Translational control of ERK signaling through miRNA/4EHP-directed silencing
Figures
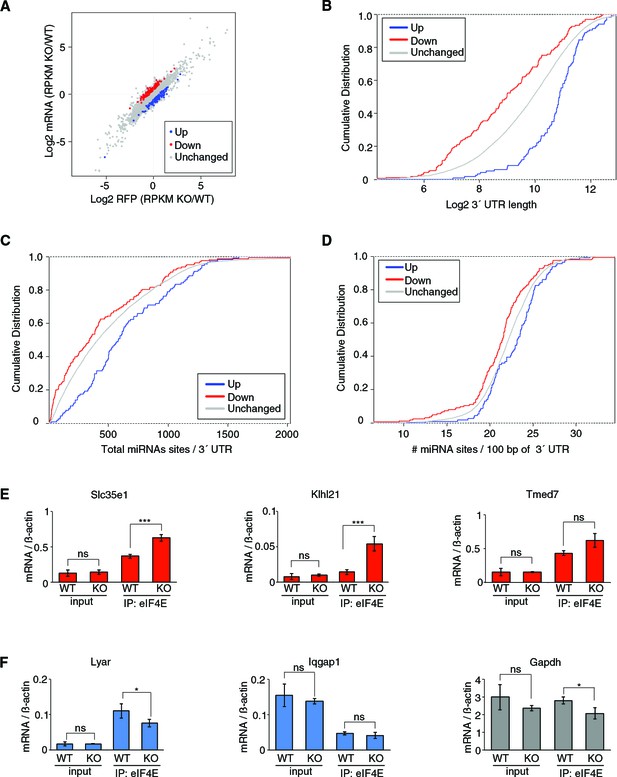
4EHP controls translation of a subset of mRNAs.
(A) The log2 ratio plot of abundance of ribosome footprints (RFP) and mRNAs in 4EHP-KO vs WT MEFs is shown. (B) Comparison of 3´ UTR length of mRNAs up- or down-regulated in 4EHP-KO MEFs. p-values: Up vs. Down: 2.26e-22, Up vs. Unchanged: 4.26e-17. (C) miRNA-binding sites in the 3´ UTR of mRNAs identified in (A). p-values: Up vs. Down: 0.000019, Up vs. Unchanged: 0.00040. (D) miRNA-binding site density (number of miRNA-binding sites per 100-nucleotide of 3´ UTR) in mRNA identified in (A). p-values: Up vs. Down: 0.000043, Up vs. Unchanged: 0.0063. (E) RNA-immunoprecipitation (RIP) analysis of the association of eIF4E with 4EHP targets in 4EHP-KO MEFs. eIF4E was immunoprecipitated using a monoclonal antibody against eIF4E from WT and 4EHP-KO MEFs. Levels of the indicated mRNAs (normalized to β-actin mRNA) in the inputs and eIF4E-bound mRNAs were analyzed by RT–qPCR. Data are mean ± SD (n = 3). The p-value was determined by two-tailed Student's t‐test: (ns) non-significant, (*) p<0.05; (**) p<0.01; (***) p<0.001.
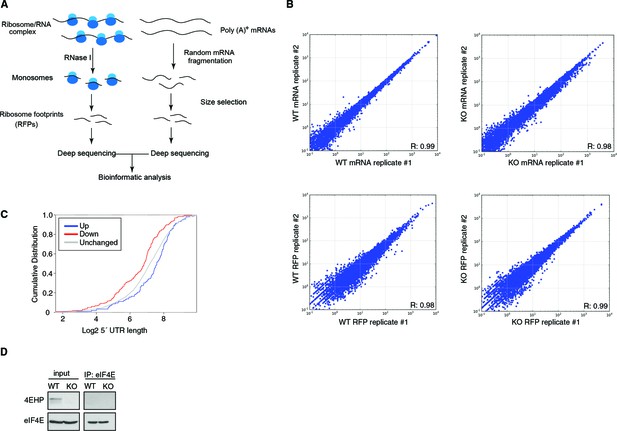
Analysis of 4EHP-sensitive mRNAs by ribosome profiling.
(A) Summary of workflow used to identify 4EHP-sensitive mRNAs by ribosome profiling. (B) Correlation between replicates in mRNA-Seq and ribosome profiling datasets. R2 indicates Pearson correlation. (C) Comparison of 5´ UTR length in mRNAs identified by Babel analysis as up- or down-regulated in 4EHP-KO MEFs. p-values: Up vs. Down: 2.68e-06, Up vs. Unchanged: 0.038. (D) WB analysis of the indicated protein in the eIF4E RIP assay (related to Figures 1E and 3A). eIF4E was immunoprecipitated using a monoclonal antibody in WT and 4EHP-KO MEFs. Precipitated proteins were separated by SDS-PAGE and probed with the specified antibodies.
