Mechanically stimulated ATP release from murine bone cells is regulated by a balance of injury and repair
Figures
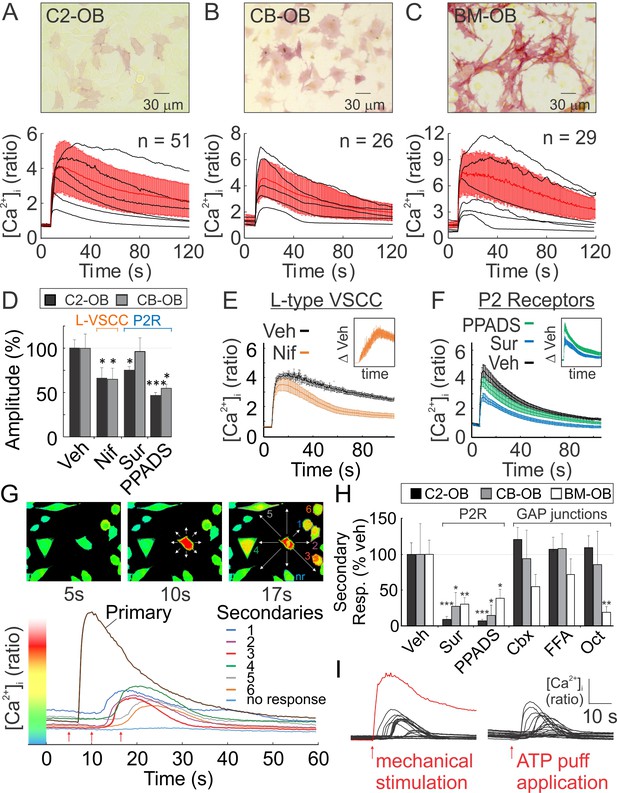
Osteoblasts are mechanosensitive
(A-C) Single Fura2-loaded C2-OB (A), CB-OB (B) or BM-OB (C) (Top: ALP staining) were stimulated with micropipette and [Ca2+]i was recorded (Figure 1—video 1). Bottom: representative traces (black), means ± SEM (red) (D) Amplitudes of mechanically evoked [Ca2+]i transients in osteoblasts pretreated with vehicle, Nif, Sur or PPADS (see Materials and methods for abbreviations, targets and doses). Means ± SEM, n = 6–15 stimulated cells, normalized to vehicle. (E, F) Contribution of L-type VSCC (E, n = 10) and P2 receptors (F, n = 12) to mechanically evoked [Ca2+]i transients in C2-OB as comparison and difference (insets) of mean ±SEM of vehicle and treatment [Ca2+]i transients. (G) [Ca2+]i in the stimulated (primary) and neighboring (secondary) C2-OB. Top: pseudocolor of 340/380 ratio, white arrows: directions of signal propagation, bottom: [Ca2+]i transients, red arrows: top panel time points. (H) Secondary responsiveness in cultures pretreated with vehicle, Sur, PPADS, Cbx, FFA or Oct. Means ± SEM, n = 7–21 stimulated primary responses, normalized to vehicle. (H) [Ca2+]i transients induced by mechanical stimulation of single C2-OB (left, red curve) or puff application of 10 µM ATP (right). For Figure 1, *significance between treatment and control by ANOVA. Source data for Figure 1 is provided in Figure 1—source data 1.
-
Figure 1—source data 1
- https://doi.org/10.7554/eLife.37812.005
Single Fura2-loaded C2-OB was stimulated with micropipette and [Ca2+]i was recorded.
Pseudocolor corresponds to 340/380 ratio, video condensed from original duration of 110 s.
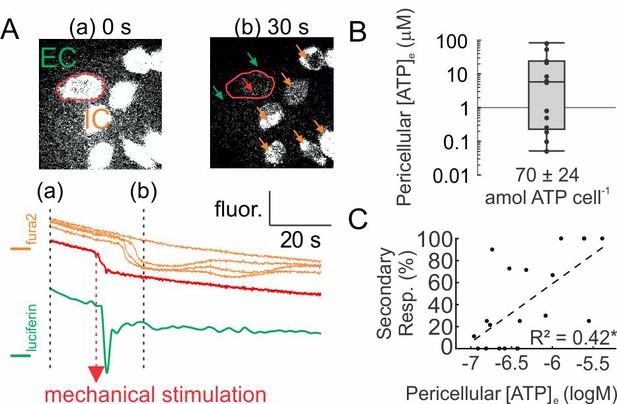
ATP release following mechanical stimulation of a single osteoblast.
(A) Fura2-loaded C2-OB bathed in luciferin/luciferase containing PS was micropipette-stimulated. Top: 380 ex/510 em images before (a) and after (b) stimulation of the cell (red outline). Bottom: intracellular (IC) Fura2 fluorescence [Ifura2] of primary (red) and secondary (orange) responders and extracellular (EC) luciferin fluorescence [Iluciferin] (green). (B) Box plot of [ATP]e in pericellular region of stimulated C2-OB, black markers: independent observations, n = 13 stimulated cells. (C) Correlation between [ATP]e and percentage of secondary responders observed in C2-OB (n = 20 stimulated primary responses), dashed line: linear regression. Source data for Figure 2 is provided in Figure 2—source data 1.
-
Figure 2—source data 1
- https://doi.org/10.7554/eLife.37812.008
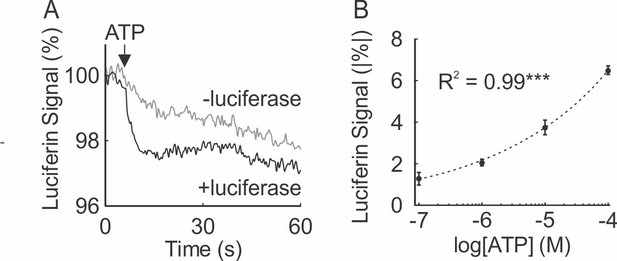
Real-time ATP measurement assay using fluorescent properties of luciferin-luciferase.
