Context-dependent signaling of coincident auditory and visual events in primary visual cortex
Figures
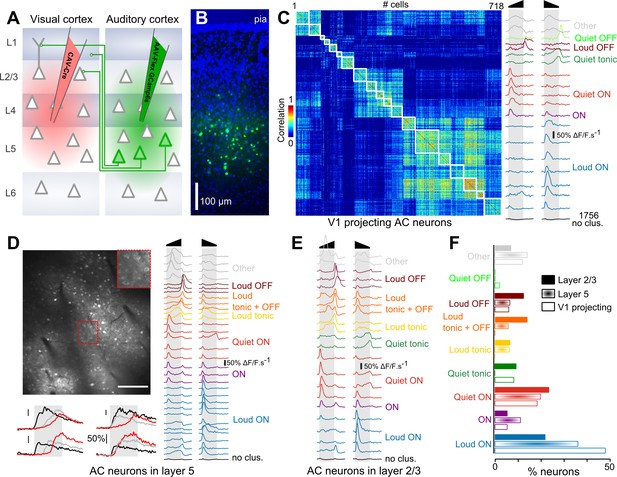
Loud onset responses dominate in V1-projecting neurons.
(A) Viral expression strategy for GCAMP6s labeling of V1-projecting neurons in AC. (B) Epifluorescence image of an AC histological section (blue = DAPI, green = GCAMP6 s) showing V1-projecting neurons (cortical layers are matched to A). (C) Matrix of sound response correlation for all cells that could be assigned to a cluster for auditory cortex neurons that project to V1. The average response traces of each cluster (mean deconvolved calcium signal across cells) are shown on the right with a color code corresponding to the functional label given to the cluster. The bottom trace (black) shows the average response for the cells that were not assigned to a cluster (non- or weakly-responsive cells). (D) Top: Example of a 1 × 0.8 mm imaging field of view showing GCAMP6s-expressing neurons in upper layer 5 of auditory cortex (depth: 400 µm) with a magnification in the top-right inset. Bottom: Example raw calcium traces for four neurons during sound presentation. Red, black and gray traces are different samples for the same neuron. Scale bars 50% ΔF/F. Right: Average response traces of each cluster obtained with neurons recorded in upper layer 5 (370 to 430 µm depth). The color code corresponds to the functional label