The fate of hippocampal synapses depends on the sequence of plasticity-inducing events
Figures
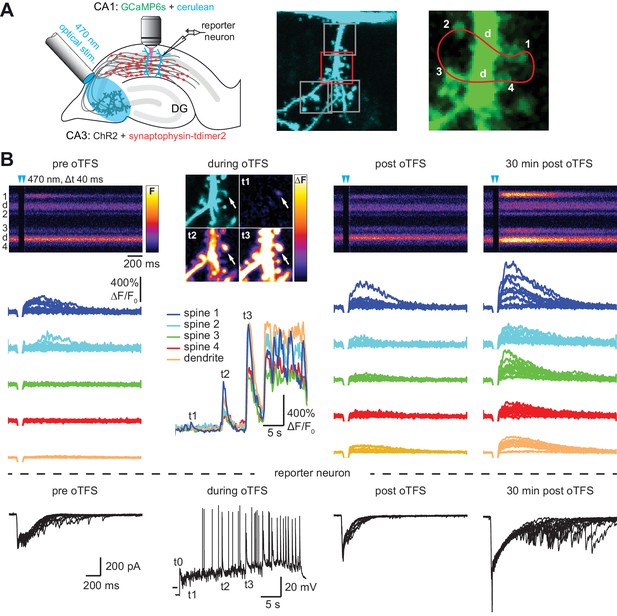
with two supplements: Channelrhodopsin-driven theta-frequency stimulation induces LTP.
(A) Left: A fiber-coupled LED (λ = 470 nm) was used to locally stimulate ChR2-expressing CA3 neurons. Spines on GCaMP6s/mCerulean-expressing CA1 pyramidal cells were imaged with two-photon microscopy. For parallel electrical recordings, a second CA1 neuron was patch-clamped (reporter neuron). Middle: oblique dendrite branching off the apical trunk filled with mCerulean. Detection of active spines was done with GCaMP6s during presynaptic optogenetic stimulation. Stimulation-induced fluorescence changes (ΔF) of GCaMP6s were analyzed in fast frame scans (squares) of oblique dendrites until a responsive spine was detected (red square). Right: Magnified view of GCaMP6s fluorescence in the dendritic section harboring an activated spine. The laser was scanned in a user-defined trajectory across multiple spines and the parental dendrite during Ca2+ imaging (red curve). (B) Fluorescence signal across time from arbitrary line scan on dendrite shown in A during ChR2-stimulation before (‘pre oTFS’), immediately (‘post oTFS’) and 30 min (‘30 min post oTFS’) after optical theta-frequency stimulation (oTFS). Temporally matched traces from multiple trials and electrophysiological recording from a reporter neuron are shown below. During oTFS the Ca2+ response was recorded in frame scan mode (‘during oTFS’). The GCaMP6s-signal (ΔF) is shown for three selected time points during oTFS. GCaMP6s-traces from the same spines and dendrite imaged in line scans are shown below together with the corresponding electrophysiological recording in voltage clamp mode from the reporter neuron.
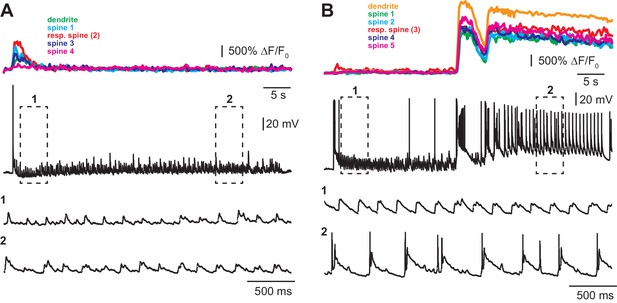
Examples of optogenetic TFS experiments.
(A) Experiment where no dendritic calcium spikes were observed in the GCaMP6s-expressing CA1 pyramidal cell (colored traces, top) and no complex spike bursts (CSBs) were triggered in the neighboring ‘reporter’ neuron (black traces). (B) Experiment with dendritic calcium spikes and synchronous CSBs in the reporter neuron.
