Regulation of subcellular dendritic synapse specificity by axon guidance cues
Figures
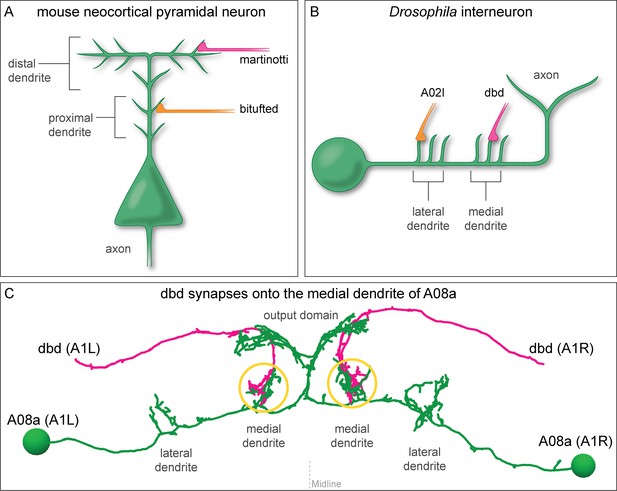
Mammalian and insect neurons display subcellular synaptic specificity.
(A) Schematic of mouse neocortical pyramidal neuron (green) with a martinotti neuron (magenta) forming synapses onto the distal dendrite and the bitufted neuron (orange) forming synapses onto the proximal dendrite. (B) Schematic of fly A08a neuron (green) with a dbd neuron (magenta) forming synapses onto the medial dendrite and an A02l neuron (orange) forming synapses onto the lateral dendrite. (C) Electron microscopy reconstruction of dbd neurons (magenta) and A08a neurons (green) morphologies in one abdominal (A) segment (A1 left and A1 right) of the Drosophila ventral nerve cord (posterior view). dbd forms synapses specifically with the medial dendritic domain, and does not synapse with the lateral dendritic domain or the output domain.

The A08a neuron receives arbor-specific synaptic inputs.
(A–C’’) Light microscopy (point scanning confocal) imaging of A08a neurons. (A) Dorsal view of the light micrograph (LM) 3D reconstruction of A08a neurons in the larval ventral nerve cord segments A1-7. The A08a neurons are visualized by 26F05(A08a)-LexA > LexAop-myr::smGdP::V5. Midline, dashed line in all panels. (A’) Posterior view of the LM 3D reconstruction of paired A08a neurons in segment A1 left/right. (B) Posterior view of a single A08a labeled by MultiColor FlpOut (MCFO), visualized by A08a-Gal4 > UAS-MCFO. (C–C’’) A08a-Gal4 drives expression of UAS-DenMark::mCherry (dendrite marker) and UAS-synaptotagmin::GFP (presynaptic marker). Note the complementary expression in the dendritic and output domains. (D–G) Electron microscopy (EM) reconstruction of A08a and four synaptic partner neurons. (D) Dorsal view of A08a neurons in segments A1-2. (D’) Posterior view of A08a neurons in segment A1. (E) A single A08a with presynaptic and postsynaptic sites labeled in red and blue highlight a distinct dendritic domain and a mixed pre- and post-synaptic output domain, respectively. (F) Synapse flow centrality analyses (Schneider-Mizell et al., 2016) shows that A08a has distinct mixed axonal (output) and dendritic compartments. (G) A08a receives dendritic arbor-specific input: dbd (yellow) and A02d (orange) synapse specifically on the medial dendrite, whereas A02l (blue) and A31x (cyan) synapse specifically on the lateral dendrite.
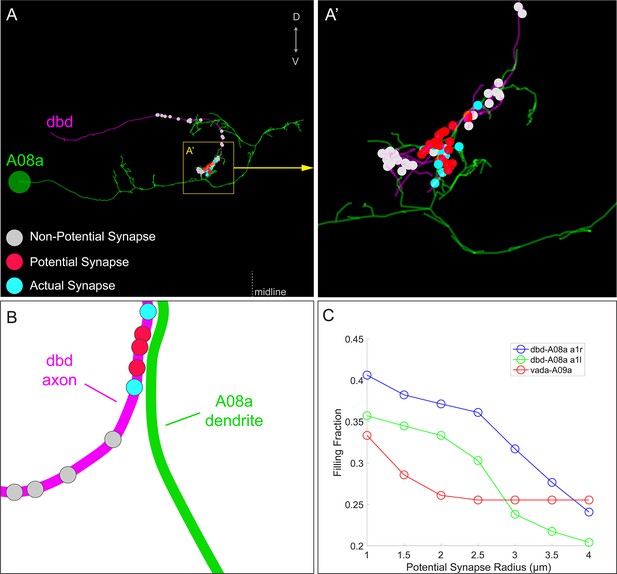
Filling fractions between dbd and A08a neurons.