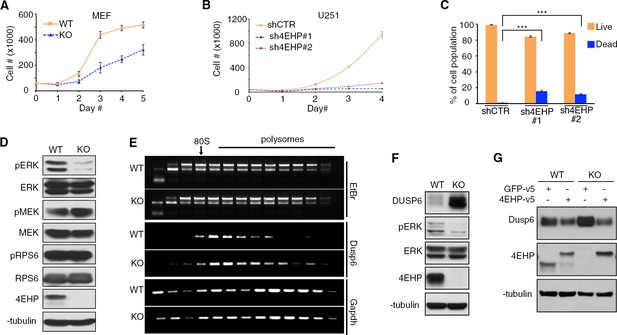
Depletion of 4EHP expression affects cell proliferation, survival, and ERK1/2 phosphorylation.
(A) Cell proliferation assay. WT and 4EHP-KO MEFs were seeded in 6-well plates and trypsinized after the indicated time points and cell numbers determined using a hematocytometer. Data are mean ± SD (n = 3). (B) Cell proliferation assay. U251 cells with stable expression of shCTR (control), sh4EHP#1, and sh4EHP#2 were seeded in 6-well plates. Cells were trypsinized after the indicated time points and cell numbers determined using a hematocytometer. Data are mean ± SD (n = 3). (C) Quantitation of cell death by FACS assay; Sub-G population was considered as ‘Dead’ and G0/1, S and G2/M population was combined as ‘Live’. Data are mean ± SD (n = 3). (D) WB for the indicated proteins in the WT and 4EHP-KO MEFs. (E) Polysome profiling/RT-PCR; RNA was extracted from each fraction (collected as described in Figure 2—figure supplement 1J), subjected to electrophoresis on agarose gel and visualized, using Ethidium Bromide (EtBr) staining. RT-PCR analyses of total RNA in each fraction was carried out with primers specific for Dusp6 and Gapdh mRNAs. (F) WB on the indicated proteins in WT and 4EHP-KO MEFs. (G) WB for the indicated proteins in the WT and 4EHP-KO MEFs, expressing a v5-tagged GFP (GFP-v5) or v5-tagged 4EHP (4EHP-v5).
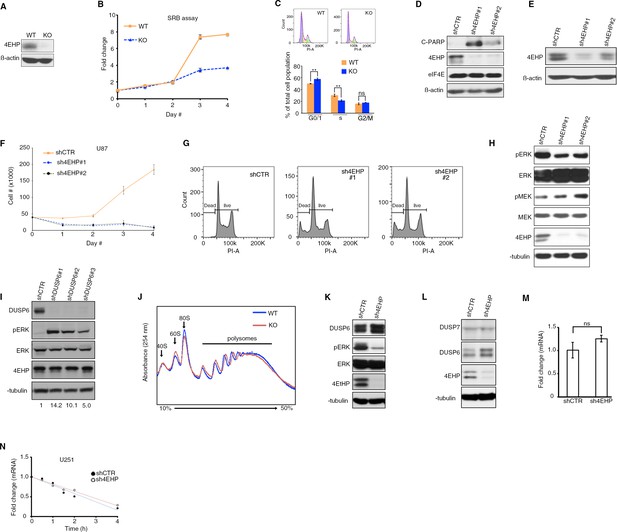
Cell proliferation and translational regulation of DUSP6 expression is affected by 4EHP depletion.
(A) WB for the indicated proteins in the WT and 4EHP-KO MEFs. (B) Cell proliferation was assessed using Sulforhodamine B (SRB ) assay . Data are mean ± SD (n = 3). (C) Top; Representative cell cycle profiles of the WT and 4EHP-KO MEFs stained with Propidium Iodide and analyzed by FACS. Bottom; quantitation of cell cycle profiles. Data are mean ± SD (n = 3). (D) WB for the indicated proteins in control and stable 4EHP-knockdown U251 cells. (E) WB for the indicated proteins in the control and stable 4EHP-knockdown U87 cells. (F) Cell proliferation assay; U87 cells with stable expression of shCTR, sh4EHP#1, and sh4EHP#2 were seeded in 6-well plates. Cells were trypsinized after the indicated time points and cell numbers determined using a hematocytometer. Data are mean ± SD (n = 3). (G) FACS assay. Representative cell cycle profiles of shCTR, sh4EHP#1, and sh4EHP#2 U251 cells stained with Propidium Iodide and analyzed by FACS. (H) WB for the indicated proteins in the control and stable 4EHP-knockdown U251 cells. (I) WB for the indicated proteins in the control and stable Dusp6-knockdown U251 cells. (J) Polysome profiling; cytoplasmic extract from WT and 4EHP-KO MEFs was fractioned by centrifugation on a 10–50% sucrose gradient. Fourteen fractions were collected while 254 nm absorbance was recorded. (K) WB for the indicated proteins in control (shCTR) and 4EHP-knockdown (sh4EHP) U251 cells. (L) WB for the indicated proteins in the control and stable 4EHP-knockdown U251 cells. (M) RT-qPCR analysis of Dusp6 mRNA in shCTR and sh4EHP U251 cells. Values are normalized to β-actin. Data are mean ± SD (n = 3). (N) RNA stability assay of Dusp6 mRNA in shCTR and sh4EHP U251 cells. The amount of RNA at different time points was determined by RT-qPCR. Values are normalized to 28S rRNA. Data are mean ± SD (n = 3).
