmer.com/pages/020/cellularimaging/products/volocity.xhtml | RRID:SCR_002668 | |
| software, algorithm | pCLAMP Software Suite | https://www.moleculardevices.com/systems/axon-conventional-patch-clamp/pclamp-11-software-suite | ||
| other | DietGel 76A | ClearH20 | Cat#72-07-5022 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34375.015