(A–A’) Posterior view of dbd (magenta) and A08a (green) neurons EM reconstruction in abdominal segment 1, left (A1L). dbd presynapses (enlarged circles) are color coded based on distance from A08a membrane and synaptic connectivity with A08a. Non-potential synapses (gray) indicate dbd presynapses that are more than 2 μm away from the center of A08a dendritic processes (skeleton). Potential synapses (red) indicate dbd presynapses less than 2 μm away from the A08a skeleton that are not connected with A08a. Actual synapses (cyan) indicate dbd presynapses that are synaptically coupled with A08a. Yellow box indicates region enlarged in A’. (B) Schematic showing how the synapse types are assigned between dbd (magenta) and A08a (green) neurons in the filling fraction analyses. The filling fraction is the number of actual synapses divided by the sum of actual and potential synapses. The distance threshold which defines non-potential synapses can be changed, allowing the filling fraction to be plotted as a function of distance (shown in C below). (C) The filling fraction plotted as a function of distance between dbd presynapses and A08a skeleton. When dbd presynapses are within 2 μm from the A08a skeleton, the percent of synapse formation (filling fraction) is 0.34 (A1L) and 0.38 (A1R). The filling fraction between a different set of neurons, v'ada and A09a, are provided for reference from Gerhard et al. (2017).
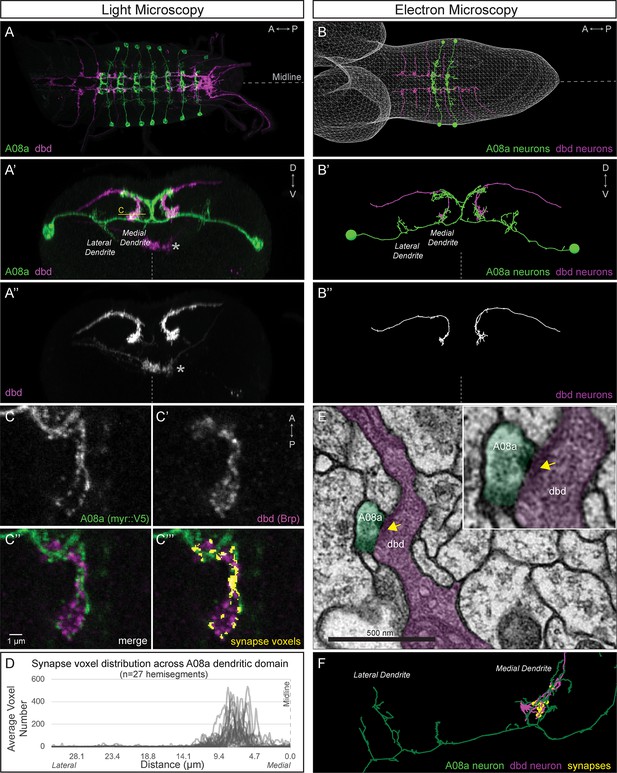
dbd and A08a neurons are synaptic partners by light and electron microscopy analyses.
(A) Dorsal view, light microscopy 3D reconstruction showing dbd (magenta) and A08a (green) neurons. A08a is visualized with A08a-LexA > LexAop-myr::smGdP::V5. dbd is visualized with dbd-Gal4 > UAS-myr::smGdP::HA. Anterior to left; midline, dashed line in all panels. (A’–A’’) Posterior view, light microscopy 3D reconstruction showing dbd and A08a neurons. dbd projects to the A08a medial dendritic arbor but not the A08a lateral dendritic arbor. Apparent colocalization of dbd with the A08a output domain is an artifact of the 3D projection. Asterisk, ventral off-target expression of dbd-Gal4. C, focal plane shown in panel C, below. (B–B’’) EM reconstruction of dbd and A08a neurons; B, dorsal view, (A1-A2); B’-B’’, posterior view, (A1). (C–C’’’) Single optical section showing a subset of dbd presynapses (magenta, labeled with dbd-Gal4 > UAS-brp-short-mstraw) positioned in close proximity to the A08a membrane (green, labeled with A08a-LexA > LexAop-myr::smGdP::V5). Voxels containing A08a membrane within 90 nm of voxels containing Brp-mstraw are defined as ‘synapse voxels’ (C’’’, yellow). (D) Quantification of synapse voxel position across A08a dendritic domain shows enrichment on the A08a medial dendritic arbor. (E) Representative chemical synapse between dbd and A08a (arrowhead) in the EM volume. (F) EM reconstruction showing that the dbd neuron (magenta) synapses specifically with the A08a medial but not lateral dendritic arbor (green); synapses, yellow circles.
