Transsynaptic interactions between IgSF proteins DIP-α and Dpr10 are required for motor neuron targeting specificity
Figures
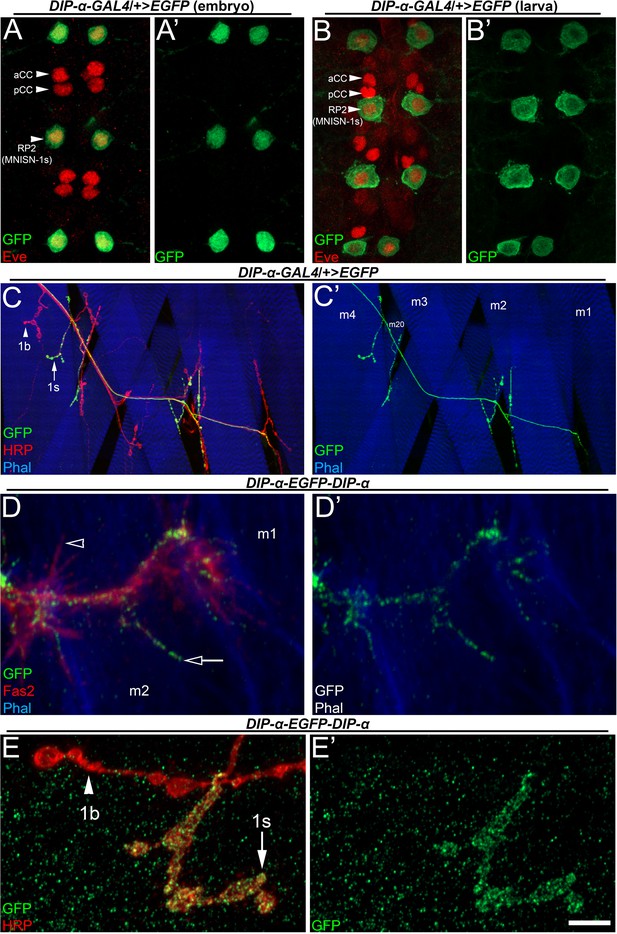
DIP-α is expressed in a subset of neurons that includes MNISN-1s/RP2.
(A–B) DIP-α-T2A-GAL4 driving EGFP expression in (A) embryonic and (B) third instar larval ventral nerve cords labeled with anti-GFP (green) and anti-Eve (red). Arrowheads denote segmentally repeating Eve expressing neurons, including RP2/MNISN-1s. (C) Dorsal larval body wall hemisegment labeled with anti-GFP (green), anti-HRP (red) and phalloidin (blue). DIP-α is only expressed in 1s neurons (arrow in C) and not 1b neurons (arrowhead). Muscles are labeled as m1-4 and m20 in C’. (D) DIP-α-EGFP-DIP-α protein trap embryo labeled with anti-GFP (green) anti-Fas2 (red), and phalloidin (blue), showing that DIP-α is enriched in MNISN-1s filopodia (arrow) but absent in others (arrowhead). (E) DIP-α-EGFP-DIP-α protein trap reveals DIP-α localization to 1s (arrow) NMJ and not 1b boutons (arrowhead), labeled with anti-GFP (green) and anti-HRP (red). Calibration bar is 16 µm in A, 13 µm in B, 50 µm in C, 3 µm in D and 4 µm in E. See also Figure 1—figure supplement 1.
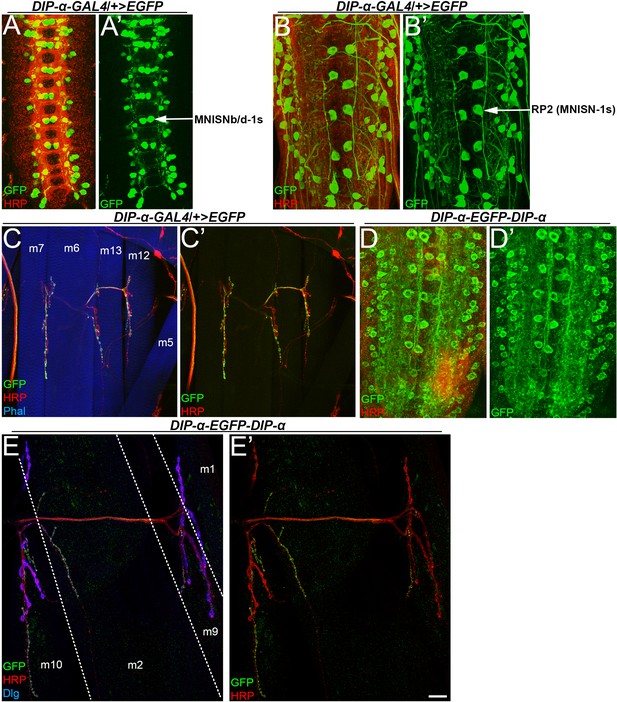
DIP-α is expressed in a subset of VNC neurons and DIP-α protein is enriched in the neuropil.