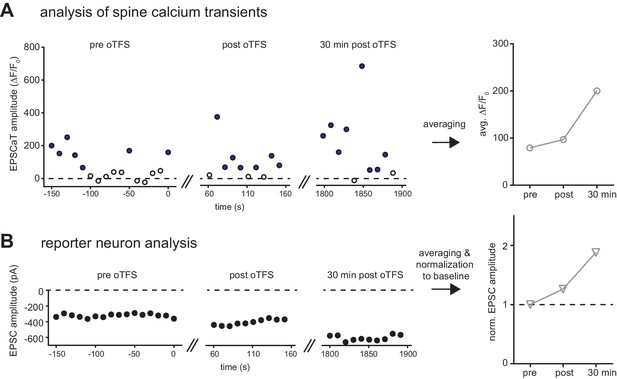
Analysis of imaging and electrophysiology data.
(A) EPSCaT amplitude of spine one is plotted for all trials shown in Figure 1B. Open circles show failures, filled circles show successes. Baseline responses (‘pre oTFS’) and responses immediately (‘post oTFS’) and 30 min after oTFS (‘30 min post oTFS’) are averaged and plotted below. (B) EPSC amplitude is plotted for the recording from the reporter neuron shown in Figure 1B. Average responses plotted below were normalized to the baseline (‘pre oTFS’).
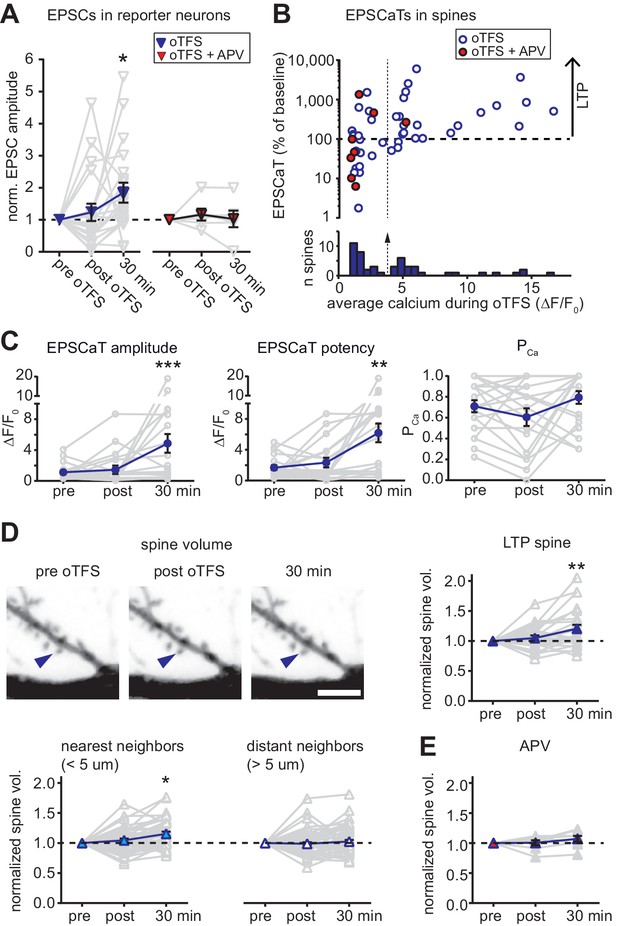
with two supplements: Characterization of oTFS-induced LTP.
(A) Changes in excitatory postsynaptic current (EPSC) amplitude in reporter neurons immediately after and 30 min after oTFS in the absence (left) or presence (right) of the NMDA receptor antagonist APV during oTFS. EPSCs were significantly increased after 30 min (p=0.012, n = 20 slice cultures). The increase was blocked by APV (p=0.69, n = 6 slice cultures). (B) Relative change of average excitatory Ca2+ transients (EPCaTs) in individual spines 30 min after the oTFS protocol plotted against the average spine Ca2+ during oTFS. In experiments indicated by filled red circles, APV was present during oTFS. (C) EPSCaT amplitude (p=0.0008, n = 20 slice cultures) and EPSCaT potency (successes only, p=0.0025) but not EPSCaT probability (PCa, p>0.05) were increased 30 min after oTFS in experiments where complex spike bursts (CSBs) were induced during oTFS. (D) Maximum intensity projections of mCerulean fluorescence in dendritic segment harboring a responding spine that was successfully potentiated (blue arrowhead). Volume of oTFS spines (p=0.002, n = 26 spines) and nearest (p=0.0001, n = 45 spines) but not distant neighbors (p=0.83, n = 58 spines) was increased 30 min after oTFS in experiments where CSBs were induced during oTFS. (E) Spine volume was not increased when NMDA receptors were blocked with APV during oTFS (p>0.05, n = 7 spines).
-
Figure 2—source data 1
Theta-frequency stimulation experiments.