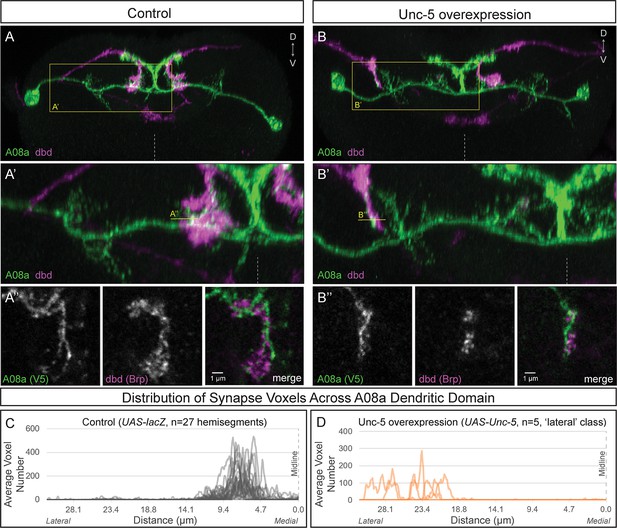
Lateralizing dbd results in Brp + putative synapses at the A08a lateral dendritic arbor.
(A–A’) In control animals, dbd membrane (magenta, labeled with dbd-Gal4 >UAS-smGdP::myr::HA) is positioned in close proximity to the A08a medial dendritic arbor membrane (green, labeled with A08a-LexA > LexAop-myr::smGdP::V5). (A) Posterior view of one segment; midline, dashed line in all panels; box, area enlarged in A’. (A’) Posterior view of dbd and the A08a medial dendritic arbor; A’’ line, optical section shown in A’’. (A’’) Single z-slice shows a subset of dbd presynapses (magenta, labeled with dbd-Gal4 > UAS-brp-short::mstraw in close proximity to the A08a medial dendritic arbor membrane. (B–B’) Overexpression of Unc-5 in dbd can lateralize the axon terminal of dbd. B’’ line, position of optical section shown in B’’ below. See Figure 4—figure supplement 1E for quantification of lateralization classes. (B’’) Single z-slice shows a subset of dbd presynapses (magenta, labeled with dbd-Gal4 >UAS-brp-short::mstraw) positioned in close proximity to A08a membrane (green, labeled with A08a-LexA > LexAop-myr::smGdP::V5). (C–D) Quantification of synapse voxel position across the dendritic domain of A08a. (C) In control animals, dbd forms synapse voxels on the medial dendritic arbor of A08a; n = 27 hemisegments from 18 animals. Data reproduced from Figure 3D. (D) In hemisegments with full lateralization of dbd (as shown in B’), dbd forms synapse voxels on the lateral dendritic arbor of A08a; n = 5 hemisegments from five animals. See Figure 4—figure supplement 1E for quantification of lateralization classes.
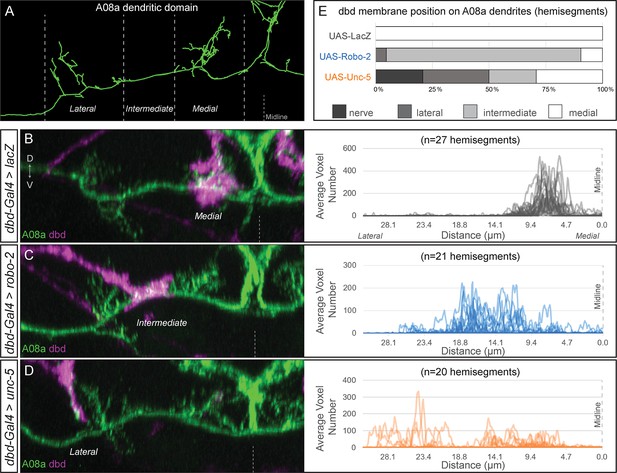
dbd axons can be variably lateralized by expression of axon guidance receptors Unc-5 and Robo-2.