(A) Ventral confocal projections of VNC in st16 embryos depicting DIP-α-GAL4 expression of EGFP. Arrow denotes MNISNb/d-1s. (B) Dorsal confocal projections of VNC in larval animals depicting DIP-α-GAL4 expression of EGFP labeled with anti-GFP (green) and anti-HRP (red). Arrow denotes MNISN-1s. (C) Ventral larval body wall muscles labeled with anti-GFP (green), anti-HRP (red), and phalloidin (blue), showing that DIP-α-GAL4 is expressed in MNISNb/d-1s motor neurons as well. (D) Ventral nerve cord from DIP-α-EGFP-DIP-α protein trap larva, labeled with anti-GFP (green) and anti-HRP (red), showing that DIP-α also localizes to the 1s dendritic processes. (E) Dorsal body wall muscles from DIP-α-EGFP-DIP-α protein trap larva, labeled with anti-GFP (green) and anti-HRP (red), showing that DIP-α localizes to type 1s boutons (arrows) on several different muscles. Calibration bar is 19 µm in A, 17 µm in B, 40 µm in C, 18 µm in D and 10 µm in E. See also Figure 1.
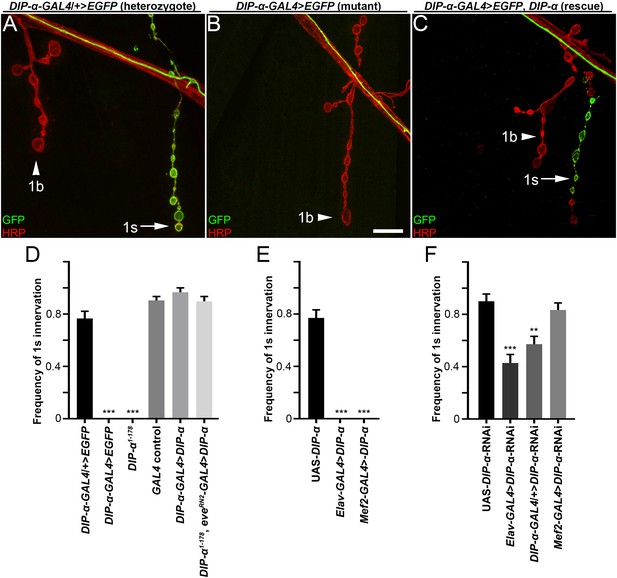
DIP-α is required for MNISN-1s branch formation on m4.
(A–C) Larval neuromuscular junctions labeled with anti-GFP (green) and anti-HRP (red) from (A) heterozygous DIP-α-GAL4 animals, (B) homozygous DIP-α-GAL4 mutants and (C) rescue of homozygous DIP-α-GAL4 mutants by expressing a UAS-DIP-α construct in DIP-α expressing cells, including MNISN-1s. DIP-α is only expressed in 1s neurons (green) (arrows in A,C) and not 1b neurons (arrowhead). (D–F) Frequency of 1s innervation of m4 from (D) mutants and rescue, (E) overexpression of UAS-DIP-α or (F) UAS-DIP-α-RNAi. n (animals/hemisegments) = (D) (12/60), (10/30), (18/100), (18/96), (9/56), (14/69), (E) (8/48), (6/30), (9/54), (F) (6/30), (12/71), (10/60), (10/59) (respectively). Calibration bar is 9 µm in A-C. **p<0.001, ***p<0.0001. Error bars represent SEM. See also Figure 2—figure supplement 1.
-
Figure 2—source data 1
Data for graphs in Figure 2.