- https://doi.org/10.7554/eLife.39151.008
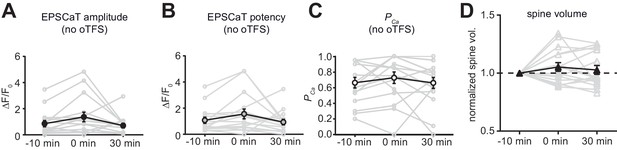
Analysis of oTFS experiments where no dendritic calcium spikes were observed during the induction protocol.
(A) The null hypothesis, no change in average EPSCaT amplitude after oTFS, could not be rejected (p>0.05, n = 17). (B) The null hypothesis, no change in EPSCaT potency after oTFS, could not be rejected (p>0.05, n = 17). (C) The probability of EPSCaTs was significantly reduced after oTFS (p=0.0098, n = 17). (A) – (C): nonparametric Friedman test followed by Dunn’s multiple comparison test. (D) The null hypothesis, no change in spine head volume after oTFS, could not be rejected (p>0.05, n = 11, Wilcoxon matched-pairs signed rank test).
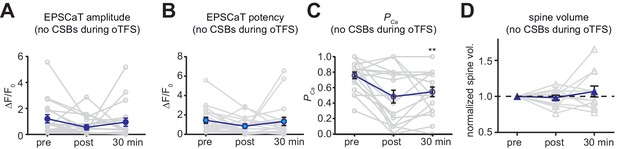
Analysis of control experiments where no oTFS was applied to responding spines.
(A) The null hypothesis, no change in average EPSCaT amplitude over time, could not be rejected (p>0.05, n = 16). (B) The null hypothesis, no change in EPSCaT potency over time, could not be rejected (p>0.05, n = 16). (C) The null hypothesis, no change in EPSCaT probability (PCa) over time, could not be rejected (p>0.05, n = 16). (A) – (C): nonparametric Friedman test followed by Dunn’s multiple comparison test. (D) The null hypothesis, no change in spine head volume after oTFS, could not be rejected (p>0.05, n = 16, RM one-way ANOVA).
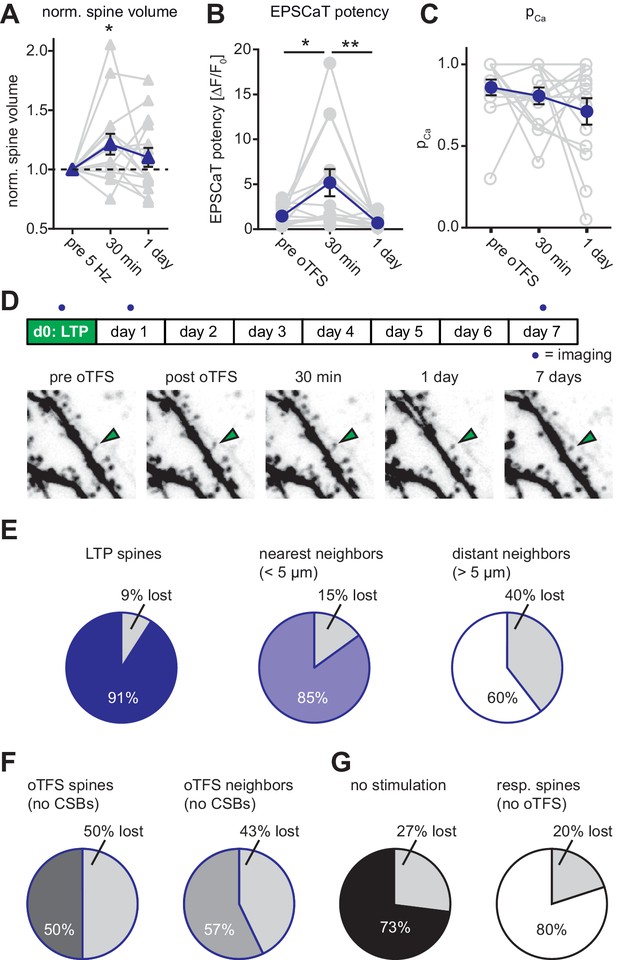
Long-term outcome of oTFS-induced LTP.