(A) The A08a neuron in the EM reconstruction can be divided into medial, intermediate, and lateral dendritic domains. (B–D) The dbd neuron membrane (magenta) can target different subcellular domains of A08a (green), posterior view of one hemisegment. (B) Control: dbd contacts the A08a medial dendritic arbor (dbd-Gal4 >UAS lacZ). Data reproduced from Figure 3D. (C) Partial lateralization example: dbd contacts the intermediate dendrite domain (dbd-Gal4 >UAS-robo-2). (D) Full lateralization example: dbd contacts that lateral A08a dendritic arbor (dbd-Gal4 >UAS-unc-5). Data reproduced from Figure 4B’,D. Right panels show the distribution of synapse voxels for each genotype. Control, UAS-lacZ, n = 27 hemisegments from 18 animals; UAS-robo-2, n = 21 hemisegments from 15 animals; UAS-unc-5, n = 20 hemisegments from 17 animals. (E) Frequency of dbd membrane lateralization by genotype. dbd will either target mostly the medial, intermediate, or lateral dendritic domains, or not enter the neuropil (‘nerve’ category). See methods for full genotypes.
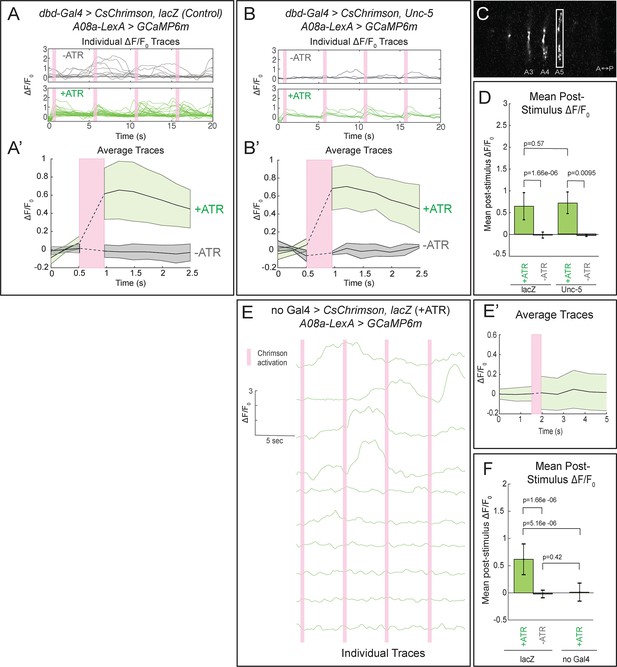
Confocal activation of Chrimson in control and lateralized dbd increases A08a GCaMP6m fluorescence.
(A–A’) In wild-type animals, Chrimson activation of dbd neurons results in increased GCaMP6m fluorescence in the A08a output domain. For all figures,+ATR is shown in green, -ATR is shown in gray, and timing of Chrimson activation (500 ms) is represented with a pink bar. (A) A08a GCaMP6m ΔF/F0 traces from individual A08a pairs resulting from wild-type dbd activation. Non-evoked spontaneous activity is present in -ATR control. (A’) Average A08a GCaMP6m ΔF/F0 traces, before and after Chrimson activation of dbd neurons. Solid black lines represent the mean ΔF/F0. Shaded regions represent the standard deviation from the mean. +ATR, n = 28 A08a pairs, from 10 animals; -ATR, n = 11 A08a pairs, from five animals. (B–B’) In animals with fully lateralized dbd, Chrimson activation of dbd results in increased GCaMP6m fluorescence in A08a axon terminals. (B) A08a GCaMP6m ΔF/F0 traces from individual A08a pairs resulting from activation of lateralized dbd. (B’) Average A08a GCaMP6m ΔF/F0 traces, before and after Chrimson activation of dbd neurons. Solid black lines represent the mean ΔF/F0. Shaded regions represent the standard deviation from the mean. +ATR, n = 6 A08a pairs, from five animals; -ATR, n = 4 A08a pairs, from three animals. (C) Example ROI used for quantification drawn around A08a axon terminals in segment A5. (D) Quantification of the mean post-stimulus ΔF/F0 for lacZ control and unc-5. Error bars represent the standard deviation from the mean. Mean post-stimulus ΔF/F0: lacZ Control +ATR, 0.62 ± 0.28, n = 28 A08a pairs, from 10 animals; lacZ Control -ATR, −0.0172 ± 0.07, n = 11 A08a pairs, from five animals; unc-5 +ATR, 0.68 ± 0.24, n = 6 A08a pairs, from five animals; unc-5 -ATR, −0.035 ± 0.02, n = 4 A08a pairs, from three animals. (E–E’) dbd-Gal4 is required to produce Chrimson-evoked responses in A08a. A08a expresses GCaMP6m in a genetic background containing UAS-lacZ and 20XUAS-CsChrimson. (E) A08a GCaMP6m ΔF/F0 traces from individual A08a pairs. (E’) Average A08a GCaMP6m ΔF/F0 traces before and after light stimulus (pink bar). Solid black line represents the mean ΔF/F0. Shaded region represents the standard deviation from the mean. +ATR is represented in green (n = 10 A08a pairs). (F) Quantification of the mean post-stimulus ΔF/F0 for lacZ control +ATR, lacZ control -ATR, and no dbd-gal4 control. Error bars represent the standard deviation from the mean. Mean post-stimulus ΔF/F0: lacZ Control +ATR, 0.62 ± 0.28, n = 28 A08a pairs, from 10 animals (Data reproduced from Figure 6D); lacZ control -ATR, −0.0172 ± 0.07, n = 11 A08a pairs, from five animals (Data reproduced from Figure 6D); No dbd-gal4Control +ATR, 0.013 ± 0.17, n = 10 A08a pairs, from five animals. Significance between two groups was determined using a Mann-Whitney test.