- https://doi.org/10.7554/eLife.42690.006
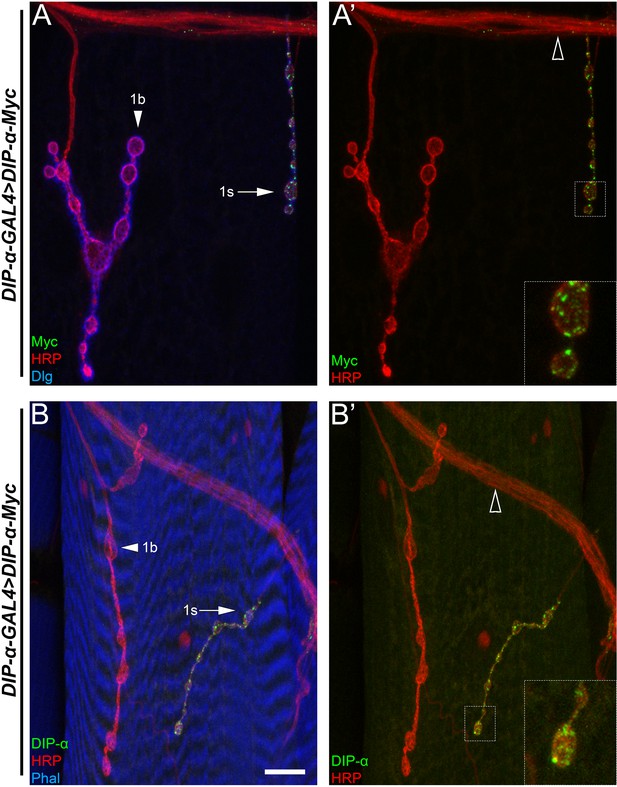
DIP-α protein from a transgene localizes normally in rescue animals.
(A,B) The DIP-α rescue construct, tagged with a c-terminal Myc tag, labeled with (A) anti-Myc (green), anti-HRP (red) and anti-Dlg (blue) or (B) anti-DIP-α (green), anti-HRP (red) or phalloidin (blue). Note localization only to 1s boutons (arrows) and not 1b boutons (arrowheads). Open arrowheads denote the ISN. Calibration bar is 10.5 µm. See also Figure 2.
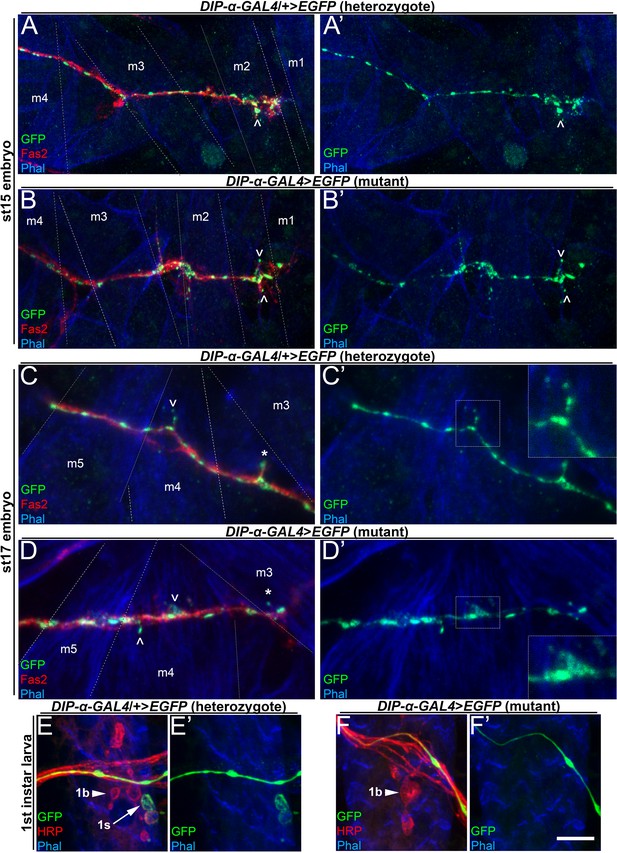
MNISN-1s axons in DIP-α mutants produce protrusions on m4 but are unable to form stable 1s branches.
(A–D) Embryonic NMJs labeled with anti-GFP (green), anti-HRP (red) and phalloidin (blue) from (A,C) heterozygous and (B,D) hemizygous DIP-α-GAL4 mutants. (A–B) are st15 embryos; (C–D) are st17 embryos. MNISN-1s protrusions over m4 form late in embryonic development (C) while protrusions over m2 form earlier (A). Note that MNISN-1s axon protrusions form properly over dorsal muscles, including m2, at st15 in (A) controls and (B) DIP-α mutants and over m4 at st17 in controls (C) and DIP-α mutants (D). Also, ^ indicates all filopodia in (A) and (B), and m4 filopodia in (C) and (D); * indicates st17 non-m4 filopodia. (E–F) First instar larva labeled with anti-GFP (green), anti-HRP (red) and phalloidin (blue) from DIP-α-GAL4 heterozygous animals and (F) DIP-α-GAL4 homozygous mutants. Note 1b boutons present in both first instar larvae (arrowhead in E,F), but only heterozygous animals have 1s boutons (arrow in E). Calibration bar is 8 µm in A,B, 4 µm in C,D and 6 µm in E,F. See also Figure 3—figure supplement 1.