(A) 1 µM ATP was added to luciferin in presence or absence of luciferase and fluorescence was recorded at 380 ex/510 em. Data were normalized to basal luciferin fluorescence. (B) [ATP]e dose dependence of luciferin-luciferase fluorescence change from baseline. Means ± SEM, n = 3 independent recordings, dashed line: linear regression, ***p<0.001.
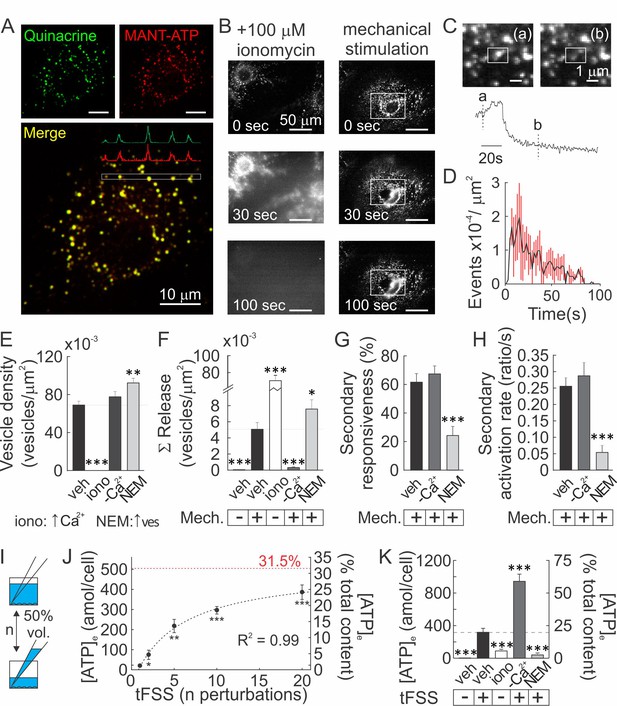
Vesicular ATP released upon mechanical stimulation is not the primary source of extracellular ATP.
(A) Confocal images of CB-OB loaded with quinacrine and MANT-ATP, Figure 3—video 1-2. (B) Changes in quinacrine-loaded CB-OB treated with ionomycin (left) or stimulated with micropipette (right, region of interest in white). (C) Vesicular release event in CB-OB (top) coincides with a sudden drop in granular quinacrine fluorescence (bottom), Figure 3—video 3-5.(D) Kinetics of vesicular release from CB-OB stimulated by micropipette at 0 s, n = 37 stimulated cells. (E–H) Quinacrine- (E, F) or Fura2- (G, H) loaded CB-OB were pre-treated (10 min) with vehicle, ionomycin (iono) or NEM or placed in [Ca2+]-depleted physiological solution, and micropipette-stimulated when indicated (+). Vesicular density (E) and cumulative release (F), secondary responsiveness (G) and [Ca2+] response activation rates (H) were determined, n = 7–37 primary cells. (I) tFSS was applied by replacing 50% media volume n times. (J, K) ATP released per cell (left axis) or as percent of cellular ATP content (right axis) was measured following CB-OB stimulation by tFSS (J, black dashed line: rational function fit; red dashed line: corresponding asymptote) or after indicated pre-treatments followed by tFSS (K, 10x media displacements, +), n = 6–8 independent cultures. For Figure 3, means ± SEM, *significance compared to vehicle (E–H), basal ATP release (J) or to tFSS-stimulated vehicle (K) by ANOVA. Source data for Figure 3 is provided in Figure 3—source data 1.
-
Figure 3—source data 1
- https://doi.org/10.7554/eLife.37812.017
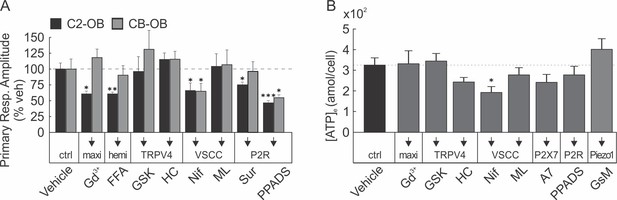
Involvement of conductive channels in osteoblast response to mechanical stimulation.
(A) Amplitudes of mechanically evoked [Ca2+]i transients in osteoblasts pretreated with vehicle, Gd3+, FFA, GSK, HC, Nif, ML, Sur and PPADS. Means ± SEM, n = 5–15 stimulated cells, normalized to vehicle. (B) CB-OB cells were stimulated by tFSS (10x) following pre-treatment with conductive channel inhibitors Gd3+, GSK, HC, Nif, ML, A7, PPADs and GsM. Means ± SEM attomoles ATP released per cell over 60 s after stimulation, n = 6–8 separate cultures, compared to vehicle. *p<0.05. **p<0.01 and ***p<0.001 indicate significance of treatment condition compared to vehicle, assessed by ANOVA followed by post-hoc Bonferroni test.
