The eukaryotic bell-shaped temporal rate of DNA replication origin firing emanates from a balance between origin activation and passivation
Figures
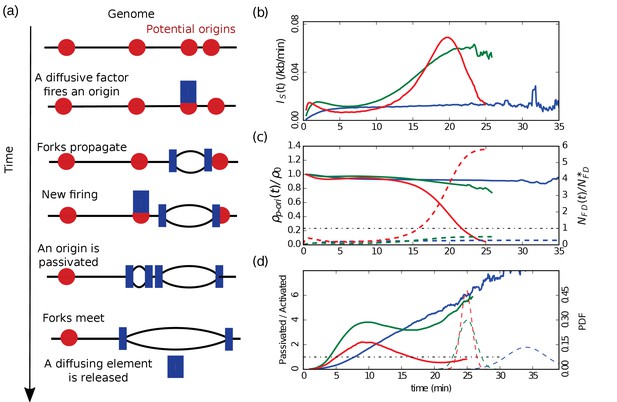
Emergence of a bell-shaped .
(a) Sketch of the different steps of our modeling of replication initiation and propagation. (b) (Equation 1) obtained from numerical simulations (Materials and methods) of one chromosome of length 3000 kb, with a fork speed kb/min. The firing factors are loaded with a characteristic time of 3 min. From blue to green to red the interaction is increased and the number of firing factors is decreased: blue ( min, , kb), green ( min, , kb), red ( min, , kb). (c) Corresponding normalized densities of p-oris (solid lines), and corresponding normalized numbers of free diffusing firing factors (dashed line): blue (), green (), red (); the horizontal dotted-dashed line corresponds to the critical threshold value . (d) Corresponding number of passivated origins over the number of activated origins (solid lines). Corresponding probability distribution functions (PDF) of replication time (dashed lines).
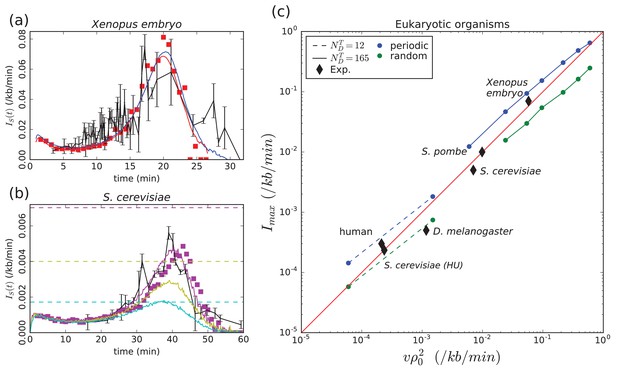
Model validation by experimental data.
(a) Xenopus embryo: Simulated (Equation (1), Materials and methods) for a chromosome of length kb and a uniform distribution of p-oris (blue: kb/min, min, , kb) or a periodic distribution of p-oris (red: kb/min, min, , kb); (red squares) 3D simulations with the same parameter values as for periodic p-ori distribution; (black) experimental : raw data obtained from Goldar et al. (2009) were binned in groups of 4 data points; the mean value and standard error of the mean of each bin were represented. (b) S. cerevisiae: Simulated (Materials and methods) for the 16 chromosomes with the following parameter values: kb/min, , min-1, when considering only Confirmed origins (light blue), Confirmed and Likely origins (yellow) and Confirmed, Likely and Dubious origins (purple); the horizontal dashed lines mark the corresponding predictions for (Equation 5); (purple squares) 3D simulations with the same parameter values considering Confirmed, Likely and Dubious origins; (black) experimental from Goldar et al. (2009). (c) Eukaryotic organisms: as a function of ; (squares and bullets) simulations performed for regularly spaced origins (blue) and uniformly distributed origins (green) (Materials and methods) with two sets of parameter values: kb, kb/min, min and (dashed line) or (solid line); (black diamonds) experimental data points for Xenopus embryo, S. cerevisiae, S. cerevisae grown in Hydroxyurea (HU), S. pombe, D. melanogaster, human (see text and Table 1). The following figure supplement is available for Figure 2.