-
Figure 3—source data 1
Data for graphs in Figure 3—figure supplement 1.
- https://doi.org/10.7554/eLife.42690.009
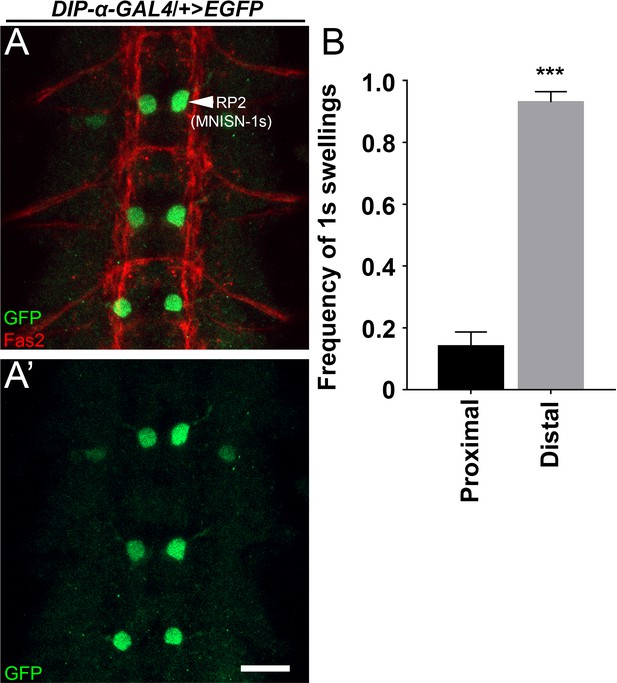
DIP-α is expressed in MNISN-1s motor neurons in st14 embryos, and 1s arbors form over distal muscles before proximal.
(A) A ventral nerve cord labeled with anti-GFP (green) and anti-Fas2 (red) from a DIP-α-GAL4 driving EGFP st14 embryo. (B) Frequency of MNISN-1s terminal swellings (varicosity precursors) from DIP-α-T2A-GAL4 driving GFP observed in st16/17 embryos. Terminal swellings are observed predominantly in distal (most dorsal). Calibration bar in A is 14 µm. n (animals/hemisegments) = (6/57). Error bars represent SEM. ***p<0.0001. See also Figure 3.
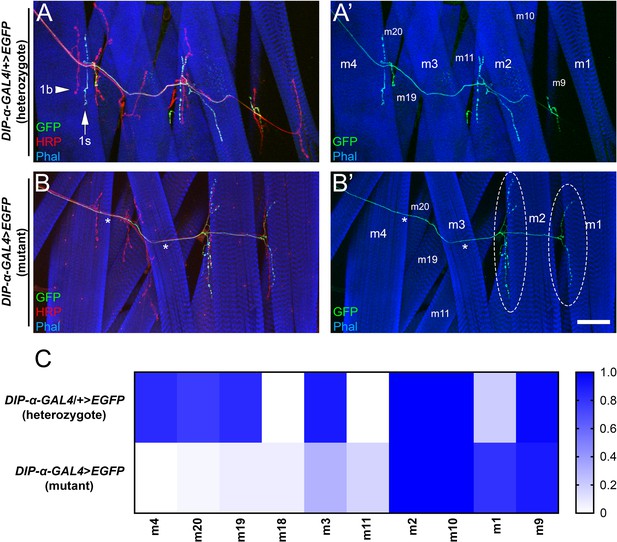
Loss of DIP-α causes selective loss of MNISN-1s branches on proximal muscles and ectopic innervation of distal muscles.
(A,B) Dorsal body wall hemisegments labeled with anti-GFP (green), anti-HRP (red) and phalloidin (blue) from (A) heterozygous DIP-α-GAL4 animals, and (B) homozygous DIP-α-GAL4 mutants. Arrow denotes 1s boutons in (A), arrowhead denotes 1b boutons, and * denote muscles in mutant that have lost 1s innervation. Overgrown 1s arbors on m2 and m1 are circled in mutants. (C) Quantification of the frequency of innervation of MNISN-1s neurons on the respective muscles from the above genotypes. n (animals/hemisegments) = (16/30), (12/30) (respectively). Calibration bar is 60 µm. See also Figure 4—figure supplement 1.
-
Figure 4—source data 1
Data for graphs in Figure 4 and Figure 4—figure supplement 1.