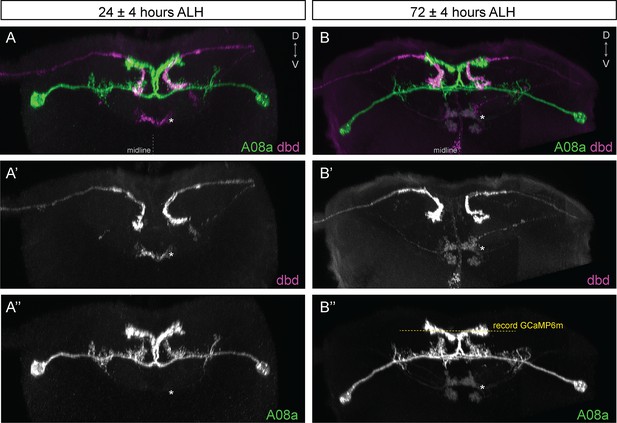
dbd and A08a neuronal morphology is similar at 24 hr and 72 hr after larval hatching (ALH).
(A–A’’) Posterior view of a 3D light microscopy reconstruction in Imaris showing dbd (magenta) and A08a (green) neurons at 24 ± 4 hr ALH. Midline, dashed line in all panels. Asterisk, ventral off-target neurons. (A’) The dbd neurons are visualized by 165(dbd)-Gal4 > UAS-myr::smGdP::HA. (A’’) The A08a neurons are visualized by 26F05(A08a)-LexA > LexAop-myr::smGdP::V5. (B–B’’) Posterior view of a 3D light microscopy reconstruction in Imaris showing dbd (magenta) and A08a (green) neurons at 72 ± 4 hr ALH. Yellow dashed line indicates the imaging focal plane used to record GCaMP6m fluorescence changes in A08a neurons. Asterisk, position of ventral off-target neurons. (B’) The dbd neurons are visualized by 165(dbd)-Gal4 > UAS-myr::smGdP::HA. (B’’) The A08a neurons are visualized by 26F05(A08a)-LexA > LexAop-myr::smGdP::V5.
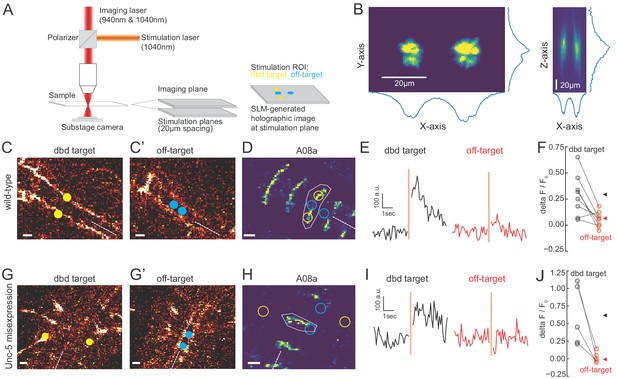
Two photon activation of dbd, but not off-target neurons, increases A08a GCaMP6m fluorescence.