used in C. The black trace stands for non- or weakly-responsive cells. (E) Average response traces of each cluster obtained with neurons recorded in layer 2/3. (F) Fraction of neurons corresponding to each cluster shown in C-E. The functional cell class distributions are significantly different across the three anatomical cell types (L5, L2/3 or V1-projecting) as assessed together and pairwise across cell type using the χ2 test of independence (p<10−64). The fraction of loud onset cells is significantly different (χ2 test of independence, performed pairwise across anatomical cell types p<10−9).
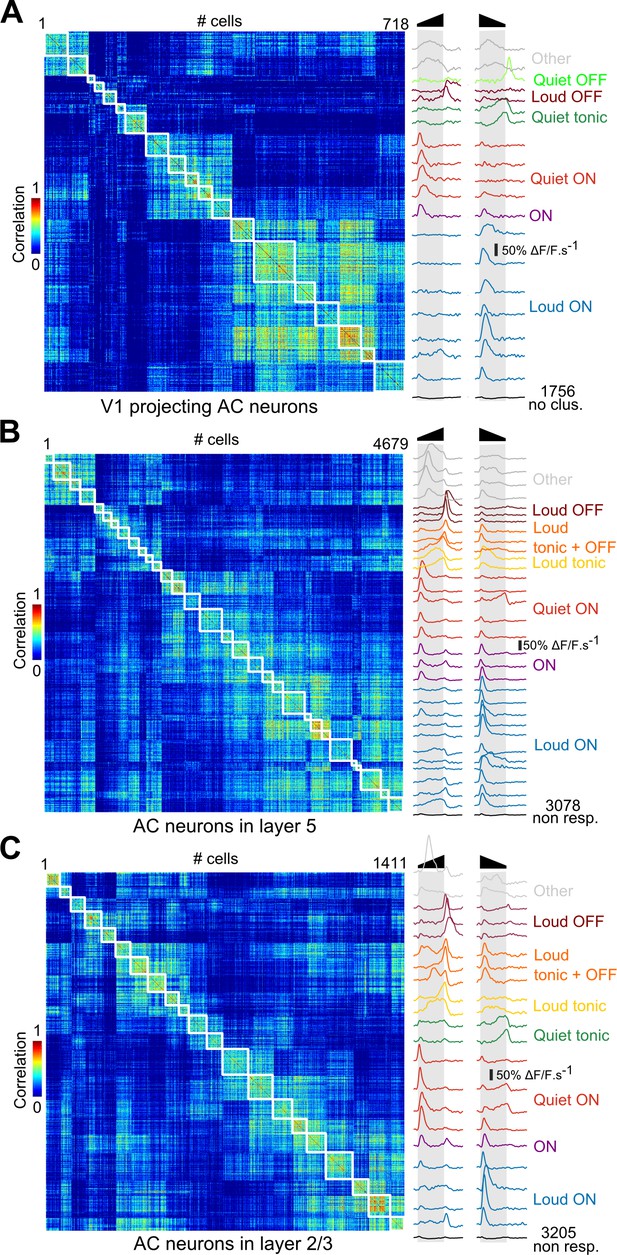
Correlation matrices for all clustered neurons.
Matrix of sound response correlation for all cells that could be assigned to a cluster, (A) for auditory cortex neurons that project to V1 (same as Figure 1C), (B) for auditory cortex neurons in upper layer 5 (370 to 430 µm depth), (C) for auditory cortex neurons in layer 2/3 (150 to 250 µm depth). The average response trace of each cluster (mean deconvolved calcium signal across cells) is shown on the right with a color code corresponding to the functional label given to the cluster. The bottom trace (black) shows the average response for the cells that were not assigned to a cluster (non- or weakly-responsive cells).
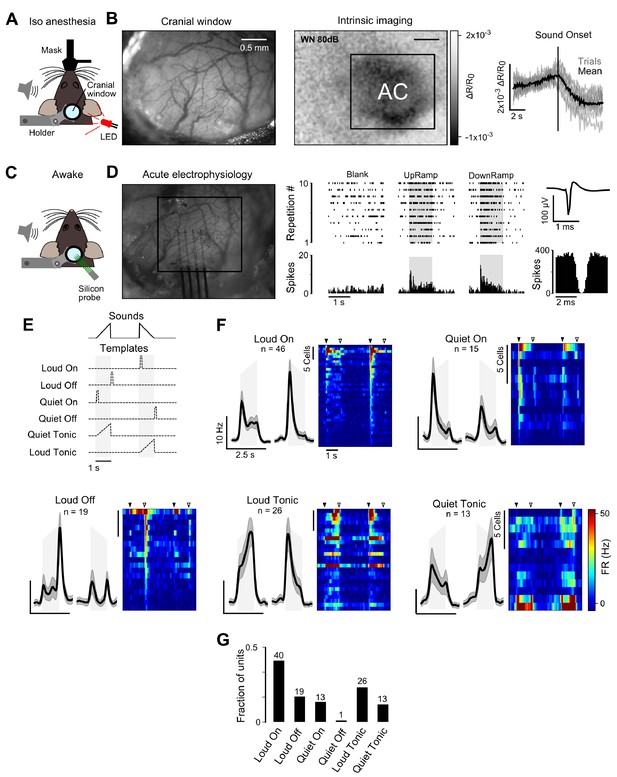
Layer 5 responses to up- and down-ramps recorded using electrophysiology.