(A) Analysis of volume changes of oTFS spines 30 min and 24 hr after oTFS. The volume increase 30 min after oTFS (p=0.03, n = 15 slice cultures) was not maintained 24 hr later (p=0.42). (B) Analysis of EPSCaT potency before, 30 min and 24 hr after oTFS. The increased potency 30 min after oTFS (p=0.015, n = 14 slice cultures) has significantly decreased again 24 hr later (p=0.005) and was similar to the condition before oTFS (p=0.55). (C) EPSCaT probability (PCa) did not change 30 min and 24 hr after oTFS (p=0.32, n = 14 slice cultures). For details on the statistical tests, please refer to the Materials and Methods section. (D) Long-term survival analysis after LTP. Spines were imaged at d0, d1 and d7. Maximum intensity projections of mCerulean fluorescence in dendritic segment harboring a responding spine that was successfully potentiated (green arrowhead). (E) Spine survival 7 days after successful LTP induction on day 0. Surviving fractions are shown for responding spines, nearest and distant neighbors. (F) Spine survival 7 days after oTFS in experiments where no complex spike bursts were induced. Directly stimulated spines and their neighbors were analyzed separately. (G) Spine survival over 7 days under baseline conditions without any optical stimulation (black) and in spines responsive to optical test pulses (resp. spines, white) which were not exposed to plasticity-inducing protocols.
-
Figure 3—source data 1
Theta-frequency stimulation: Spine volume changes.
- https://doi.org/10.7554/eLife.39151.010
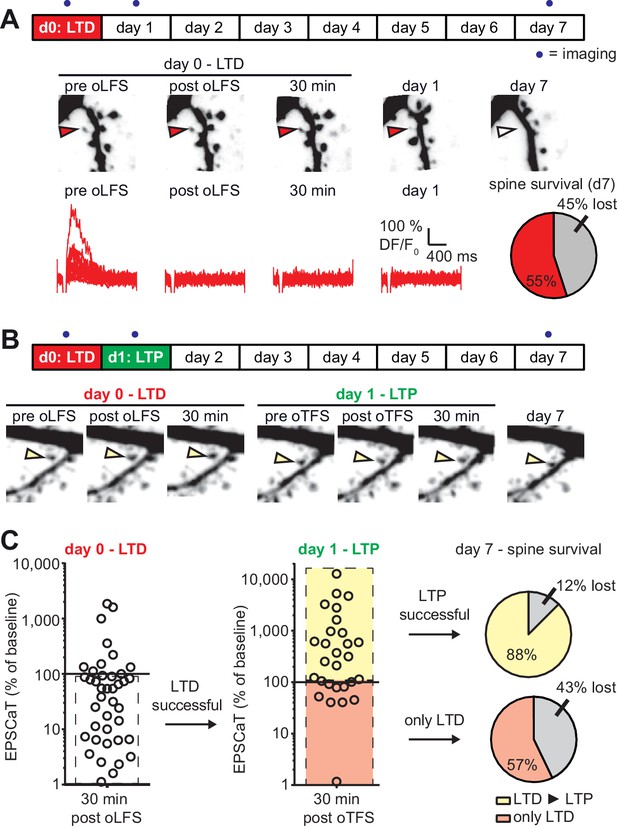
with one supplement: LTD-induced spine elimination is reversed by LTP or sustained synaptic transmission.
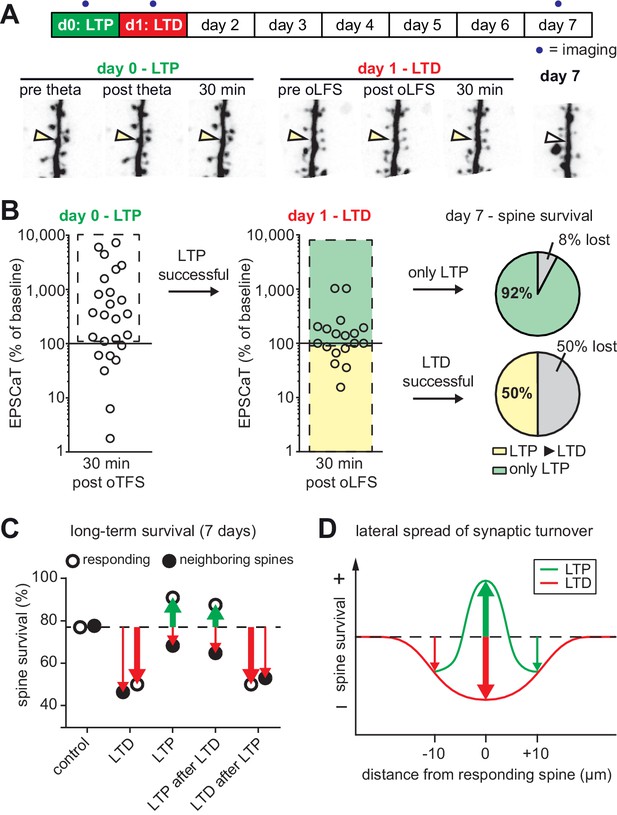
(A) Long-term survival analysis after LTD. Spines were imaged at d0, d1 and d7. Below: Maximum intensity projections of mCerulean fluorescence in dendritic segment harboring a responding spine that was successfully depressed (red arrowhead). Open arrowhead on day seven indicates position of eliminated spine. Corresponding EPSCaT traces from indicated time points are shown in red. Pie chart shows quantification of spine survival after 7 days. (B) LTP 24 hr after LTD. Below: Dendritic segment harboring a responding spine that was successfully depressed on day 0 and potentiated on day 1 (yellow arrowhead). (C) Assessment of synaptic weight changes induced by oLFS on day 0 and oTFS on day 1. Dashed box