- https://doi.org/10.7554/eLife.42690.012
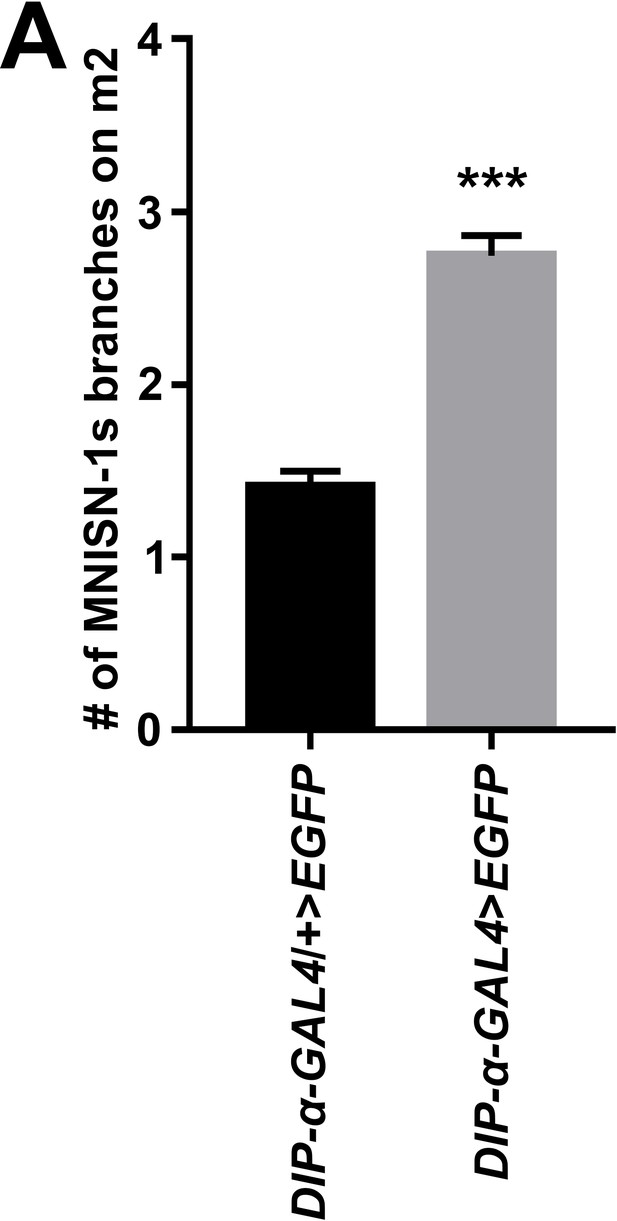
Quantification of MNISN-1s arbor branches on m2.
(A) Quantification of the number of MNISN-1s branches on m2 in controls (DIP-α-T2A-GAL4/+) and hemizygous nulls (DIP-α-T2A-GAL4/Y). n (animals/hemisegments) = (13/54), (15/59) (respectively). Error bars represent SEM. ***p<0.0001. See also Figure 4.
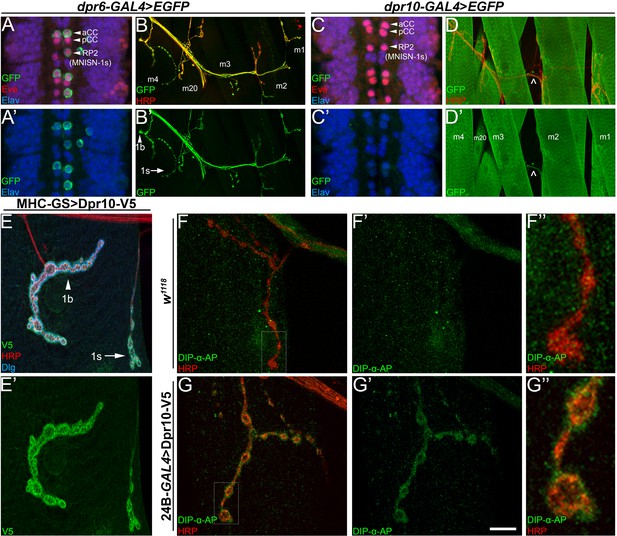
Dpr10 is expressed in muscles and can localize to the SSR and bind to DIP-α.