(A) Sketch of optical imaging of intrinsic signals experimental setup. (B) Localization of AC by intrinsic imaging. Left, 5 mm cranial window on the right hemisphere of an anesthetized mouse under green LED light. Middle and Right, Mean intrinsic signal response map and mean + single trial response traces for White Noise stimulation (WN) from the mouse shown on the left. Responses traces are derived from the region of interest delineated on the left image. (C) Sketch of silicon probe-based electrophysiology experiments in awake mice. (D) Left: Cranial window of the same animal as in B, after placing the probe into AC. Recordings were usually performed at 400, 600 700 and 800 µm in a single penetration for each animal. Middle: A raster plot and a peristimulus histogram of the responses to up- and down-ramp presentations for a sample single unit. Right: Spike waveform and autocorrelogram of spike train for the same unit. (E) Correlation-based, template matching method to detect the six main response types observed in two-photon calcium imaging (onset, offset, tonic response with a quiet or loud intensity tuning). Because of the small number of cells, only these prominent, simple response types were selected. They capture also some of the composite response types (ON, Loud Tonic + OFF) which are more difficult to capture using a binary model. For each unit (n = 410 isolated units in 4 mice and 15 recording locations), the correlation between its average response histogram and the six templates (represented by dashed lines) was computed. Each cell was assigned to the template to which its response was most correlated provided that the correlation was above 0.4 (112 cells passed this threshold). (F) Mean response profiles smoothed with a Gaussian filter, σ = 30 ms) for the different groups of cells identified by template matching. On the right, the heat map depicts the mean response of each unit to up- (left) and down- (right) ramps. Black and white triangles correspond to sound onsets and offsets respectively. Note that a small fraction of neurons classified as Loud or Quiet ON are equally responsive for both types of onset, corresponding to the ON cluster label defined for two-photon calcium imaging data. (G) Histogram of the fractions of neurons that were assigned to each of the six templates shown together with the number of neurons in each group. The plot shows that Loud ON responses types are dominant in layer 5, as seen with two-photon calcium imaging (Figure 1F).
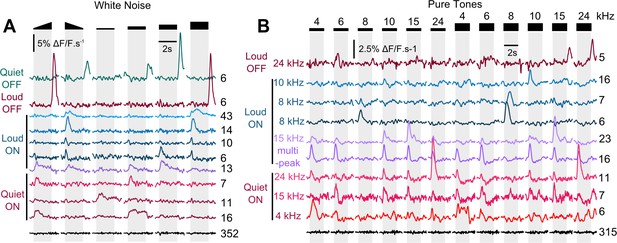
AC neurons projecting to V1 are tuned both to intensity and frequency.
(A) Response profiles to up- and down-ramping white noise sounds (ramping range 60–85 dB SPL) and to 2s-long constant white noise stimuli at 50, 60 70 and 80 dB SPL, for 11 functional clusters of V1-projecting neurons labeled with different colors. Cell count per cluster is shown on the right. Some clusters respond to the same features and are labeled with similar colors (blue colors for Loud onsets, red colors for quiet onsets). The cluster labeled in black corresponds to all neurons that show no response to the stimuli. These clusters are derived from a dataset of 484 neurons, imaged in six sessions in two mice (different from the dataset shown in Figure 1). Gray shading: up or down ramping sound presentation. The cluster responding only to loud or only to quiet onsets or offsets in ramped stimuli display amplitude tuning with the constant stimuli. (B) Response profiles to constant 2s-long pure tones (4, 6, 8, 10, 15, 24 kHz) at 60 (left) and 80 dB SPL (right) for 10 functional clusters of V1-projecting neurons labeled with different colors. Cell count per cluster is shown on the right. We represented clusters responding to the same intensity range and sound epoch (onset or offset) with similar colors (blue colors for Loud onsets, red colors for quiet onsets). The cluster labeled in black corresponds to all neurons that show no response to the stimuli. These clusters are derived from a dataset of 412 neurons imaged in 6 sessions in two mice. This data shows that neurons responding to a particular sound epoch and intensity range can be themselves tuned to different frequencies.
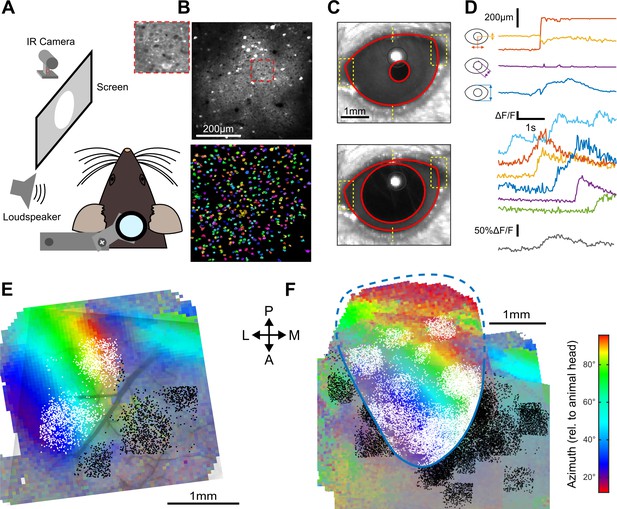
Retinotopically mapped two-photon imaging fields in V1.
