Flavodiiron proteins 1–to-4 function in versatile combinations in O2 photoreduction in cyanobacteria
Figures
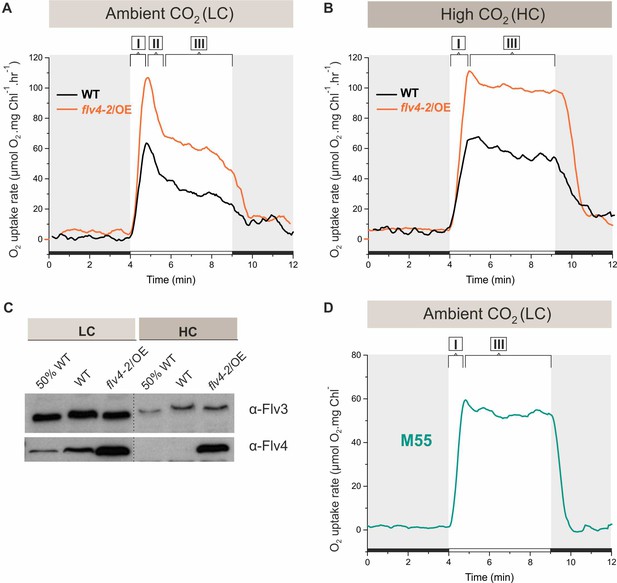
O2 reduction rates and Flv3 and Flv4 protein accumulation in cells grown in low (LC) and high CO2 (HC).
(A, B) O2 reduction rate of WT, flv4-2/OE and (D) the M55 mutant (∆ndhB) was recorded in darkness (gray background) and under illumination (white background). The experiment was conducted in three independent biological replicates and a representative plot is shown. (Figure 1—source data 1). (C) Immunoblot detection of Flv3 and Flv4 in WT and flv4-2/OE. Pre-cultures were grown in BG-11, pH 8.2 under 3% CO2 (HC) for 3 days, after that cells were harvested and resuspended in fresh BG-11, pH 8.2 at OD750 = 0.2. The experimental cultures were grown under HC or under LC. For the MIMS experiments the cells were harvested and resuspended in fresh BG-11, pH 8.2 at 10 µg Chl a mL−1. O2 photoreduction was recorded during the transition from darkness to high-light intensity of 500 µmol photons m−2s−1. In order to create comparable conditions for MIMS measurements, LC-grown cells were supplemented with 1.5 mM NaHCO3 prior to the measurements. Independent experiments performed on WT cells grown in BG-11 lacking Na2CO3, but supplied with 1.5 mM NaHCO3 prior to MIMS measurement showed no significant difference in O2 photoreduction rates (Figure 1—figure supplement 2), thus allowing confident comparison of the MIMS results. Different phases of O2 photoreduction kinetics are indicated as {I}, {II}, {III}. 50% WT, corresponds to 1:2 diluted WT total protein sample.
-
Figure 1—source data 1
O2 reduction rates of WT, flv4-2/OE and M55 mutants grown in different CO2 levels.
- https://doi.org/10.7554/eLife.45766.005
-
Figure 1—source data 2
Oxygen exchange rates of WT and mutant cells.
- https://doi.org/10.7554/eLife.45766.006
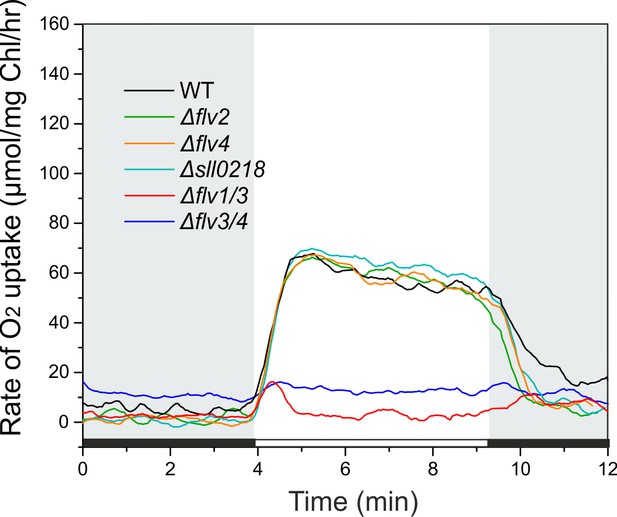
O2 reduction rates under high CO2.