(A) Schematic of two photon microscope used for Ca+2 imaging and holographic photostimulation. We used a separate imaging (940 or 1040 nm) and stimulation laser (1040 nm). Holographic photostimulation patterns were constructed with a spatial light modulator (SLM). Stimulation targeted either dbd neurons (yellow circles) or off-target neurons (blue circles), separated on average by 20 µm in the z-axis. (B) XY and XZ profile of fluorescence induced by a holographic stimulation pattern consisting of two 10 µm diameter circles separated center-to-center by 26 µm. Profiles were obtained by moving objective (and therefore stimulation pattern) systematically relative to a fixed slide with a ~1 µm fluorescent coating while imaging with a sub-stage camera. Blue lines indicate fluorescence summed across respective axes (arbitrary units). (C–F) Targeting of Chrimson stimulation and Ca+2 imaging of A08a neurons in wild-type 72 hr ALH larvae. (C–C’) Two photon image (1040 nm) of fluorescent mCherry marker at two imaging planes 20 µm apart. Stimulation ROIs used for targeting dbd (C, yellow dots) and off-target (C’, cyan dots) neurons are overlaid. Dashed white line indicates midline. Scale bars, 10 μm. (D) Summed GCaMP6m fluorescence in A08a neurons (940 nm). White polygon depicts spatial region used to quantify fluorescence for traces in E. The stimulation regions shown in C are overlaid (outlines: yellow, dbd; cyan, ventral off-targets). Scale bars, 10 μm. (E) Example Ca+2 responses from the wild-type larva shown in C,D. Black trace shows raw A08a fluorescence (arbitrary units) prior to and following 150 ms holographic stimulation of dbd targets. Red trace shows A08a fluorescence in response to ventral off-target stimulation. Stimulation timing depicted with pink rectangle. (F) Mean Ca+2 responses (ΔF/F0 ) in A08a for each animal to dbd stimulation (black dots) or ventral off-target stimulation (red dots). Triangles are means for each group (dbd, 0.29 +/-. 07; off-target, 0.06 ± 0.07). N = 8 animals. Scale bars, 10 μm. (G–J) Targeting of Chrimson stimulation and Ca+2 imaging of A08a neurons in Unc-5 misexpression larvae at 72 hr ALH. (G–G’) Two photon image (1040 nm) of fluorescent mCherry marker at dbd (G) and off-target imaging planes (G’), separated by 20 µm. Stimulation ROIs overlaid (dbd, G, yellow dots; off target, G’, cyan dots). (H) Summed GCaMP6m fluorescence in A08a neurons. Stimulation regions and measurement region plotted as in D. (I) Example Ca+2 responses from Unc-5 larva shown in G, H. Plotting conventions as in E. Black trace shows raw A08a fluorescence in response to dbd stimulation; red trace is A08a fluorescence in response to off-target stimulation. (J) Mean Ca+2 responses (ΔF/F0 ) in A08a for each animal to dbd stimulation (black dots) or ventral off-target stimulation (red dots). Triangles are means for each group (dbd, 0.60 ± 0.17; off-target, 0.02+/.03). N = 5 animals. Scale bars, 10 μm.

Lateralized dbd forms direct, monosynaptic connections with the A08a lateral dendrite.
(A) TTX eliminates spontaneous rhythmic neuronal activity in A08a (in which activity is part of an inter-segmental activity wave moving in the anterior or posterior direction representing fictive motor waves; Itakura et al., 2015). Representative traces show the ΔF/F0 for individual pairs of A08a neurons over the course of 3 min in lacZ control animals. Purple trace shows A08a ΔF/F0 without TTX present. Black trace shows A08a ΔF/F0 in the presence of 3 μM TTX, in which 20/20 A08a pairs from eight animals where rhythmic activity was eliminated. In 8/20 of these A08a pairs, non-rhythmic, non-intersegmentally coordinated changes in GCaMP6m fluorescence were observed, exemplified by the gray trace (see Discussion). (B) Experiment to test for monosynaptic dbd-A08a connectivity. TTX eliminates action-potential-mediated activity, preventing stimulation of non-Chrimson expressing neurons. Light-activation of Chrimson induces action-potential-independent neurotransmitter release from dbd. If dbd is monosynaptically connected to A08a, increases