(A) Sketch of the experimental setup. (B) (top) Example of a 0.5 × 0.5 imaging field of view showing GCAMP6s-expressing neurons in V1 (top with magnification in inset) and the ROIs that were automatically detected as putative neurons (bottom, see Materials and methods). (C) Examples of eye tracking images. (D) (top) Eye tracking showing a large saccade during a blank trial. The blue, purple, and yellow-red traces indicate apertures between eye lids, pupil diameter and x-y motion of pupil center. (bottom) Examples of raw GCAMP6s traces from individual neurons recorded concomitantly in V1 (each color is a single neuron, frame rate: 31 Hz) and population average (gray) showing saccade-related neuronal activity. (E) Example of a retinotopic map obtained with Fourier intrinsic imaging (see Materials and methods) and segregation between neuron locations inside (white) and outside (black) V1. The color code indicates the azimuth in the visual field. (F) Registered maps across nine mice.
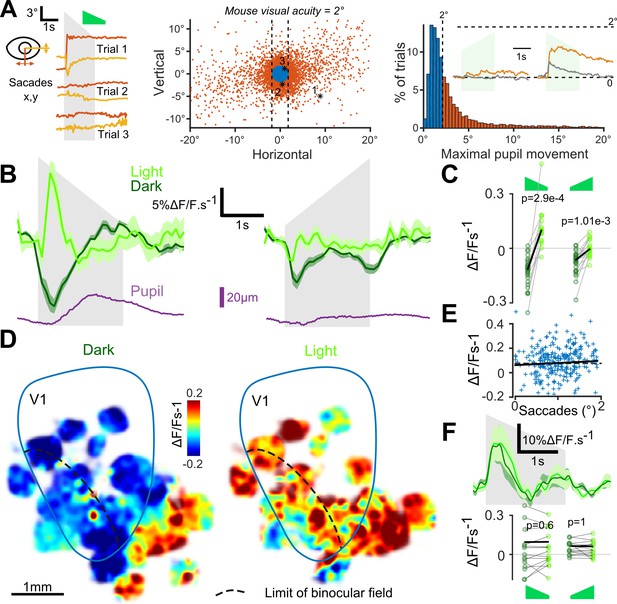
Auditory responses in V1 switch sign in the presence of visual inputs.
(A) (left) Horizontal and vertical pupil movements recorded during three presentations of the down-ramp (gray shading). (center and right) Distribution of saccades across all mice (n = 7) and trials. The blue portion indicates trials where pupil movement did not exceed visual acuity in the mouse (2° of visual angle: 84% of all trials). The trials with larger eye movements (red) were removed from the analysis (trial filtering). Inset: average saccade responses to up-ramps and down-ramps, before (orange) and after (gray) trial filtering. (B) Averaged deconvolved calcium traces of V1 neurons in the light (light green) and in the dark (dark green) (6207 neurons, n = 17 sessions in seven mice). The purple line is the average pupil diameter. Left: Down ramp responses, Right: Up ramp responses. Up- and down ramping gray shadings indicate the timing of up- and down ramping sounds. (C) Average V1 responses are larger in the light than in the dark (n = 17 recording sessions in seven mice, Wilcoxon signed rank test). (D) Smoothed maps (Gaussian filter, σ = 320 µm) of the local responses to the down-ramping sound, averaged across sessions and animals after registration with respect to the retinotopic map. (E) Single trial saccade amplitudes (below the 2° visual acuity threshold) do not correlate with the amplitude of V1 population responses to sounds (Pearson correlation coefficient 0.05, p=0.42). (F) (top) Mean deconvolved signal for 2474 V1 projecting cells recorded in AC in light and in dark conditions. (bottom) Average onset response (0 to 500 ms) to up- and down-ramps for each recording session, showing no significant difference between light and dark conditions (Wilcoxon sign test, p=0.6 and p=1, n = 15 sessions).