in left graph indicates all experiments where LTD was successfully induced on day 0. Only these spines were considered in the LTP experiment on day 1 (middle). Yellow shaded box indicates all experiments where LTP was successfully induced on day 1 (after LTD on day 0; LTD ► LTP). Red shaded box indicates experiments where oTFS did not lead to LTP (only LTD). Pie charts show quantification of spine survival after 7 days for these two conditions.
-
Figure 4—source data 1
Low-frequency stimulation followed by theta-freuquency stimulation.
- https://doi.org/10.7554/eLife.39151.013
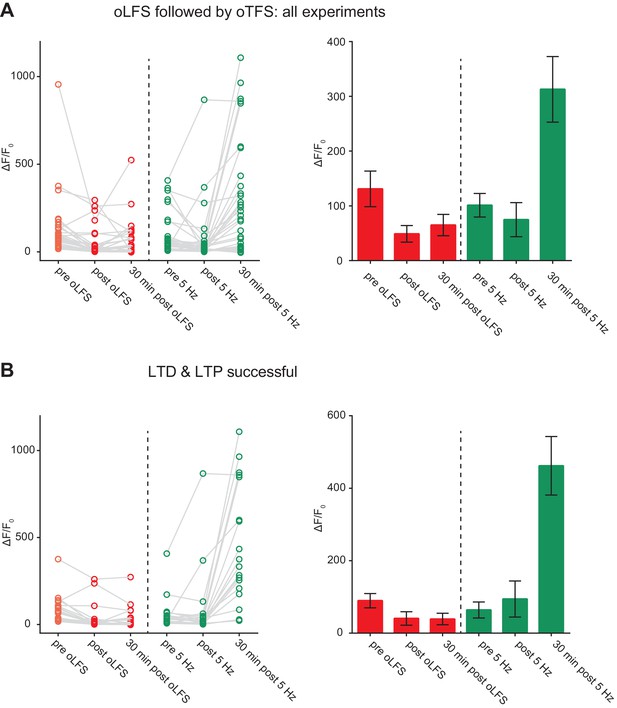
LTD followed by LTP.
(A) Outcome of all experiments where optogenetic low-frequency stimulation (oLFS) on day 0 was followed by optogenetic theta-frequency stimulation (oTFS) on day 1 (n = 30). (B) Outcome of selected experiments (subset of A, n = 18) where oLFS induced LTD (on day 0) and oTFS induced LTP of spine calcium signals (on day 1). Survival rate of this group (88%) is shown in Figure 4c.
with one supplement: The most recent plasticity event fully accounts for synaptic tenacity.
(A) Long-term survival analysis of experiments where LTD was induced 24 hr after LTP. Maximum intensity projections of mCerulean fluorescence in dendritic segment harboring a responding spine that was successfully potentiated on day 0 and depressed on day 1 (yellow arrowhead). (B) Assessment of synaptic weight changes induced by oTFS on day 0 and oLFS on day 1. Dashed box in left graph indicates all experiments where LTP was successfully induced on day 0. Only these spines were considered in the LTD experiment on day 1 (middle). Yellow shaded box indicates all experiments where LTD was successfully induced on day 1 (after LTP on day 0, LTP ► LTD). Note the low probability of depression after potentiation. Green shaded box encompasses experiments where oLFS did not lead to LTD or even led to LTP (only LTP). Pie charts show quantification of spine survival after 7 days for these two conditions. (C) Comparison of spine survival 7 days after various plasticity paradigms. Stimulated spines are shown as open circles; non-stimulated neighbors within 10 µm are shown as filled circles. Values for ‘control’ and ‘LTD’ are from Wiegert and Oertner, 2013. (D) LTP stabilizes the spine carrying the potentiated synapse, but reduces the average lifetime of more distant (>5 µm) spines on the same dendrite.
