Functional and structural characterization of an ECF-type ABC transporter for vitamin B12
Figures
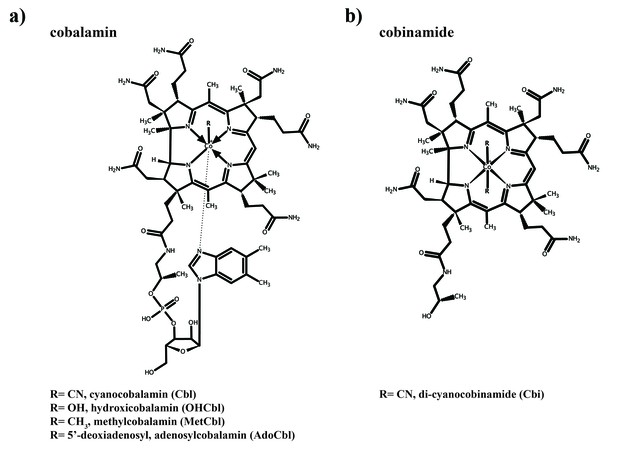
Structures of cobalamin and cobinamide.
(a) Cobalamin structure, represented in the base-on conformation with the 5’,6’-dimethyl-benzimidazole ribonucleotide moiety (α-ligand) coordinating the central cobalt ion. The variable β-ligands are denoted as R in the lower left corner. (b) Structure of cobinamide, which lacks the DMBI moiety and has two cyano groups coordinating the cobalt ion from each side of the corrin ring.
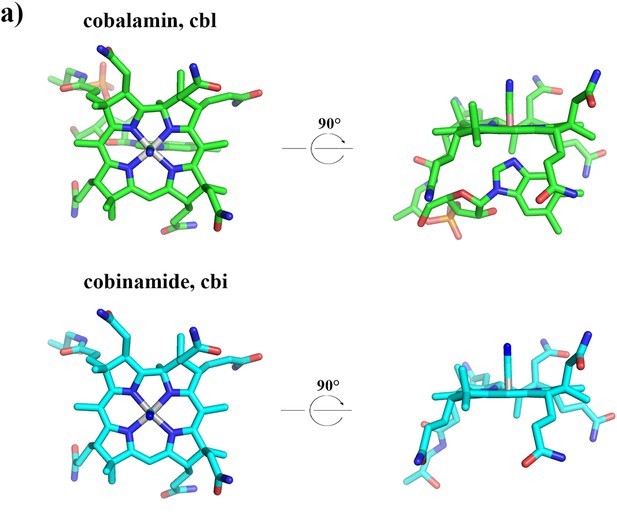
3D structures of cobalamin and cobinamide.
Cyanocobalamin (green) and mono-cyanocobinamide (cyan) are shown in stick representation. The central cobalt ion is colored in grey, nitrogen atoms in blue, oxygen atoms in red and phosphate atoms in orange.
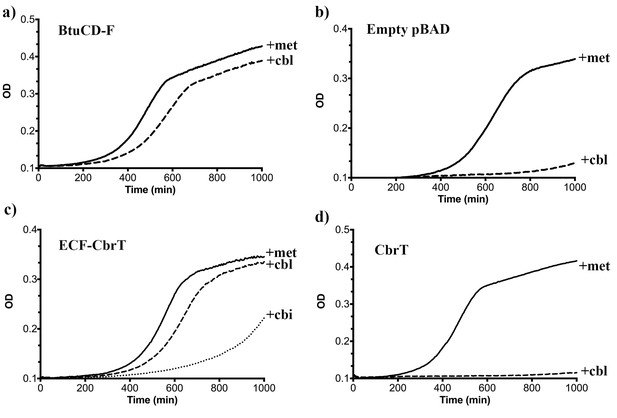
ECF-CbrT supports cobalamin-dependent growth of an E.coli deletion strain.
(a) The triple knock out strain E. coli ΔFEC expressing the BtuCDF ABC-transporter (positive control) grows in the presence of 50 μg/ml L-methionine or 1 nM CN-Cbl with lag-times of 300 min or 380 min, respectively, (b) E. coli ΔFEC carrying only the empty expression vector (negative control) grows only in the presence of 50 μg/ml L-methionine but not with 1 nM CN-Cbl. The lag-times of the negative controls are 450 min or >1000 min, respectively. (c) E. coli ΔFEC expressing the entire ECF-CbrT transporter supports growth in the presence of either 1 nM Cbl or 1 nM Cbi with a lag-time of 470 min or 730 min, respectively. The lag-time in the presence of 50 μg/ml L-methionine is 410 min. (d) Expression of the solitary S-component CbrT without its cognate ECF-module is not able to support growth of E. coli ΔFEC in the presence of 1 nM CN-Cbl.