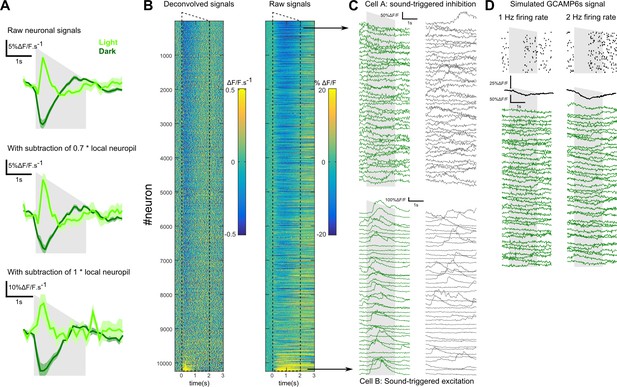
Robust inhibition in response to sounds in V1.
(A) Mean V1 population responses to a down-ramping sound in lit and dark conditions measured without neuropil subtraction (top), or with the conventional neuropil subtraction (factor 0.7 applied to neuropil signal, middle) or with a larger correction (factor 1 applied to neuropil signal, bottom). In all cases, a clear decrease in fluorescence is observed in the dark. (B) Heat map showing the deconvolved and raw calcium signal responses to the down-ramp for all recorded V1 neurons. Inhibition is observed robustly across cells. (C) Single-trial, raw calcium signal responses to a down-ramp (left) and spontaneous activity (right) for a cell inhibited (top) and another excited by the sound (bottom). The inhibition corresponds to both a decrease in fluorescence and a decrease in the occurrence of spontaneous positive calcium events. (D) Simulations of GCAMP6s ΔF/F signal for Poisson spike trains at 1 Hz and 2 Hz (see on top raster plots for 40 instantiations), with a 1 s period where all spikes are removed (inhibition). In the simulation, each spike produced a transient of peak amplitude ΔF/F = 10% and modeled as a difference of exponentials with rise and decay time constants of 70 ms and 1870 ms respectively. All parameters are derived from Deneux et al. (2016a). All transients were summed (convolution of the spike train with the single spike kernel) and Gaussian white noise (5% standard deviation) was added to produce the simulated calcium traces. The plots, for the 40 spike trains shown on the top, show that a 1 s firing pause during a low Poisson firing rate yielded trial-averaged (black trace) and single-trial traces highly compatible with inhibition traces observed in vivo, for example in C. Thus, inhibitory responses observed in calcium signals from V1 neurons could result from the downward modulation of a low, basal firing rate (of the order of 1 Hz) in each cell commonly observed in awake cortex (Hromádka et al., 2008).
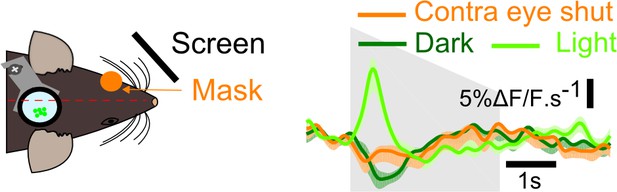
Mean V1 responses (2226 neurons, n = 13 sessions in two additional mice) to a down-ramping sound (gray shading) in the dark, in the light and with the contralateral eye reversibly occluded as depicted in the sketch on the left.
This suggests that the absence of visual inputs to the visual hemifield corresponding to the imaged V1 area is sufficient to obtain sound-induced inhibition.
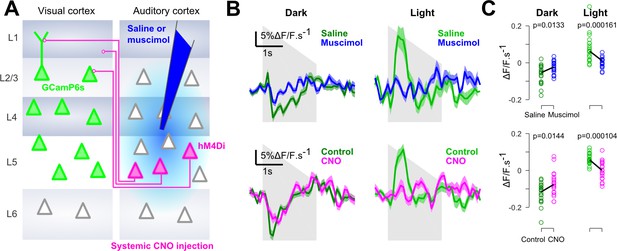
Auditory responses in V1 are caused by direct AC projections.