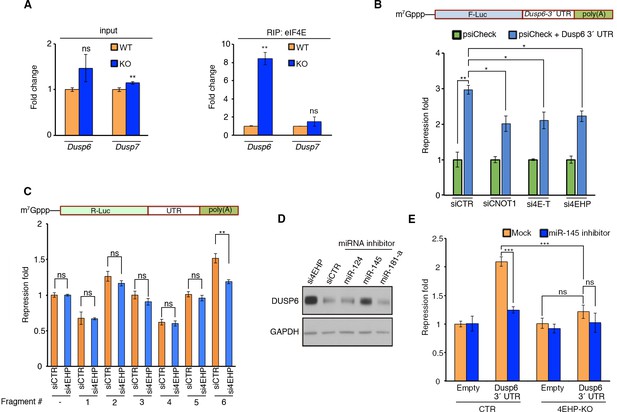
4EHP enables miRNA-mediated silencing of Dusp6 mRNA.
(A) RIP analysis of the association of eIF4E with Dusp6 mRNA in WT and 4EHP-KO MEFs. eIF4E was immunoprecipitated using a monoclonal antibody. Levels of the indicated mRNAs (normalized to β-actin mRNA) in the inputs and eIF4E-bound mRNAs were analyzed by RT–qPCR. Data are mean ± SD (n = 3). (B) Top; Schematic representation of the psiCHECK-FL-Dusp6 3´ UTR reporter. Bottom; CTR, CNOT1, 4E-T, or 4EHP-knockdown cells were co-transfected with psiCHECK-FL-Dusp6 3´ UTR reporter or the psiCHECK reporter (as control) in HEK293T cells. Luciferase activity was measured 24 hr after transfection. Firefly (F-Luc) values were normalized against Renilla (R-Luc) levels, and repression fold was calculated for the psiCHECK-FL-Dusp6 3´ UTR reporter relative to psiCHECK reporter level for each condition. Data are mean ± SD (n = 3). (C) The psiCHECK reporter (control) or psiCHECK-RL with truncated fragments of the Dusp6 3´ UTR were transfected into the HEK293T cells. Luciferase activity was measured 24 hr after transfection. R-Luc values were normalized against F-Luc levels, and repression fold was calculated for the psiCHECK-RL-Dusp6 3´ UTR reporter relative to psiCHECK reporter level for each condition. Data are mean ± SD (n = 3). (D) WB for the indicated proteins in U251 cells transfected with si4EHP or the indicated miRNA inhibitors. (E) The psiCHECK reporter (control) or psiCHECK-FL-Dusp6 3´ UTR were co-transfected along with the mock or miR-145 inhibitor in the control (CTR) or 4EHP-KO HEK293 cells. Luciferase activity was measured 24 hr after transfection. F-Luc values were normalized against R-Luc levels, and repression fold was calculated relative to the psiCHECK reporter/control inhibitor for each condition. Data are mean ± SD (n = 3). The p-values was determined by two-tailed Student's t‐test: (ns) non-significant, (*) p<0.05; (**) p<0.01; (***) p<0.001.
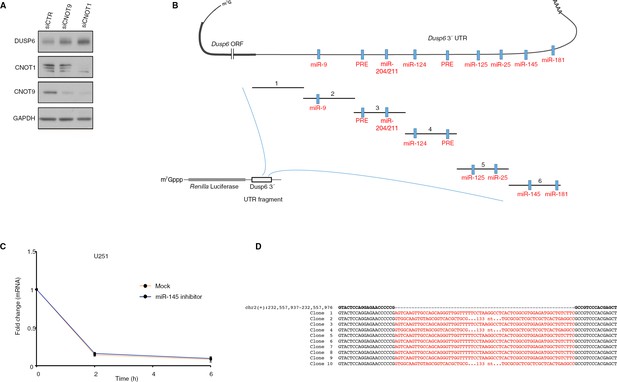
Repression of DUSP6 expression by CCR4-NOT complex.