-
Figure 2—source data 1
Data file for the experimental Xenopus in Figure 2 (a).
- https://doi.org/10.7554/eLife.35192.006
-
Figure 2—source data 2
Data file for the experimental S.
cerevisae in Figure 2 (b).
- https://doi.org/10.7554/eLife.35192.007
-
Figure 2—source data 3
Data file for the experimental parameters used in Figure 2 (c).
- https://doi.org/10.7554/eLife.35192.008
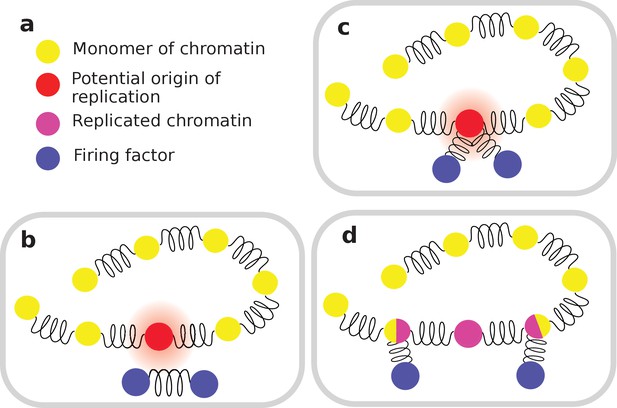
Different steps of the interaction between diffusing elements and origins of replication.
(a) Definition of the color coding; (b) once in the vicinity of an origin of replication, a firing factor can be captured; (c) it is then splitted; (d) the two forks then travel in opposite direction, each carrying half of the diffusing firing factor.
Tables
Experimental data for various eukaryotic organisms with genome length (), replication fork velocity (kb/min), number of p-oris (), (kb) and (Mbmin).
All data are from Goldar et al. (2009), except for S. cerevisiae grown in presence or absence of hydroxyurea (HU) which were computed from the replication profile of Alvino et al. (2007). For S. cerevisiae and S. pombe, Confirmed, Likely, and Dubious origins were taken into account. For D. melanogaster, was obtained from the same Kc cell type as the one used to estimate . For Xenopus embryo, we assumed that a p-ori corresponds to a dimer of MCM2-7 hexamer so that was estimated as a half of the experimental density of MCM3 molecules reported for Xenopus sperm nuclei DNA in Xenopus egg extract (Mahbubani et al., 1997). For human, we averaged the number of origins experimentally identified in K562 (62971) and in MCF7 (94195) cell lines.
| Ref. | ||||||
|---|---|---|---|---|---|---|
| S. cerevisiae | 12.5 | 1.60 | 829 | 0.066 | 6.0 | Sekedat et al. (2010) and Siow et al. (2012) |
| S. cerevisiae in presence of HU | 12.5 | 0.05 | 829 | 0.066 | 0.24 | Alvino et al. (2007). Same and as S. cerevisiae in normal growth condition. |
| S. pombe | 12.5 | 2.80 | 741 | 0.059 | 10.0 | Siow et al. (2012) and Kaykov and Nurse (2015) |
| D. melanogaster | 143.6 | 0.63 | 6184 | 0.043 | 0.5 | Ananiev et al. (1977) and Cayrou et al. (2011) |
| human | 6469.0 | 1.46 | 78000 | 0.012 | 0.3 | Conti et al. (2007) and Martin et al. (2011) |
| Xenopus sperm | 2233.0 | 0.52 | 744333 | 0.333 | 70.0 | Mahbubani et al. (1997) and Loveland et al. (2012) |
Additional files
-
Supplementary file 1
This file provides: the parameter values used for all the simulations in Figures 1 and 2; the list of all the symbols used in the main text and their meanings.
- https://doi.org/10.7554/eLife.35192.010
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35192.011