(A) Schematics of the AC inactivation experiment (blue) and of the chemogenetic inactivation experiment in which V1-projecting neurons in AC are specifically silenced (magenta). (B) (top) Mean deconvolved calcium signal of V1 neurons in dark (dark green) or in light (light green) during saline injections (n = 765 neurons in four experiments and three mice) or muscimol inactivation of AC (n = 825 neurons in five experiments, same three mice). (bottom) Same as top graphs but for chemogenetic inactivation of V1-projecting neurons in AC (same n = 743 neurons before and after CNO activation, in three experiments and two mice). Shading indicates SEM. (C) Mean deconvolved calcium signal from the population responses shown in B for the saline (green) versus muscimol (blue) conditions and for Control (green) versus CNO (magenta) conditions (computed from 200 to 500 ms after sound onset for the negative responses in the dark, and from 200 to 1000 ms for the positive response in the light). The p-values are derived from a two-sided Wilcoxon sign test (n = 40 stimulus repetitions).
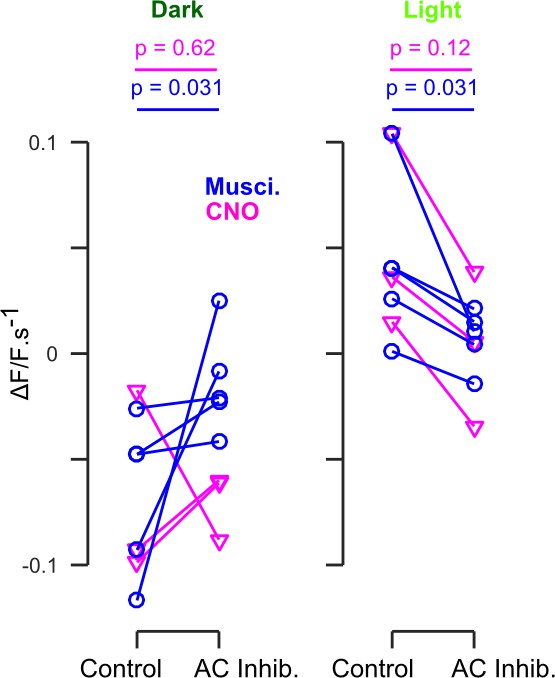
Distribution of AC inactivation effects across experiments for the data shown in Figure 4B and C.
p-values indicate the result of a one-sided Wilcoxon signed-rank test for chemogenetic (n = 3, magenta) and muscimol (n = 5, blue) experiments.
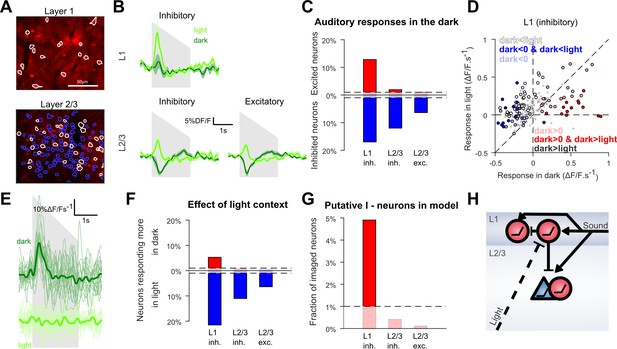
Context-dependent inhibition by sound is mediated by a sub-population of L1 interneurons.
(A) In vivo two-photon images of GAD2-positive V1 neurons in L1 and L2/3 expressing td-Tomato. Superimposed are the contours of the active regions of interest identified by GCAMP6s imaging, blue: putative pyramidal cells, white: putative GABAergic cells. (B) Mean responses to the down-ramping sound (gray shading) for inhibitory and excitatory neurons in L2/3 and L1. (C) Fraction of neurons significantly excited (red) or inhibited (blue) by sounds for each layer and cell type (Wilcoxon rank sum test, p<0.01: the dashed lines indicate chance level). (D) Scatter plot of the mean responses of all L1 inhibitory neurons to the down-ramping sound in the light against in the dark. Small gray dots indicate neurons that do not significantly respond in any condition. Larger, colored dots indicate significantly responding neurons. Dark blue indicates neurons significantly inhibited in the dark and responding more in light than in dark. Dark red dots indicate neurons significantly activated in the dark and responding more in dark than in light. Neurons responding equally in dark and light are marked with light blue (negative response) and light red (positive response) dots. Neurons that are non-responsive in the dark but respond more or less in the light are marked in white and dark, respectively. (E) Single trial deconvolved traces for an L1 interneuron responding to a down-ramping sound (gray shading) in the dark and inactive in the light. (F) Fractions of neurons responding significantly more in light than in dark (blue) or more in dark than in light (red) (Wilcoxon rank sum test, p<0.01). (G) Fraction of neurons that can mediate context-dependent inhibition in each layer that is interneurons significantly excited by sounds in the dark and responding significantly less in the light than in the dark. (H) Simplified schematic of the proposed mechanism for context-dependent auditory responses in V1. Cells unaffected by visual context are not displayed.