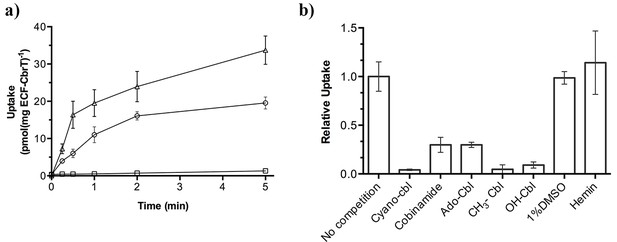
57Co-cyanocobalamin (cyano-Cbl) and 57Co-cobinamide (Cbi) transport by purified and reconstituted ECF-CbrT.
(a) ATP-dependent uptake of radiolabeled CN-Cbl and Cbi by ECF-CbrT in proteoliposomes. Proteoliposomes were loaded with either 5 mM Mg-ATP (circles for CN-Cbl, triangles for Cbi) or 5 mM Mg-ADP (CN-Cbl, squares). (b) Competition assay using Cbl-analogues. The initial uptake rate at 1 nM 57Co-cyanocobalamin (CN-Cbl) was measured. Competing compounds (adenosyl-cobalamin (Ado-Cbl); methyl-cobalamin (CH3-Cbl); hydroxyl-cobalamin (OH-Cbl); cobinamide or hemin) were added at a concentration of 250 nM. The uptake was normalized to a condition without competitor (10 pmol*mg−1*min−1). Since hemin is not readily soluble in an aqueous solution, we added 1% (v/v) DMSO during the assay, which did not affect the transporter activity. All competition experiments were performed in triplicate and the error bars indicate the standard deviation (s.d.).
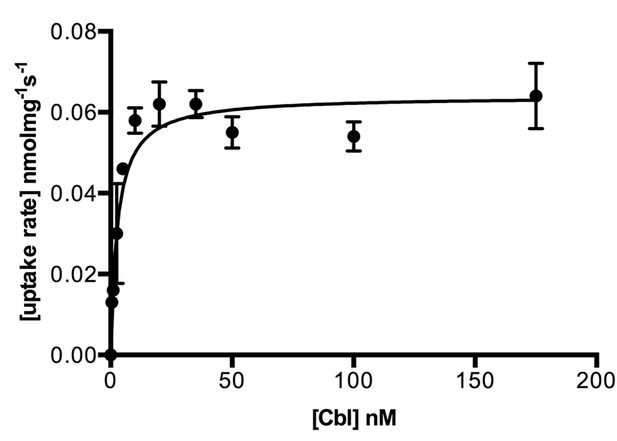
Kinetics of cobalamin uptake by ECF-CbrT.
Transport rates are shown as a function of the cobalamin concentration. Proteoliposomes were loaded with 5 mM Mg-ATP. Experiments were performed in triplicate and error bars indicate the s.d.’s. Values for the initial uptake rates were obtained by fitting the Michaelis-Menten function to the data, R2 = 0.979. The estimated KM value is 2.1 ± 0.4 nM and the Vmax is 0.06 ± 0.01 pmolmg−1s−1.
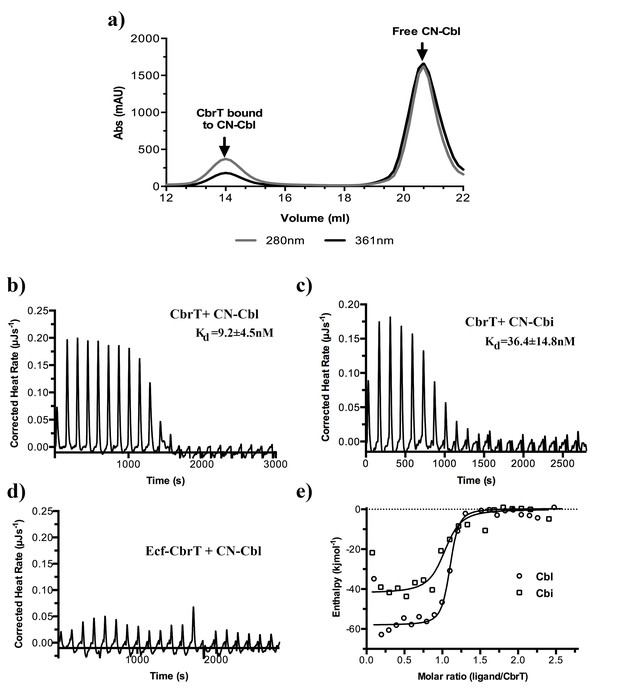
Cobalamin and cobinamide binding to CbrT.