Confocal Z-stack of quinacrine- (left) and MANT-ATP (middle)-loaded CB-OBcells with merged images (right), ex. 1.
https://doi.org/10.7554/eLife.37812.011Confocal Z-stack of quinacrine- (left) and MANT-ATP (middle)-loaded CB-OB cells with merged images (right), ex. 2.
https://doi.org/10.7554/eLife.37812.012Single quinacrine-loaded CB-OB was stimulated with micropipette and vesicular release events were recorded, ex. 1.
https://doi.org/10.7554/eLife.37812.014Single quinacrine-loaded CB-OB was stimulated with micropipette and vesicular release events were recorded, ex. 2.
https://doi.org/10.7554/eLife.37812.015Single quinacrine-loaded CB-OB was stimulated with micropipette and vesicular release events were recorded, ex. 3.
https://doi.org/10.7554/eLife.37812.016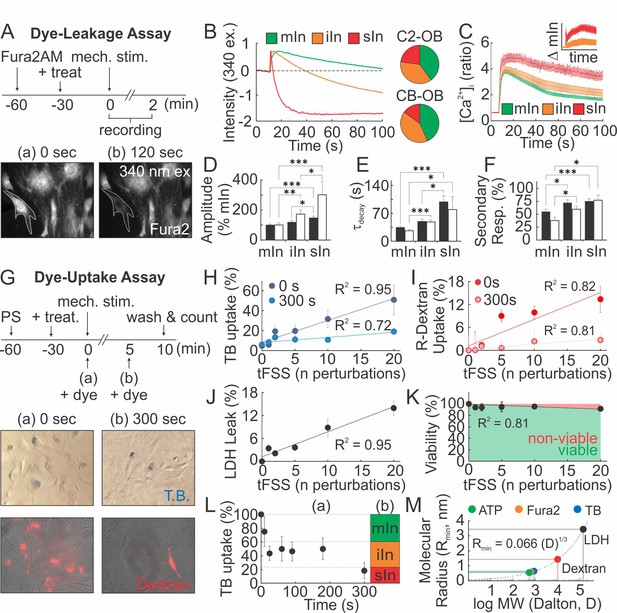
Mechanical stimuli regularly and reversibly compromise the integrity of osteoblast cell membrane.
(A–F) Membrane permeability in micropipette-stimulated Fura2-loaded osteoblasts. (A) Top: Schematic of dye-leakage assay. Bottom: 340 ex/510 em before (a) and after (b) CB-OB stimulation (white outline). (B) Representative Fura2 traces in C2-OB with minor (mIn), intermediate (iIn) or severe (sIn) cell injury and relative occurrence frequency (right, n = 35–40 stimulated cells). (C) C2-OB [Ca2+]i responses and differences (inset) for mIn (green), iIn (orange) and sIn (red), n = 8–14 stimulated cells. (D–F) Primary [Ca2+]i response amplitudes (D) and decay constants (E), and secondary responsiveness (F) in CB-OB (black; n = 40 stimulated cells) or C2-OB (white; n = 35 stimulated cells), grouped by cell injury status. (G–K) Membrane permeability in tFSS-stimulated osteoblasts. (G) Top: schematic of dye-uptake assay. Bottom: CB-OB stained with TB (top) or R-dextran (bottom) prior to (0 s, left) or after (300 s, right) tFSS application (10x). Uptake of TB (H) or R-dextran (I) added prior to (0 s) or after (300 s) tFSS stimulation of CB-OB (n = 4 independent cultures). (J) Leakage of LDH from CB-OB 5 min after tFSS (n = 8 independent cultures, normalized to total LDH). (K) Viability of C2-OB 1 hr after tFSS assessed by alamarBlue (n = 8–16 independent cultures). (L) Uptake of TB added at indicated times after micropipette-stimulation of C2-OB (L-a, n = 4–23 stimulated cells) compared to relative frequencies of mIn, iIn and sIn assessed by Fura2-leakage assay (L-b). (M) Calculated minimum membrane lesion radius Rmin required for permeability to LDH, R-dextran, TB, Fura2 and ATP. For Figure 4, means ± SEM, dashed lines: linear regression, *significance by ANOVA. Source data for Figure 4 is provided in Figure 4—source data 1.
-
Figure 4—source data 1
- https://doi.org/10.7554/eLife.37812.020
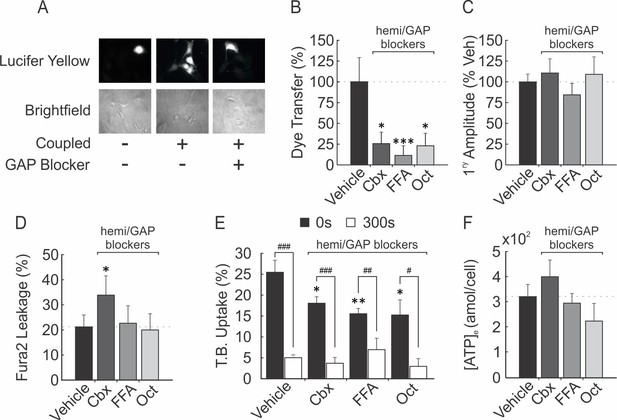
Involvement of hemichannels in ATP release and membrane resealing in murine osteoblasts
(A–B) To demonstrate the presence of functional GAP-junctions and validate GAP-junction/hemi channel blockers, C2-OB were pretreated (10 min) with vehicle (n = 22), Cbx (n = 11), FFA (n = 6) and Oct (n = 10) and scraped and stained with LY. Sample sizes are number of regions of interest imaged from three independent cultures. Fluorescence (A, top) and bright field (A, bottom) images show dye transfer and cell-cell coupling, respectively. Mean ± SEM percentage of cells that were not initially scraped but were coupled and LY-positive following LY staining, normalized to vehicle (B). (C–D) Fura2-loaded CB-OB pretreated with GAP-junction/hemi-channel blockers were mechanically stimulated with glass micropipette and [Ca2+]i elevation amplitude (C, mean ± SEM amplitude, normalized to vehicle) and percentage of sIn cells (D, mean ± SEM percentage) was determined (n = 6–9 stimulated cells). (E–F) Membrane injury of CB-OB following tFSS (10x resuspensions) was assessed by T.B. uptake at 0 and 300 s (E, n = 5–8 separate cultures, means ± SEM), and ATP release ([ATP]e) was measured using bioluminescence assay (F, n = 6 separate cultures, means ±SEM attomoles released per cell over 60 s after simulation) following pre-treatment with GAP-junction/hemichannel blockers. Comparisons to vehicle; *p<0.05, **p<0.01 and ***p<0.001, or as specified; #p<0.05, ##p<0.01 and ###p<0.001, indicate significance assessed by ANOVA followed by post-hoc Bonferroni test.