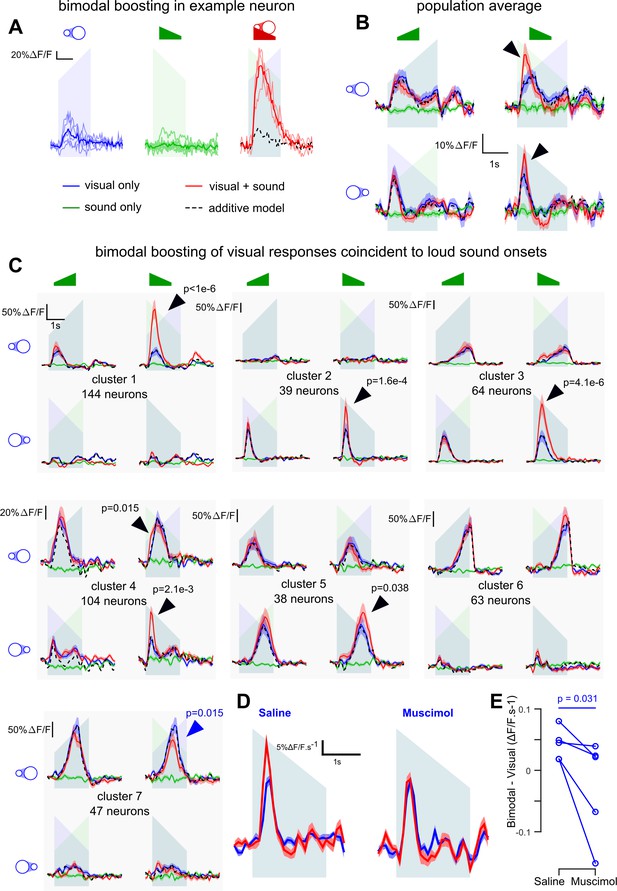
V1 neurons are boosted by the coincidence of loud auditory onsets with visual onsets.
(A) Sample neuron in V1. Raw GCAMP6s traces for a visual stimulus (blue), a sound (green) and a combination of both (red) show a large neuronal response for the bimodal condition compared to the additive prediction (dashed black line) based on unimodal responses. Individual trials (thin lines) and their average (thick lines) show that the multisensory responses are robust for this neuron. (B) Average deconvolved calcium responses (mean ±sem) in V1 for up- and down-ramping sounds (green), looming and receding visual stimuli (blue) and the four bimodal combinations. Black dashed lines: linear prediction. (C) Uni- and bimodal responses, displayed as in B., of the seven different functional cell types identified by hierarchical clustering. (D) Average deconvolved responses to the receding disk in absence (blue) and presence (red) of a simultaneous down-ramping sound, upon saline injection in AC (left, n = 765 neurons in four experiments and three mice) and upon muscimol inactivation of AC (right, n = 825 neurons in five experiments, same three mice). (E) Distribution of AC inactivation effects across experiments for the data shown in D, one-sided Wilcoxon signed-rank test (n = 5). Throughout the figure the ramping green shadings mark the presentation of up- or down-ramping sounds, and the ramping blue shadings the presentation of looming and receding visual stimuli.