(a) Co-purification of CN-cobalamin with CbrT. The elution peak of the size exclusion column at a volume of 14 ml contains purified CbrT. The protein absorbs at 280 nm and CN-Cbl at 361 nm, showing that CbrT is eluted bound to CN-Cbl. (b) and (c) ITC measurements of Cbl and Cbi binding to CbrT. The determined Kd values for Cbl and Cbi were averaged from triplicate measurements and the error is s.d. (d) ITC measurement showing the absence of Cbl-binding to the full complex, ECF-CbrT. Fitting of single binding site models to the data is shown in panel (e).
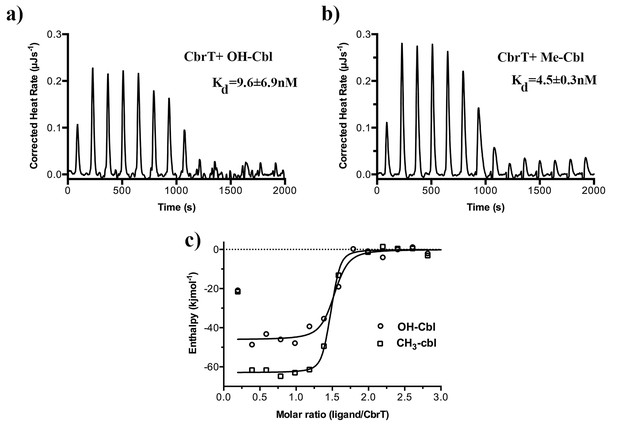
Binding of Cbl-analogs to CbrT.
ITC measurements of OH-Cbl (a) and CH3-Cbl (b) binding to CbrT. The determined Kd values were averaged from duplicate measurements. Fitting of single binding site models to the data is shown in panel c).
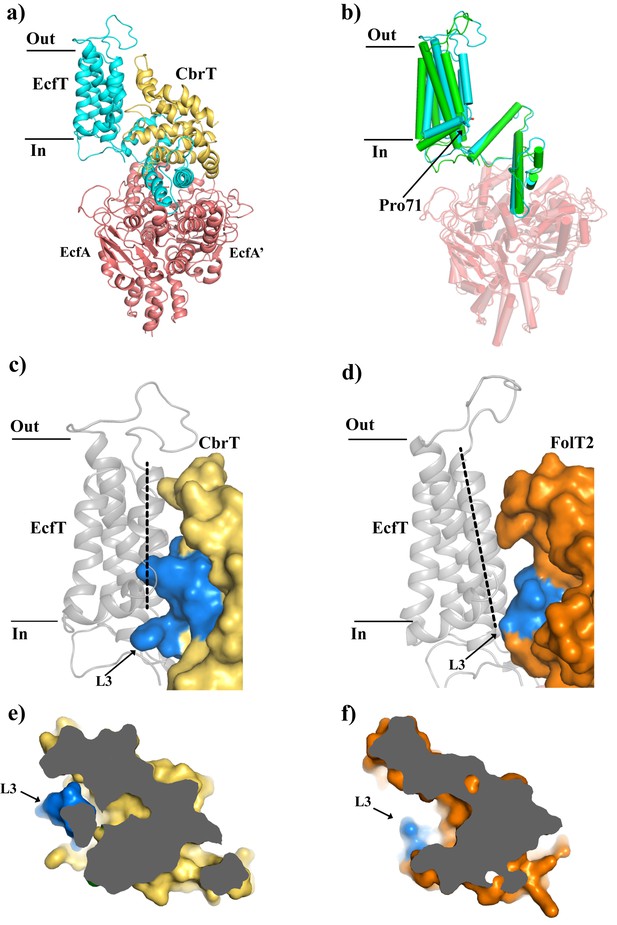
Comparison of the structures of ECF-CbrT and ECF-FolT from L.delbrueckii.