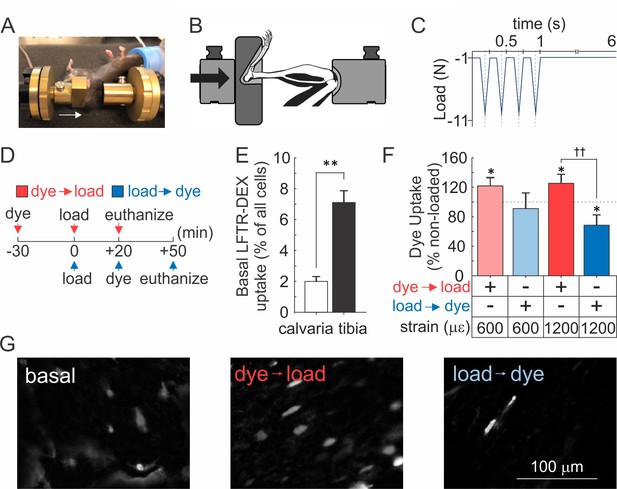
Osteocyte membranes are reversibly disrupted during in vivo cyclic compressive loading of the tibia.
(A, B) Left tibia of anesthetized mouse was positioned in loading device as shown in picture (A) and schematic (B). Arrows indicate direction of load. (C) The triangle waveform included 0.15 s symmetric active loading/unloading, with a 0.1 s rest phase (−1 N) between load cycles and a 5 s rest inserted between every four cycles. A maximum force of −5.5 N or −11 N was applied, which engenders 600 µε or 1200 µε, respectively at the periosteal surface of the tibia mid-diaphysis in these mice. (D) Experimental design schematic: animals were injected with LFTR-Dex 30 min before (red) or 20 min after (blue) in vivo cyclic tibial loading (5 min). (E) Proportion of cells exhibiting LFTR-Dex uptake in calvariae (n = 5 animals) and control tibiae (n = 20 from 10 animals), normalized to average total cell number, means ± SEM, **p<0.01. (F) Dye uptake in tibia injected before (red) or after (blue) loading at 600 µε or 1200 µε, means ± SEM, n = 5/strain/dye protocol, normalized to non-loaded contralateral tibia, *significance compared to non-loaded tibia, ††significance between dye protocols, by ANOVA. (G) Contrast enhanced images of dye uptake in control (left), and 1200 µε loaded tibia injected with dye before (middle) or after (right) loading. Source data for Figure 5 is provided in Figure 5—source data 1.
-
Figure 5—source data 1
- https://doi.org/10.7554/eLife.37812.023
In vivo cyclic compressive loading was applied to the left tibia.
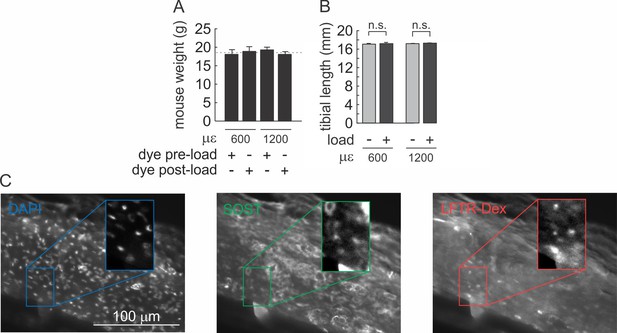
(A) Average weights of 10-week-old female C57Bl/6J mice for each experimental cohort. Mean ± SD, n = 5 animals per group. (B) Impact of loading on tibial length compared to contralateral non-loaded tibia for 600 µε (n = 10 animals) and 1200 µε (n = 10 animals) loading regime. For (A) and (B), no significant difference was demonstrated by ANOVA. (C) Immunofluorescence images of nuclear DAPI (left), anti-sclerostin (middle) and LFTR-Dex uptake (right) in non-loaded tibia. Magnified regions are contrast-enhanced.
Recording of in vivo cyclic compressive loading being applied to left tibia of anesthetized C57Bl/6J mouse.
https://doi.org/10.7554/eLife.37812.026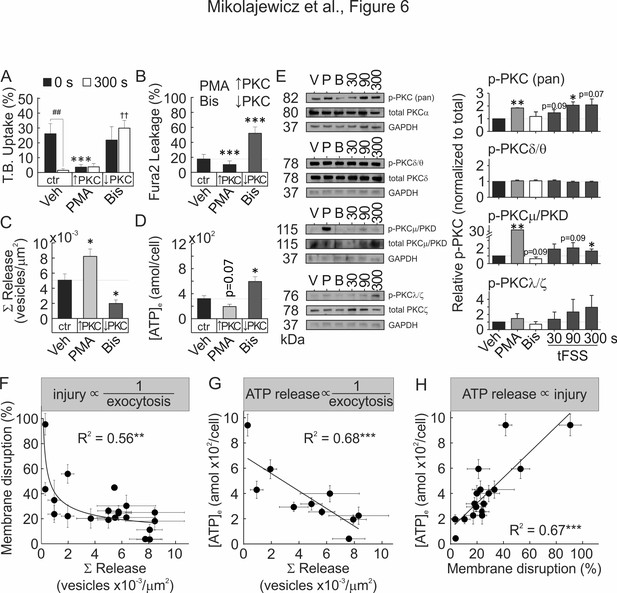
Membrane resealing depends on PKC-regulated vesicular exocytosis.