(A,C) Embryonic ventral nerve cord from (A) dpr6-GAL4 and (C) dpr10-GAL4 driving EGFP expression, labeled with anti-GFP (green), anti-Eve (red) and anti-Elav (blue). dpr6 is expressed in aCC and RP2 (MNISN-1s) neurons, while dpr10 is not expressed in these Eve+ cells (see also Figure 5—figure supplement 1A). (B,D) Larval dorsal body wall hemisegments from (B) dpr6-GAL4 or (D) dpr10-GAL4 driving EGFP expression, labeled with anti-GFP (green), and anti-HRP (red). Muscles are labeled m4-m1, m20. Dpr10 is also expressed in unknown neurons ( ^ in 5D denotes axon). (E) Larval m4 NMJ from animals expressing Dpr10-V5 using MHC-GeneSwitch-GAL4 in the absence of RU486 (low-level muscle expression only), labeled with anti-V5 (green), anti-HRP (red) and anti-Dlg (blue). 1b and 1s neurons are marked with arrowhead and arrow, respectively. (F,G) Larval m4 NMJ from either (F) control w1118 or (G) 24B-GAL4>UAS-Dpr10-V5 (high level pan-muscle expression). Live tissue was incubated with DIP-α-AP5 protein (see Materials and methods) and then labeled with anti-AP (green) and anti-HRP (red). Calibration bar is 15 µm in A,C, 60 µm in B,D, 12 µm in E, and 18 µm in F,G. See also Figure 5—figure supplement 1.
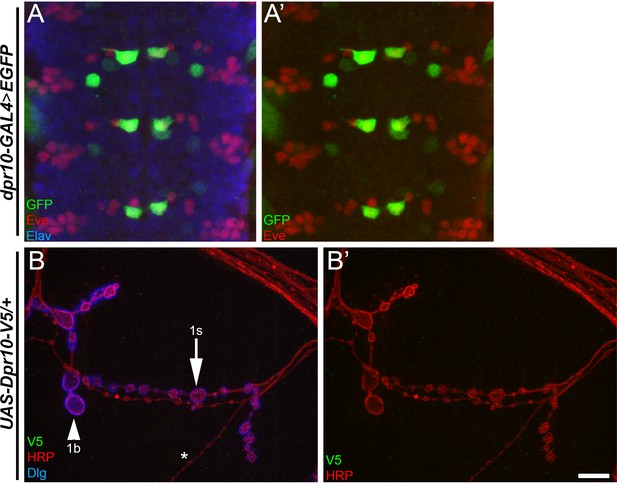
Dpr10 is expressed in a subset of neurons in the embryonic ventral nerve cord, and anti-V5 staining in a control sample.
(A) An embryonic ventral nerve cord from dpr10-GAL4 > EGFP labeled with anti-GFP (green) and anti-Elav (red) from sections below the MNISN-1s neuron cell bodies seen in Figure 4. (B) A larval m4 NMJ in a UAS-Dpr10-V5/+ (no GAL4 driver) labeled with anti-V5 (green), anti-HRP (red) and anti-Dlg (blue). Note the lack of anti-V5 signal. 1s boutons (arrow), 1b boutons (arrowhead), and type two boutons (asterisk). Calibration bar is 11 µm in A and 8 µm in B. See also Figure 5.
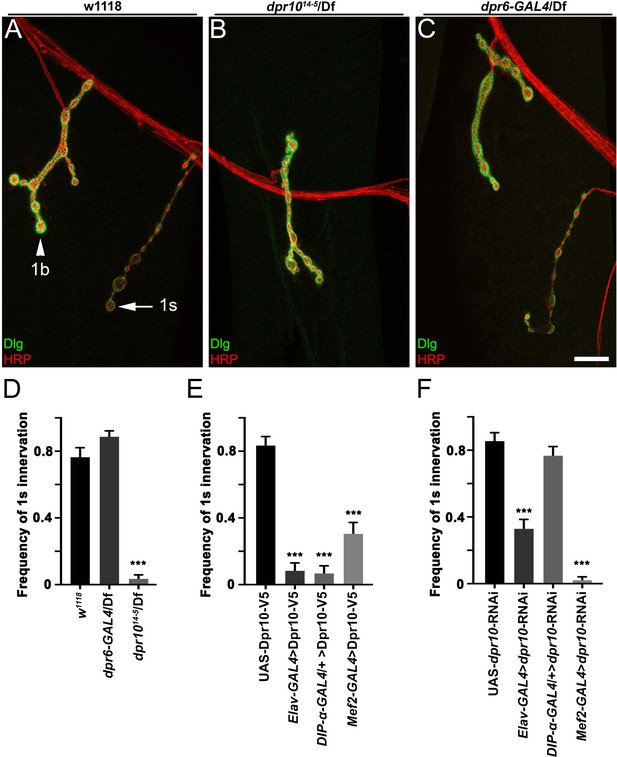
Dpr10 is required for MNISN-1s innervation of muscle 4.