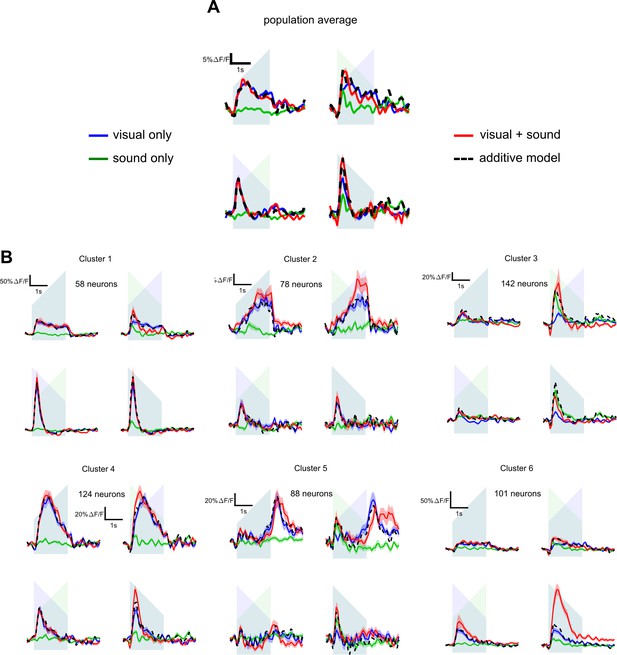
Bimodal responses in the area anteromedial to V1.
(A) Population responses (3076 neurons, nine sessions, four mice) to the four unimodal stimuli and four bimodal stimuli in the area anteromedial to V1 (see Figure 3D). Down-ramping sounds alone elicit strong onset responses which at the population level sum linearly with visual responses. (B) Result of the clustering of uni- and multimodal responses in the anteromedial area. Six clusters were identified, five of which integrate mostly linearly auditory and visual responses and only one (bottom right) shows non-linear auditory-visual responses. Interestingly also, one cluster shows auditory responses that are stronger than visual responses (top right), corroborating the idea that auditory input to the associative region anteromedial to V1 has also a driving role and not only a modulatory role as we observe in V1.
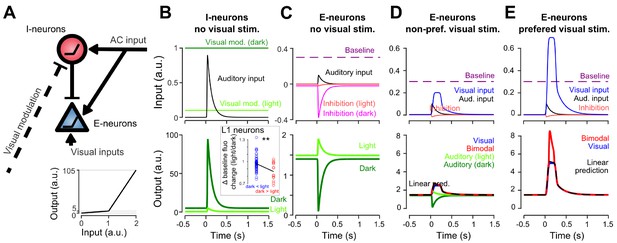
Non-linear inhibitory and excitatory neurons reproduce gated-inhibition and bimodal boosting.
(A) Sketch of a minimal model for the switch between negative and positive sound responses in dark vs in light and boost of visual responses to sounds. An inhibitory (I-neurons) population endowed with a non-linear response function (bottom) receives a positive auditory input and a negative visual input. When both inputs are active (lit condition), the auditory drive to I-neurons is below threshold and no inhibition is delivered to the excitatory non-linear neuronal population (E-neurons). In the dark condition, the visual modulation is reduced releasing sound triggered inhibition. Both I- and E-neurons are non-linear with a low and a high output gain. Auditory and visual inputs are typically converted with a low-gain, while coincident auditory and preferred-visual inputs pass the threshold and yield a high-gain output (boosting). (B) Input currents and output response of I-neurons during auditory simulation in light and dark conditions. Inset: Change in baseline fluorescence between light and dark conditions in L1 interneurons. A significant baseline decrease in light is observed for interneurons that are more sound responsive in dark than in light (i.e. putative I-neurons, red) as compared to interneurons that are more responsive in the light (Wilcoxon rank sum test, p=0.00989, n1 = 57 ‘dark <light’ cells, n2 = 14 ‘light <dark’ cells). (C) Same for E-neurons. (D) Input currents and output response of E-neurons during visual simulation alone (blue) and bimodal stimulation for a sub-threshold (non-preferred) visual input. Superimposed are the auditory responses in light and dark from panel (C) (E) Same as in D. but for a preferred, supra-threshold visual input.
Additional files
-
Source code 1
Custom analysis scripts.
- https://doi.org/10.7554/eLife.44006.016
-
Transparent reporting form
- https://doi.org/10.7554/eLife.44006.017