(A–D) CB-OB were pretreated with vehicle, PMA or Bis, and membrane injury (A, B) vesicular exocytosis (C) and ATP release (D) were assessed. (A) TB uptake at 0 and 300 s after tFSS (10x), n = 5–8 independent cultures. (B) Fura2 leakage, determined as percentage of sIn cells after micropipette-stimulation, n = 9–16 stimulated cells. (C) Cumulative vesicular release over 100 s after micropipette-stimulation, n = 6–10 stimulated cells. (D) ATP release over 60 s following 10x tFSS stimulation, n = 8 independent cultures. (E) Immunoblotting for PKC isoform phosphorylation in CB-OB treated with vehicle, PMA or Bis (30 min); or 30 s, 90 s or 300 s after tFSS (10x). Shown are immunoblots (left), complete gels in Figure 6—figure supplement 4, and densitometry (right) of phosphorylated and total conventional (pPKC pan), atypical (pPKC ζ/λ), novel (pPKC δ/θ), and PKD/PKCµ isoforms. Phosphorylated PKC isoforms were normalized by total PKC levels and reported relative to vehicle, n = 3 independent cultures. (F–H) Relationships between vesicular release (pooled data from vesicular release experiments), membrane disruption (pooled from membrane integrity experiments) and ATP release (pooled from 10x tFSS experiments) following mechanical stimulation of CB-OB, solid line - regression. For Figure 6, data are means ± SEM, *significance compared to vehicle (0 s vehicle for A), †significance compared to 300 s vehicle (A) and #significance of indicated comparisons (A), by ANOVA or by regression. Source data for Figure 6 is provided in Figure 6—source data 1.
-
Figure 6—source data 1
- https://doi.org/10.7554/eLife.37812.032
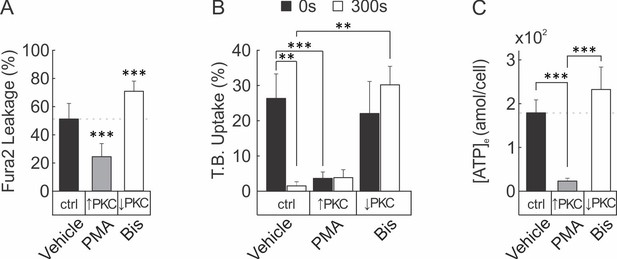
PKC regulates membrane resealing and ATP release in C2-OB cell line.
(A–C) C2-OB were pre-treated (30 min) with vehicle, PMA or Bis. (A) Membrane permeability of micropipette-stimulated Fura2-loaded C2-OB was assessed by dye-leakage. Means ± SEM percentage of sIn cells, n = 11 stimulated cells. (B) Membrane permeability of C2-OB stimulated with tFSS (10x resuspensions) was assessed by staining at 0 or 300 s with T.B. Means ± SEM percentage of dye-positive cells, n = 7 separate cultures. (C) Amount of ATP released ([ATP]e) from tFSS-stimulated (10x resuspensions) C2-OB was assessed by bioluminescence assay. Means ± SEM attomoles ATP release per cell, 60 s after stimulation. Compared to vehicle (unless otherwise specified), **p<0.01 and ***p<0.001 indicate significance assessed by ANOVA followed by post-hoc Bonferroni test.
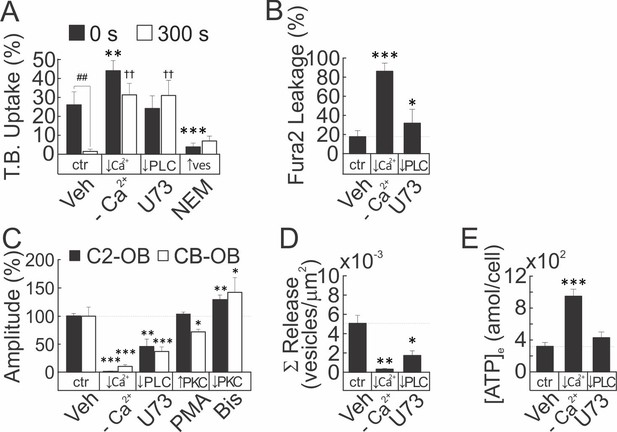
Membrane resealing, vesicular exocytosis and ATP release are Ca2+/PLC-dependent processes.
(A–E) CB-OB were pretreated with vehicle, calcium-depleted physiological solution (-Ca2+), U73, NEM, PMA or Bis where indicated, and mechanically induced membrane injury (A, B) amplitude of [Ca2+]i elevations (C), vesicular exocytosis (D) and ATP release (E) were assessed. (A) TB uptake at 0 and 300 s after tFSS (10x), n = 5–8 separate cultures. (B) Fura2 leakage following micropipette stimulation, determined as percentage of sIn cells after micropipette-stimulation, n = 9–16 stimulated cells. (C) Amplitudes of micropipette-stimulated [Ca2+]i elevations in Fura2-loaded C2-OB and CB-OB cells, n = 9–16 stimulated cells. (D) Cumulative vesicular release over 100 s after micropipette-stimulation, n = 6–10 stimulated cells. (E) ATP release over 60 s following 10x tFSS stimulation, n = 8 separate cultures. For Figure 6—figure supplement 2, data are means ±SEM, ***significance compared to vehicle (0 s vehicle for A), ††significance compared to 300 s vehicle (A) and ## significance of indicated comparisons (A) by ANOVA or by regression.