(a) Cartoon representation of ECF–CbrT from the perspective of the plane of the membrane. Cytoplasmic ATPases, EcfA and EcfA’, are colored in red, EcfT in cyan and CbrT in yellow. (b) Structural differences between the membrane domains of EcfT. The structures of ECF-CbrT (cyan) and ECF-FolT2 (green) from L. delbrueckii were superimposed by structural alignment of the ATPase units. Pro71 of EcfT is represented in sticks. (c) and (d) Surface representation of CbrT (c, yellow) and FolT2 (d, orange) interacting with EcfT (cartoon representation coloured in grey) with loop 3 of the S-components colored in blue. A dashed line highlights the movement of transmembrane helix 3 of EcfT. (e) and (f) Loop 3 obstructs access to the substrate binding cavity in CbrT but not in FolT2. (e) Slice-through of CbrT in surface representation, viewed from the plane of the membrane. Loop 3 is colored in blue. The ECF module has been omitted for clarity. (f) Same slice through representation like in (e) but for FolT2.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Lactobacillus delbrueckii subsp. bulgaricus) | cbrT | NA | LDB_RS00385 | |
| Strain, strain background (E. coli) | MC1061 | Casadaban, M. J., and Cohen, S. N. (1980). Analysis of gene control signals by DNAfusion and cloning in Escherichia coli.Journal of Molecular Biology, 138(2),179–207 PMID 6997493 | E. coli ΔFEC was constructed in this paper with the following deletions ΔbtuF, ΔmetE, and ΔbtuC:: KmR. Strain requires either L-methionine or cobalmin/cobinamide plus expression of an appropiate cobalmin/cobinamide transporter. Strain can be made available upon reasonable request. | |
| Strain, strain background (E. coli) | JW0154 | Coli Genetic Stock Center Yale | E. coli ΔFEC was constructed in this paper with the following deletions ΔbtuF, ΔmetE, and ΔbtuC::KmR. Strain requires either L-methionine or cobalmin/cobinamide plus expression of an appropiate cobalmin/cobinamide transporter. Strain can be made available upon reasonable request. | |
| Strain, strain background (E. coli) | JW3805 | Coli Genetic Stock Center Yale | E. coli ΔFEC was constructed in this paper with the following deletions ΔbtuF, ΔmetE, and ΔbtuC::KmR. Strain requires either L-methionine or cobalmin/cobinamide plus expression of an appropiate cobalmin/ cobinamide transporter. Strain can be made available upon reasonable request. | |
| Strain, strain background (E. coli) | JW1701 | Coli Genetic Stock Center Yale | E. coli ΔFEC was constructed in this paper with the following deletions ΔbtuF, ΔmetE, and ΔbtuC::KmR. Strain requires either L-methionine or cobalmin/cobinamide plus expression of an appropiate cobalmin/ cobinamide transporter. Strain can be made available upon reasonable request. | |
| Strain, strain background (E. coli) | ΔFEC | This paper | E. coli ΔFEC was constructed in this paper with the following deletions ΔbtuF, ΔmetE, and ΔbtuC::KmR. Strain requires either L-methionine or cobalmin/cobinamide plus expression of an appropiate cobalmin/ cobinamide transporter. Strain can be made available upon reasonable request. | |
| Biological sample (Lactobacillus delbrueckii) | Lactobacillus delbrueckii subsp. bulgaricus genomic DNA | DSMZ | DSM 20081 | |
| Recombinant DNA reagent | pBAD24_CbrT | This paper | Expression plasmids for CbrT and ECF-CbrT in E. coli. Plasmids can be provided upon reasonable request. | |
| Recombinant DNA reagent | p2BAD_ECF_CbrT | This paper | Expression plasmids for CbrT and ECF-CbrT in E. coli. Plasmids can be provided upon reasonable request. | |
| Chemical compound, drug | CN-Cbl | Acros | 405920050 | |
| Chemical compound, drug | OH-Cbl | Sigma-Aldrich | 95200–1G | |
| Chemical compound, drug | Met-Cbl | Sigma-Aldrich | M9756-250G | |
| Chemical compound, drug | Ado-Cbl | Sigma-Aldrich | C0884-250MG | |
| Chemical compound, drug | Cbi | Sigma-Aldrich | C3021-50MG | |
| Chemical compound, drug | hemin | Sigma-Aldrich | 51280–1G | |
| Chemical compound, drug | 57Co-cyanocobalamin | MP-Biomedicals | 06B-430000 | |
| Chemical compound, drug | perchloric acid | Sigma-Aldrich | 311421–50 ML | |
| Software, algorithm | Origin 8 | Company | ||
| Other | ECF-CbrT coordinate file and structure factors | this paper | accession numberPDB ID code 6FNP | Crystal structure of ECF-CbrT |
Additional files
-
Supplementary file 1
Data collection, phasing and refinement statistics.
- https://doi.org/10.7554/eLife.35828.010
-
Supplementary file 2
Primer list used in this study.
- https://doi.org/10.7554/eLife.35828.011
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35828.012