in A08a GCaMP fluorescence will result. (C–C”) Wild-type dbd has excitatory, monosynaptic connection to A08a medial dendritic arbor. (C) A08a GCaMP6m ΔF/F0 traces from individual A08a pairs resulting from wildtype dbd activation in the presence of TTX. (C’) Average A08a GCaMP6m ΔF/F0 traces in the presence of 3 μM TTX, before and after Chrimson activation of dbd neurons. Solid black lines represent the mean ΔF/F0. Shaded regions represent the standard deviation from the mean. +ATR, n = 20 A08a pairs, from nine animals; -ATR, n = 9 A08a pairs, from four animals. (C”) Quantification of the mean post-stimulus ΔF/F0 for lacZ control and lacZ +TTX animals. Mean post-stimulus ΔF/F0: lacZ Control +ATR, 0.62 ± 0.28, n = 28 A08a pairs, from 10 animals (Data reproduced from Figure 5D); lacZ control -ATR, −0.0172 ± 0.07, n = 11 A08a pairs, from five animals (Data reproduced from Figure 5D); lacZ control +TTX + ATR, 1.48 ± 0.70, n = 20 A08a pairs, from nine animals; lacZ control +TTX ATR, 0.019 ± 0.055, n = 9 A08a pairs, from four animals. (D–D”) Lateralized dbd has excitatory, monosynaptic connection to A08a lateral dendritic arbor. (D) GCaMP6m ΔF/F0 traces from A08a pairs after activation of lateralized dbd in the presence of TTX. (D’) Average A08a GCaMP6m ΔF/F0 traces in the presence of 3 μM TTX, before and after Chrimson activation (pink bar) of dbd neurons. Solid black lines represent the mean ΔF/F0. Shaded regions represent the standard deviation from the mean. +ATR, n = 17 A08a pairs, from 14 animals; -ATR, n = 5 A08a pairs, from four animals. (D”) Quantification of the mean post-stimulus ΔF/F0 for Unc-5 and Unc-5 +TTX animals. Mean post-stimulus ΔF/F0: Unc-5 +ATR, 0.68 ± 0.24, n = 6 A08a pairs, from five animals (Data reproduced from Figure 5D); Unc-5 -ATR, −0.035 ± 0.02, n = 4 A08a pairs, from three animals (Data reproduced from Figure 5D); Unc-5 +TTX +ATR, 2.00 ± 0.76, n = 17 A08a pairs, from 14 animals; Unc-5 +TTX ATR, 0.023 ± 0.03, n = 5 A08a pairs, from four animals. Significance between two groups was determined using a Mann-Whitney test.
Videos
dbd and A08a neurons can be visualized with light microscopy.
Synaptic partners dbd (magenta) and A08a (green) can be genetically labeled (165(dbd)-Gal4 > UAS-myr::smGdP::HA and 26 F05(A08a)-LexA > LexAop-myr::smGdP::V5 respectively).
Functional connectivity between dbd and A08a (lacZ control).
Top: +ATR. A08a in WT controls exhibits stimulus-evoked changes in fluorescence. Video shows A08a axon terminals in a fictive brain preparation, anterior to the left. Bottom: -ATR. A08a does not exhibit stimulus-evoked changes in fluorescence in the absence of ATR. ‘ON’ indicates presentation of 561 nm light stimulus. Frames acquired at 4 frames/s, displayed at 0.5x speed.
Functional connectivity between lateralized dbd and A08a (unc-5).
Top: +ATR. A08a exhibits stimulus-evoked changes in fluorescence to lateralized dbd. Video shows A08a axon terminals in a fictive brain preparation, anterior to the left. Bottom: -ATR. A08a does not exhibit stimulus-evoked changes in fluorescence in the absence of ATR. ‘ON’ indicates presentation of 561 nm light stimulus. White arrows indicate segments confirmed to have fully lateralized dbd’s in both left and right hemisegments. Frames acquired at 4 frames/s, displayed at 0.5x speed.
Spontaneous A08a rhythmic activity (lacZ control, -TTX, -ATR).
A08a exhibits spontaneous rhythmic activity in the absence of TTX. Video shows A08a axon terminals in a fictive brain preparation, anterior to the left. Video was acquired at two frames/second and recorded over 5 min. Video is displayed at 2x speed.
TTX abolishes spontaneous A08a rhythmic activity (lacZ control,+TTX, -ATR).
A08a spontaneous rhythmic activity is eliminated in the presence of 3 µM TTX. Video shows A08a axon terminals in a fictive brain preparation, anterior to the left. Video was acquired at two frames/second and recorded over 5 min. Video is displayed at 2x speed.
Tables
Summary of inputs to A08a medial and lateral dendritic arbors from the first instar larval EM reconstruction.