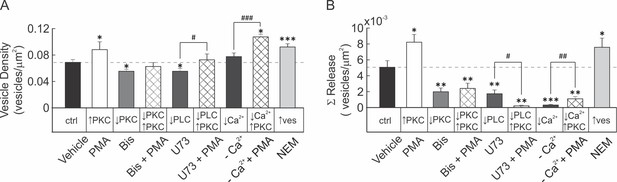
Basal vesicular density and mechanically-stimulated vesicular release in compact bone-derived osteoblasts.
(A–B) Quinacrine-loaded CB-OB were pretreated (30 min) with PMA, Bis, PMA + Bis, U73, U73 + PMA, [Ca2+]e-free PS, [Ca2+]e-free PS + PMA and NEM. Basal vesicular density (A) and cumulative vesicular release in response to stimulation by glass micropipette (B) were determined. Mean ± SEM vesicular density or cumulative vesicular release, normalized to unit area (n = 11–16 stimulated cells). Comparisons with vehicle; *p<0.05, **p<0.01 and ***p<0.001, or as specified; #p<0.05, ##p<0.01 and ###p<0.001, indicate significance assessed by ANOVA followed by post-hoc Bonferroni test.
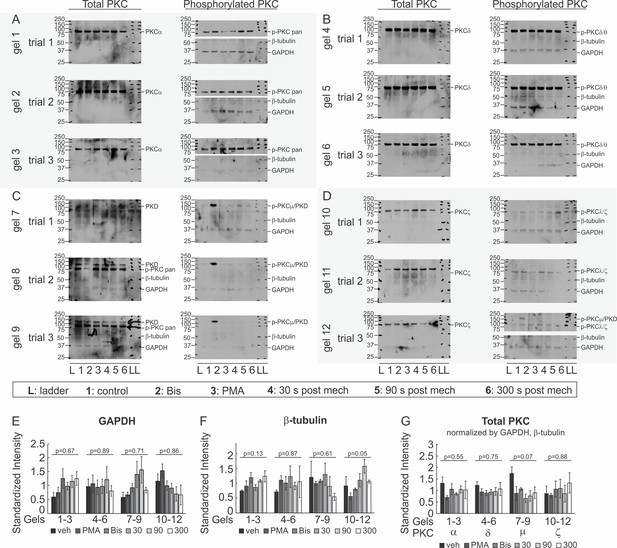
Immunoblot analysis of PKC isoforms.
(A–D) Immunoblots for phospho- and total- conventional PKC (A), novel PKC (B), PKC/PKCµ (C) and atypical PKC (D) isoforms, and GAPDH and β-tubulin in cell lysates extracted from CB-OB treated with vehicle, PMA or Bis (30 min); or 30 s, 90 s or 300 s after tFSS (10x). (E–G) GAPDH (E) and β-tubulin (F) band intensities were averaged and used to normalize total PKC levels (G). Differences in loading controls and total PKC levels were assessed by ANOVA and p-values are reported, n = 3 cultures isolated from different mice.
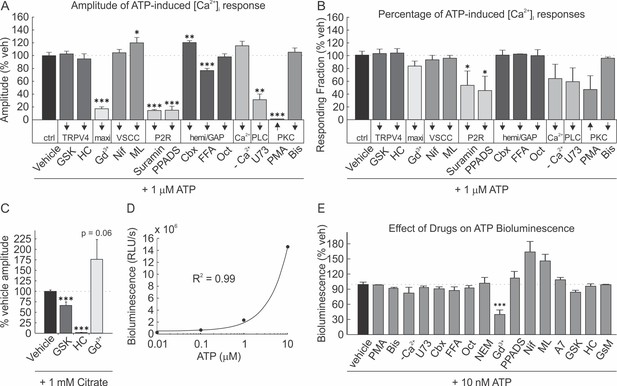
Effect of drug treatments on [Ca2+]i elevations and ATP bioluminescence assay.
(A, B) Effect of drugs on P2 receptor-mediated [Ca2+]i elevation amplitude (A) and responsiveness (B). 1 µM ATP was applied to Fura2-loaded C2-OB pre-treated for 10 mins with vehicle (n = 30), GSK (n = 28), HC (n = 30), Gd3+ (n = 18), Nif (n = 46), ML (n = 45), Sur (n = 51), PPADS (n = 36), Cbx (n = 47), FFA (n = 45), Oct (n = 39) or 30 mins with [Ca2+]e-free PS (n = 62), U73 (n = 29), PMA (n = 18), or Bis (n = 48). Mean ±SEM response normalized to vehicle. Compared to vehicle, *p<0.05, **p<0.01 and ***p<0.001 indicate significance assessed by ANOVA followed by post-hoc Bonferroni test. (C) Effect of drugs on citrate-mediated [Ca2+]i elevation amplitude. 1 mM citrate was applied as TRP agonist to Fura2-loaded C2-OB pretreated for 10 mins with GSK (n = 48), HC (n = 18) or Gd3+ (n = 29). Mean ±SEM amplitude normalized to vehicle condition. For A-C, sample sizes are number of quantified cells from at least three independent cultures. (D) Calibration curve relating ATP standard concentrations to measured bioluminescence. Mean ± SEM relative light units per sec (RLU/s). Solid line, linear regression. (E) Effect of drugs on ATP bioluminescence assay. 10 nM ATP-dependent luciferin/luciferase bioluminescence was measured in the presence of PMA, Bis, [Ca2+]e-free PS, U73, Cbx, FFA, Oct, NEM, Gd3+, PPADS, Nif, ML, A7, GSK, HC, or GsM. Mean ± SEM RLU/s as percentage of vehicle condition. Compared to vehicle, **p<0.01 and ***p<0.001 indicate significance assessed by ANOVA followed by post-hoc Bonferroni test.