(A–C) Larval m4 NMJs labeled with anti-DLG (green) and anti-HRP (red) from (A) control w1118 animals, (B) dpr1014-5/Df null mutants and (C) dpr6-GAL4/Df. The difference in anti-Dlg staining intensity allows us to differentiate 1b and 1s NMJs. 1b and 1s neurons are marked with arrowhead and arrow, respectively. (D–F) Frequency of 1s innervation of m4 from (D) dpr6 and dpr10 mutants and (E) overexpression of UAS-Dpr10-V5 and (F) UAS-dpr10-RNAi. n (animals/hemisegments) = (D) (8/62), (12/70), (11/60) and (E) (8/48), (6/36), (5/30), (8/47), (F) (8/48), (8/48), (10/60), (8/48) (respectively). Calibration bar is 16 µm in A-C. Error bars represent SEM. ***p<0.0001. See also Figure 6—figure supplement 1.
-
Figure 6—source data 1
Data for graphs in Figure 6 and Figure 6—figure supplement 1.
- https://doi.org/10.7554/eLife.42690.017
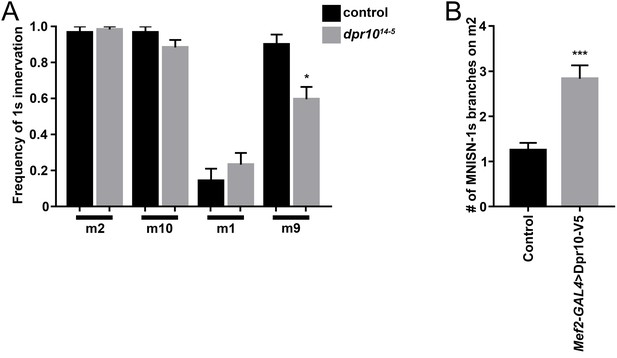
MNISN-1s innervation of dorsal muscles and branching on m2.
(A) Frequency of MNISN-1s innervation of dorsal muscles from control (w1118) and dpr10 mutants. (B) Quantification of MNISN-1s branches on m2 in control (UAS-Dpr10/+) and muscle overexpressedDpr10-V5. n (animals/hemisegments) = (A) (7/30), (7/32) and (B) (6/30), (12/62) (respectively). Error bars represent SEM. *p<0.05, ***p<0.0001. See also Figure 6.
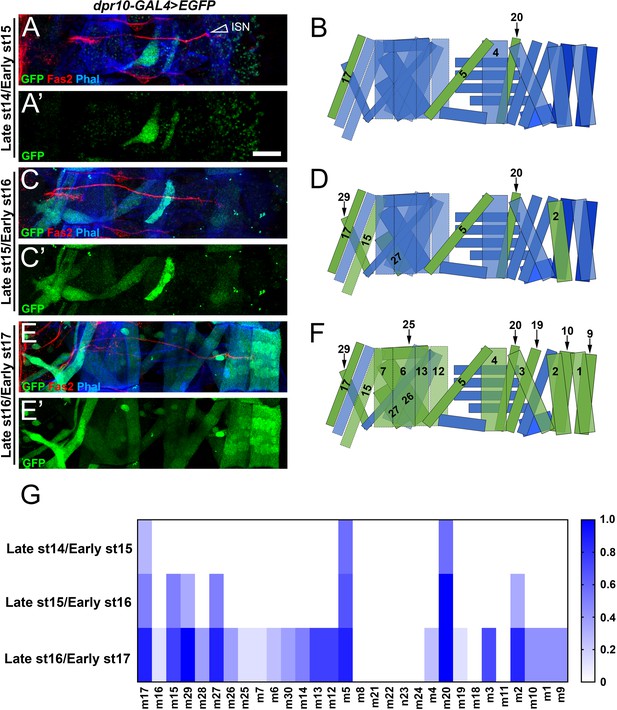
Temporal and spatial expression of dpr10 in embryonic muscles.
(A,C,E) Embryonic dpr10-GAL4>EGFP abdominal body wall hemisegments labeled with anti-GFP (green), anti-Fas2 (red) and phalloidin (blue) from (A) late st14/early st15, (C) late st15/early st16, and (E) late st16/early st17. Open arrowhead denotes Intersegmental Nerve (ISN), which carries dorsal neurons. (B,D,F) Cartoon representation of an embryonic hemisegment. Green muscles are those that express dpr10 at the respective developmental stage while blue muscles do not express dpr10. (G) Heat map quantification of the frequency of dpr10-GAL4>EGFP expression in each muscle at each developmental stage, where 1 represents 100% probability of expression. n (embryos) = 7, 6, 8 (respectively). Calibration bar is 19 µm.