(A) WB for the indicated proteins in control or siRNA transfected U251 cells. (B) Diagram of Dusp6 mRNA 3´ UTR, predicted miRNA binding sites, pumilio responsive element (PRE), and truncation fragments of the UTR created for cloning into the reporter construct used in Figure 3C. (C) RNA stability assay of Dusp6 mRNA in Mock and miR-145-inhibitor transfected cells. The quantity of RNA at different time points was determined by RT-qPCR. Values are normalized to 28S rRNA. Data are mean ± SD (n = 3). (D) Sequence alignment of 10 cloned PCR products amplified from the genomic segment of 4EHP targeted by 5´-TGAGCTCGTGGGACGGCCGG sgRNA showing the disruption of the coding sequence (related to Figure 3E).
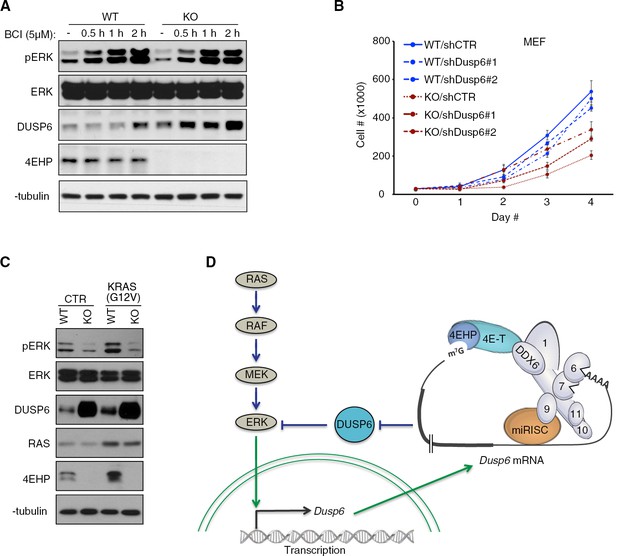
De-repression of DUSP6 in 4EHP-depleted cells impedes on ERK activity and cell proliferation.
(A) Time course WB analyses of BCI-treated WT and 4EHP-KO MEFs. (B) Cell proliferation assay. WT and 4EHP-KO MEFs with stable expression of shCTR, shDusp6#1, and shDusp6#2 were seeded in 6-well plates. Cells were trypsinized after the indicated time points and cell numbers determined using a hematocytometer. Data are mean ± SD (n = 3). (C) WB for the indicated proteins in the WT and 4EHP-KO MEFs, with stable expression of a constitutively active mutant of KRAS (G12V). (D) Model of regulation of MAPK/ERK pathway activity by 4EHP through translational control of the Dusp6 mRNA. Upon phosphorylation by MEK, ERK translocates to the nucleus and activates the Dusp6 gene. The Dusp6 transcript is then exported to the cytoplasm and translated. miRNAs control the translation of Dusp6 mRNA via the CCR4-NOT/4E-T/4EHP complex and thus regulate the MAPK/ERK pathway activity.
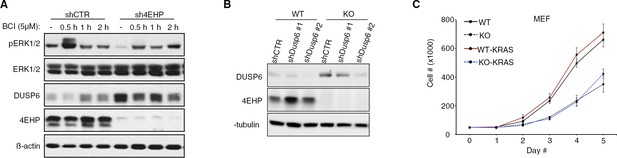
DUSP6-mediated repression of ERK activity and cell proliferation in 4EHP-depleted cells.
(A) Time course analyses of BCI-treated control and 4EHP-knockdown U251 cells by WB for the indicated proteins. (B) WB for the indicated proteins in the control or Dusp6-knockdown WT and 4EHP-KO MEFs. (C) Cell proliferation assay. WT and 4EHP-KO MEFs, with stable expression of a constitutively active mutant KRAS (G12V) were seeded in 6-well plates. Cells were trypsinized after the indicated time points and cell numbers determined using a hematocytometer. Data are mean ± SD (n = 3).
Additional files
-
Supplementary file 1
mRNAs differentially translated in 4EHP-KO vs WT MEFs identified by the ribosome profiling assay.
- https://doi.org/10.7554/eLife.35034.010
-
Supplementary file 2
Dusp6 3´ UTR isolated from U251 human glioblastoma cell line.
Highlighted sequence represent the translation stop codon.
- https://doi.org/10.7554/eLife.35034.011
-
Supplementary file 3
List of primers used in this study.
- https://doi.org/10.7554/eLife.35034.012
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35034.013