-
Figure 5—source data 1
Theta-frequency stimulation followed by low-freuquency stimulation.
- https://doi.org/10.7554/eLife.39151.016
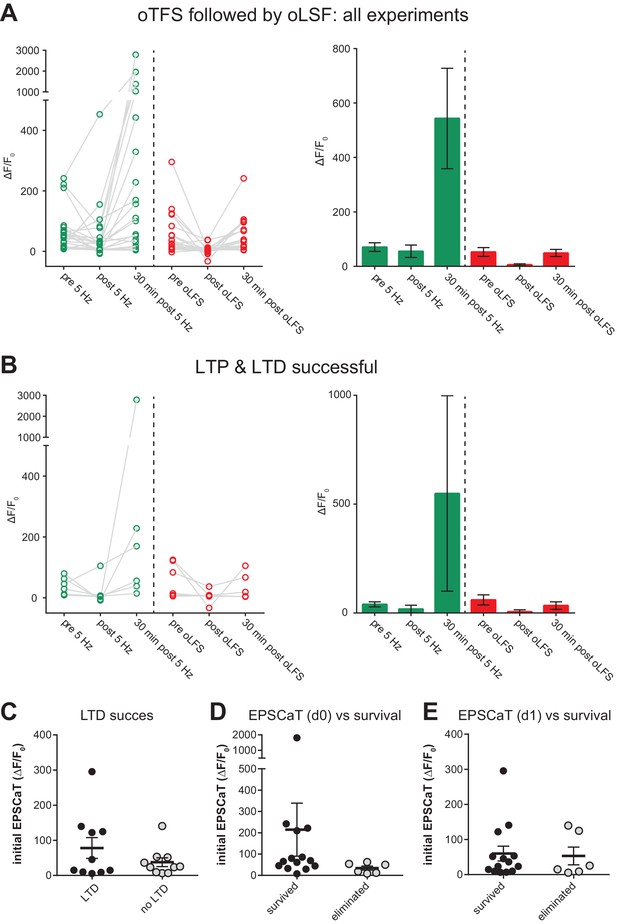
LTP followed by LTD.
(A) Outcome of all experiments where oTFS on day 0 was followed by oLFS on day 1 (n = 20). (B) Outcome of selected experiments where oTFS induced LTP (on day 0) and oLFS induced LTD of spine calcium signals (on day 1, n = 6). Survival rate of this group (50%) is shown in Figure 5b. (C) Initial EPSCaT amplitude on day one did not predict success of LTD induction (p>0.05, n = 20). (D) Initial EPSCaT amplitude on day 0 (before oTFS) did not predict 7 day survival (p>0.05, n = 20). (E) EPSCaT amplitude on day1 (before oLFS) did not predict 7 day survival (p>0.05, n = 20).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Rattus norvegicus, male) | Wistar | Charles River | Crl:WI | bred in the animal facility, UKE Hamburg |
| Strain, strain background (R. norvegicus, male) | Wistar | Janvier | RjHAN:WI | bred in the animal facility, UKE Hamburg |
| Genetic reagent (Clamydomonas reinhardtii) | ChR2(ET/TC) | doi: 10.1073/pnas.1017210108 | channelr hodopsin | |
| Genetic reagent (Aequorea victoria) | GCaMP6s | doi: 10.1038/nature12354 | calcium indicator | |
| Genetic reagent (A. victoria) | mCerulean | doi: 10.1038/nbt945 | fluorescent protein | |
| Transfected construct (R. norvegicus) | ChR2(ET/TC)−2A- synaptophysin- tdimer2 | doi: 10.1073/pnas.1315926110 | transfection of CA3 neurons | |
| Recombinant DNA reagent | rAAV2/7 | Vector Facility UKE Hamburg | viral vector | |
| Chemical compound, drug | APV | Tocris Bioscience | CAS Number 79055-68-8 | NMDA receptor blocker |
| Software, algorithm | ScanImage3.8 | DOI: 10.1186/1475-925X-2–13 | modified for arbitrary line scans |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.39151.017