-
Figure 7—source data 1
Data for graphs in Figure 7.
- https://doi.org/10.7554/eLife.42690.019
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (D. melanogaster) | w1118 | Bloomington Drosophila Stock center (BDSC) | ||
| Genetic reagent (D. melanogaster) | Mef2-GAL4 | PMID: 9671578 | Gift of Hugo Bellen | |
| Genetic reagent (D. melanogaster) | DIP-α-T2A-GAL4 | PMID: 21985007 | Gift of Hugo Bellen | |
| Genetic reagent (D. melanogaster) | DIP-α-EGFP-DIP-α | PMID: 26687361 | Gift of Hugo Bellen | |
| Genetic reagent (D. melanogaster) | DIP-α1-178 | PMID: 30467079 | Gift of Lawrence Zipursky | |
| Genetic reagent (D. melanogaster) | dpr1014-5 | PMID: 30467079 | Gift of Lawrence Zipursky | |
| Genetic reagent (D. melanogaster) | UAS-DIP-α-Myc | PMID: 30467079 | Gift of Lawrence Zipursky | |
| Genetic reagent (D. melanogaster) | UAS-Dpr10-V5 | PMID: 30467079 | Gift of Lawrence Zipursky | |
| Genetic reagent (D. melanogaster) | dpr10-T2A-GAL4 | PMID: 21985007 | Gift of Hugo Bellen | |
| Genetic reagent (D. melanogaster) | dpr6-T2A-GAL4 | PMID: 21985007 | Gift of Hugo Bellen | |
| Genetic reagent (D. melanogaster) | UAS-2XEGFP | PMID: 12324968 | BDSC #6874 | |
| Genetic reagent (D. melanogaster) | Elav-GAL4 | PMID: 22319582 | BDSC #8765 | |
| Genetic reagent (D. melanogaster) | 24B-GAL4 | PMID: 8223268 | BDSC #1767 | |
| Genetic reagent (D. melanogaster) | MHC-GeneSwitch | PMID: 11675495 | Gift of Haig Keshishian | |
| Genetic reagent (D. melanogaster) | EveRN2-GAL4 | PMID: 14624243 | BDSC #7470 | |
| Genetic reagent (D. melanogaster) | UAS-dpr10-RNAi | PMID: 17625558 | Vienna Drosophila Resource Center #103511 | |
| Genetic reagent (D. melanogaster) | UAS-DIP-α-RNAi | PMID: 26320097 | BDSC #38965 | |
| Genetic reagent (D. melanogaster) | Df(3L)BSC673 | BDSC #26525 | Deficiency covering dpr6 and dpr10 | |
| Antibody | Goat anti-HRP-TRITC | Jackson Immunological Research | #123-025-021 | 1:50 |
| Antibody | Goat anti-HRP-Alexa405 | Jackson Immunological Research | #123-475-021 | 1:50 |
| Antibody | Mouse anti-Dlg | Developmental Studies Hybridoma Bank | #4F3 | 1:100 |
| Antibody | Mouse anti-Fas2 | Developmental Studies Hybridoma Bank | #1D4 | 1:100 |
| Antibody | Mouse anti-DIP-α | PMID: 30467079 | Gift of Lawrence Zipursky | 1:20 |
| Antibody | Mouse anti-V5 | ThermoFisher | #R960-25 | 1:400 |
| Antibody | Rabbit anti-GFP | ThermoFisher | #A11122 | 1:1000 |
| Antibody | Rabbit anti-Dlg | PMID: 9354326 | Gift of Vivian Budnik | 1:40,000 |
| Antibody | Rabbit anti-Myc | Cell Signaling Technology | #71D10 | 1:200 |
| Antibody | Goat anti-Mouse-Alexa488 | ThermoFisher | #A11029 | 1:500 |
| Antibody | Goat anti-Mouse-Alexa568 | ThermoFisher | #A11031 | 1:500 |
| Antibody | Goat anti-Rabbit-Alexa488 | ThermoFisher | #A11008 | 1:500 |
| Antibody | Goat anti-Rabbit-Alexa568 | ThermoFisher | #A11036 | 1:500 |
| Chemical compound, drug | Phalloidin-Alexa647 | ThermoFisher | #A22287 | 1:100 |
| Antibody | Rabbit anti-alkaline phosphatase | Abcam | #ab16695 | 1:100 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.42690.020