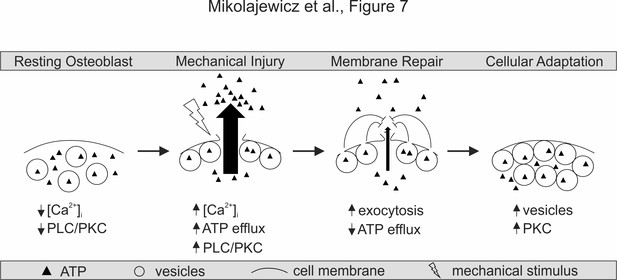
Proposed model for mechanically stimulated ATP release in osteoblasts.
Resting Osteoblasts: Under basal conditions, the cell membrane is intact and intracellular free calcium levels and PLC/PKC signaling are minimal. Mechanical injury: Mechanical stimulation results in disruption of the cellular membrane, which leads to the influx of calcium, activation of PLC/PKC signalling, and the efflux of ATP. Membrane repair: The disrupted membrane is rapidly repaired through a process involving Ca2+/PLC/PKC-dependent vesicular exocytosis, thereby limiting ATP release. Cellular adaptation: Elevated PKC levels result in priming of the vesicular pathway, likely regulating cellular resilience and responsiveness to subsequence cycles of mechanical stimulation.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (M. musculus) | BMP2-transfected C2C12 myoblast cells (C2C12-BMP2; C2-OB) | Dr. M. Murshed, McGill University | osteoblast cell line | |
| Strain, strain background (M.musculus, female) | 10 week-old C57Bl/6J | Jackson Laboratory | Stock #: 000664; RRID: IMSR_JAX:000644 | primary osteoblast source, in vivo studies |
| Software, algorithm | Calcium Analyser | DOI: 10.3389/ fphys.2016.00525 | MATLAB algorithm for calcium analysis | |
| Chemical compound, drug | MANT-ATP | AnaSpec | Cat. AS-64610 | fluorescent ATP analog, 5 µM (overnight, 37°C) |
| Chemical compound, drug | GsMTx-4; GsM | Alomone Labs | Cat. STG-100 | Piezo1 inhibitor, 1 µM (10 min, room temperature) |
| Chemical compound, drug | U73122; U73 | Calbiochem | Cat. 662035 | PLC inhibitor, 10 µM (10 min, room temperature) |
| Antibody | GAPDH (D16H11, Rabbit antibody) | Cell Signaling Technology | Cat. 5174; RRID:AB_10622025 | WB, 1:1000 dilution, 5% BSA/TBST (1 hr, room temperature) |
| Antibody | β-tubulin (9F3, Rabbit antibody) | Cell Signaling Technology | Cat. 2128; RRID:AB_823664 | WB, 1:1000 dilution, 5% BSA/TBST (1 hr, room temperature) |
| Antibody | p-PKC (pan; βII Ser660, Rabbit antibody) | Cell Signaling Technology | Cat. 9371; RRID:AB_330848 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | PKCα (Rabbit antibody) | Cell Signaling Technology | Cat. 2056; RRID:AB_2284227 | WB, 1:1000 dilution,5% BSA/TBST (over night, 4°C) |
| Antibody | p-PKCδ/θ (Ser643/676, Rabbit antibody) | Cell Signaling Technology | Cat. 9376; RRID:AB_330848 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | PKCδ (D10E2, Rabbit anitbody) | Cell Signaling Technology | Cat. 9616; RRID:AB_10949973 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | p-PKD/PKCµ (Ser744/748, Rabbit antibody) | Cell Signaling Technology | Cat. 2054; RRID:AB_330848 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | PKD/PKCµ (D4J1N, Rabbit antibody) | Cell Signaling Technology | Cat. 90039 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | p-PKCζ/λ (Thr410/403, Rabbit antibody) | Cell Signaling Technology | Cat. 9378; RRID:AB_330848 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | PKCζ (C24E6, Rabbit antibody) | Cell Signaling Technology | Cat. 9368; RRID:AB_10693777 | WB, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Antibody | anti-rabbit IgG, HRP-linked secondary antibody) | Cell Signaling Technology | Cat. 7074; RRID:AB_2099233 | WB, 1:1000 dilution, 5% BSA/TBST (1 hr, room temperature) |
| Chemical compound, drug | Minimum Essential Medium (MEM) α; αMEM | Gibco | Cat. 12,000–022 | cell culture reagent |
| Chemical compound, drug | Trypan Blue Solution, 0.4%; TB | Gibco | Cat. 15250061 | in vitro injury assay dye |
| Commercial assay or kit | Alamar Blue Cell Viability Reagent | Invitrogen | Cat. DAL1025 | cell viability assay |
| Chemical compound, drug | DAPI | Invitrogen | Cat. D1306; RRID:AB_2629482 | IF, nucleur stain, 1:5000 dilution, H20 (5 min, room temperature) |
| Chemical compound, drug | Fura2 AM | Invitrogen | Cat. F1221 | ratiometric calcium-binding dye (30 min, room temperature) |
| Chemical compound, drug | D-Luciferin potassium salt | Invitrogen | Cat. L2916 | luciferase substrate, ATP bioluminescence assay |
| Chemical compound, drug | Lucifer Yellow CH, Lithium Salt; LY | Invitrogen | Cat. L453 | GAP junction permeable dye, 10 µM (2 min, 37°C) |