Neurons with the most synapses to A08a medial and lateral arbors shown. Neurons with fewer synapses also show specificity for medial or lateral dendritic arbors.
| A08a inputs (hemisegment) | Pre-synapse number | A08a arbor targeted | |
|---|---|---|---|
| Total | With A08a | ||
| dbd (A1L) | 79 | 10 | Medial only |
| dbd (A1R) | 78 | 13 | Medial only |
| A02d (A1L) | 66 | 22 | Medial only |
| A02d (A1R) | 63 | 8 | Medial only |
| A02l (A1L) | 38 | 12 | Lateral only |
| A02l (A1R) | 31 | 4 | Lateral only |
| A31x (A1L) | 19 | 3 | Lateral only |
| A31x (A1R) | 26 | 9 | Lateral only |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Species (Drosophila melanogaster) | 26F05-LexA | BDSC | 54702 | Expressed in A08a neurons |
| Species (D. melanogaster) | 26F05-Gal4 | BDSC | 49192 | Expressed in A08a neurons |
| Species (D. melanogaster) | 165-Gal4 | W. Grueber | N/A | Expressed in dbd neurons |
| Species (D. melanogaster) | UAS-LacZ | BDSC | 8529 | Control transgene |
| Species (D. melanogaster) | UAS-LacZ | BDSC | 8530 | Control transgene |
| Species (D. melanogaster) | UAS-unc-5::HA | B. Dickson | N/A | UAS drives unc-5 |
| Species (D. melanogaster) | UAS-robo-2::HA | BDSC | 66886 | UAS drives robo-2 |
| Species (D. melanogaster) | UAS-bruchpilot(short)-mstrawberry | S. Sigrist | N/A | UAS drives fluorescently labeled truncated bruchpilot |
| Species (D. melanogaster) | 10xUAS-IVS-myr::smGdP::HA, 13xLexAop2-IVS-myr::smGdP::V5 | BDSC | 64092 | UAS drives HA membrane tag, LexAop drives V5 membrane tag |
| Species (D. melanogaster) | UAS-MCFO | BDSC | 64090 | UAS drives multi-colored-flip-out |
| Species (D. melanogaster) | UAS-DenMark, UAS-syt.eGFP | BDSC | 33064 | UAS drives DenMark, UAS drives synaptotagmin::GFP |
| Species (D. melanogaster) | 13XLexAop2-IVS-p10-GCaMP6m, 20xUAS-CsChrimson-mCherry | V. Jayaraman | N/A | LexAop drives GCamp6m, UAS drives Chrimson |
| Antibody, monoclonal | Mouse anti-V5 | Invitrogen, Carlsbad, CA, | Cat. R96025, Lot 1949337 | (1:1000) |
| Antibody, polyclonal | Rabbit anti-mCherry | Novus Biologicals, Littleton, CO | Cat. NBP2-25157, Lot 102816 | (1:500) |
| Antibody, monoclonal | Rat anti-HA | Roche Holding, AG, Basel, Switzerland | Cat. 11867423001, Lot 27573500 | (1:100, after suggested dilution) |
| Antibody, monoclonal | Rat anti-OLLASDyLight-650conjugated antibody | Novus Biologicals, Littleton, CO | Cat. NBP1-06713C, Lot F-090517c | (1:100) |
| Antibody, polyclonal | Chicken anti GFP | Aves Labs, Inc, Tigard, OR | Cat. GFP-1020, Lot. GFP697986 | (1:1000) |
| Antibody, polyclonal | Rabbit anti-mCherry | Novus Biologicals, Littleton, CO | Cat. NBP2-25157, Lot 102816 | (1:500) |
| Antibody, secondary | Alexa Fluor 488 AffiniPure Donkey Anti-Mouse IgG (H + L) | Jackson ImmunoResearch, West Grove, PA | Cat. 715-545-151 | (1:400) |
| Antibody, secondary | Rhodamine RedTM-X (RRX) AffiniPure Donkey Anti-Rabbit IgG (H + L) | Jackson ImmunoResearch, West Grove, PA | Cat. 711-295-152 | (1:400) |
| Antibody, secondary | Alexa Fluor 647 AffiniPure Donkey Anti-Rat IgG (H + L) | Jackson ImmunoResearch, West Grove, PA | Cat. 712-605-153 | (1:400) |
| Antibody, secondary | Alexa Fluor 488 AffiniPure Donkey Anti-Chicken IgY (IgG) (H + L) | Jackson ImmunoResearch, West Grove, PA | Cat. 703-545-155 | (1:400) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.43478.022