| Chemical compound, drug | Rhodamine B-conjugated 10 000 MW Dextran; R-dextran | Invitrogen | Cat. D1824 | in vitro injury assay dye |
| Chemical compound, drug | lysine-fixable Texas Red-conjugated 10 000 MW Dextra; LFTR-Dex | Invitrogen | Cat. D1863 | in vivo injury assay dye |
| Antibody | Sclerostin (Goat antibody) | R and D Systems | Cat. AF1589; RRID:AB_2195345 | IF, 1:1000 dilution, 5% BSA/TBST (over night, 4°C) |
| Chemical compound, drug | Collagenase P from Clostridium histolyticum | Roche | Cat. 11213857001 | enzymatic digest of bone fragments |
| Commercial assay or kit | Cytotoxicity Detection KitPLUS; LDH | Roche | Cat. 04 744 926 011 | LDH leakage assay |
| Antibody | Donkey anti-goat IgG-FITC | Santa Cruz | Cat. Sc-2024; RRID:AB_631727 | IF, 1:1000 dilution,5% BSA/TBST (1 hr, room temperature) |
| Chemical compound, drug | Adenosine 5’-triphosphate magnesium salt; ATP | Sigma-Aldrich | Cat. A9187 | |
| Chemical compound, drug | Bisindolylmaleimide II; Bis | Sigma-Aldrich | Cat. B3056 | PKC inhibitor, 1 µM (10 min, room temperature) |
| Chemical compound, drug | Carbenoxolone disodium salt; Cbx | Sigma-Aldrich | Cat. C4790 | Hemichannel blocker, 10 µM (10 min, room temperature) |
| Chemical compound, drug | Fast Red Violet LB Salt | Sigma-Aldrich | Cat. F3381 | alkaline phosphatase assay reagent |
| Chemical compound, drug | Gadolinium (III) chloride; Gd3+ | Sigma-Aldrich | Cat. 439770 | Mechanically-sensitive channel blocker, 10 µM (10 min, room temperature) |
| Chemical compound, drug | GSK2193874; GSK | Sigma-Aldrich | Cat. SML0942 | TRPV4 inhibitor, 100 nM (10 min, room temperature) |
| Chemical compound, drug | HC-067047; HC | Sigma-Aldrich | Cat. SML0143 | TRPV4 inhibitor,100 nM (10 min, room temperature) |
| Chemical compound, drug | L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate | Sigma-Aldrich | Cat. A8960 | osteoblast differentiation reagent,50 µg/mL |
| Chemical compound, drug | Luciferase from Photinus pyralis | Sigma-Aldrich | Cat. L9420 | ATP bioluminescence assay reagent |
| Chemical compound, drug | ML218; ML | Sigma-Aldrich | Cat. SML0385 | T-type VSCC inhibitor, 10 µM (10 min, room temperature) |
| Chemical compound, drug | Naphthol AS-MX phosphate disodium salt | Sigma-Aldrich | Cat. N5000 | alkaline phosphatase assay reagent |
| Chemical compound, drug | N-ethylmaleimide; NEM | Sigma-Aldrich | Cat. E3876 | Vesicular activator, 1 mM (10 min, room temperature) |
| Chemical compound, drug | Nifedipine; Nif | Sigma-Aldrich | Cat. N7634 | L-type VSCC inhibitor, 10 µM (10 min, room temperature) |
| Chemical compound, drug | 1-octanol; Oct | Sigma-Aldrich | Cat. 297877 | Connexin blocker, 1 mM (10 min, room temperature) |
| Chemical compound, drug | phorbol 12-myristate 13-acetate; PMA | Sigma-Aldrich | Cat. P8139 | PKC activator, 100 nM (10 min, room temperature) |
| Chemical compound, drug | Quinacrine dihydrochloride | Sigma-Aldrich | Cat. Q3251 | Vesicle dye, 10 µM (15 min, room temperature) |
| Chemical compound, drug | Flufenamic Acid; FFA | Tocris Bioscience | Cat. 4522 | Hemichannel blocker, 10 µM (10 min, room temperature) |
| Chemical compound, drug | Ionomycin calcium salt; Iono | Tocris Bioscience | Cat. 1704 | calcium ionophore, 100 µM |
| Chemical compound, drug | PPADS tetrasodium salt; PPADS | Tocris Bioscience | Cat. 0625 | P2R inhibitor,100 µM (10 min, room temperature) |
| Chemical compound, drug | Suramin hexasodium salt; Sur | Tocris Bioscience | Cat. 1472 | P2R inhibitor, 100 µM (10 min, room temperature) |
| Chemical compound, drug | A 740003; A7 | Tocris Bioscience | Cat. 3701 | P2X7 inhibitor, 1 µM (10 min, room temperature) |
| Chemical compound, drug | Dulbecco’s Modified Eagle Medium; DMEM | Wisent Bio Products | Cat. 319–020 CL | cell culture reagent |
| Chemical compound, drug | Fetal bovine Serum; FBS | Wisent Bio Products | Cat. 080152 | cell culture reagent |
| Chemical compound, drug | Penicillin Streptomycin | Wisent Bio Products | Cat. 450–201-EL | antibiotic, 1% |
| Chemical compound, drug | Sodium Pyruvate | Wisent Bio Products | Cat. 600–110 UL | cell culture reagent |
| Chemical compound, drug | Collagenase Type II | Worthington Biochemical Corporation | Cat. LS004176 | enzymatic digest of bone fragments |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.37812.025