Cells were grown under 3% CO2 (BG-11, pH 8.2), harvested and resuspended in fresh BG-11 at 10 µg Chl a mL−1 . O2 uptake was recorded during the transition from dark to high-light (500 µmol photons m−2 s−1).
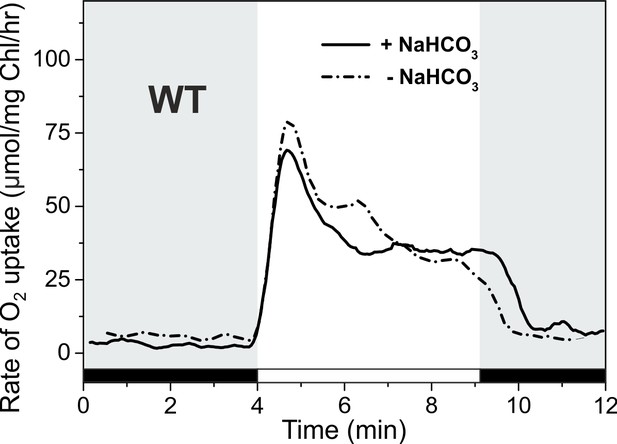
O2 reduction rates during the dark-to-light transition of WT cells with and without addition of 1.5 mM NaHCO3 prior MIMS measurements.
The cells were harvested and inoculated in the fresh BG-11 7.5 without Na2CO3. Prior to MIMS measurement, cells were supplemented with 1.5 mM NaHCO3 (solid line), or measured in the absence of an additional carbon source.
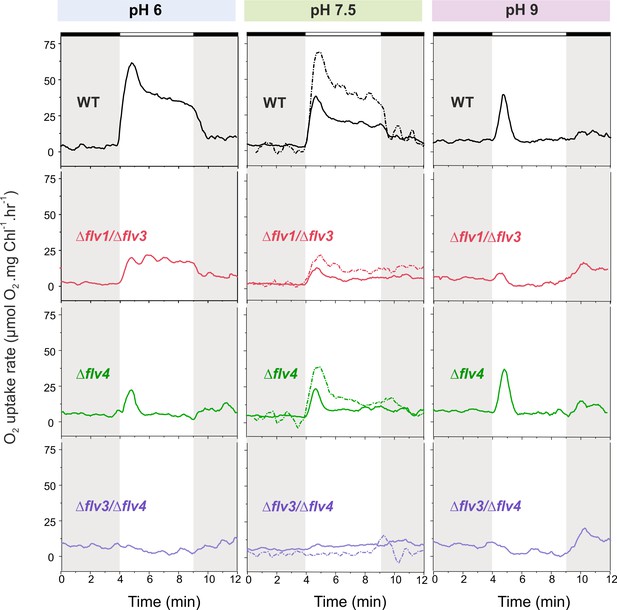
O2 reduction rates of WT and FDP mutants grown at different pH levels.
O2 reduction rate was recorded in darkness (gray background) and under illumination with actinic white light at an intensity of 500 µmol photons m−2 s−1 (white background). Pre-cultures were grown in standard BG-11 medium (containing Na2CO3 at a final concentration of 0.189 mM) under HC for 3 days at different pH levels. For MIMS experiments, cells were shifted to LC at OD750≈0.2 (same pH) and grown for 4 days before measurements. Exceptions were: (i) pH 6 experimental cultures were inoculated from pH 8.2 pre-cultures; and (ii) pH 7.5 pre-culture was shifted to LC in standard BG-11 containing Na2CO3 at a final concentration of 0.189 mM or in BG-11 without Na2CO3 (dotted line ‘- Na2CO3’). The experiment was conducted in three independent biological replicates (except experiment at pH 6 with n = 2 independent biological replicates) and a representative plot is shown. (Figure 2—source data 1). In order to create comparable conditions for MIMS measurements, all cells were supplemented with 1.5 mM NaHCO3 prior to the measurements.
-
Figure 2—source data 1
O2 reduction rates of WT and FDP mutants grown at different pH levels.
- https://doi.org/10.7554/eLife.45766.009
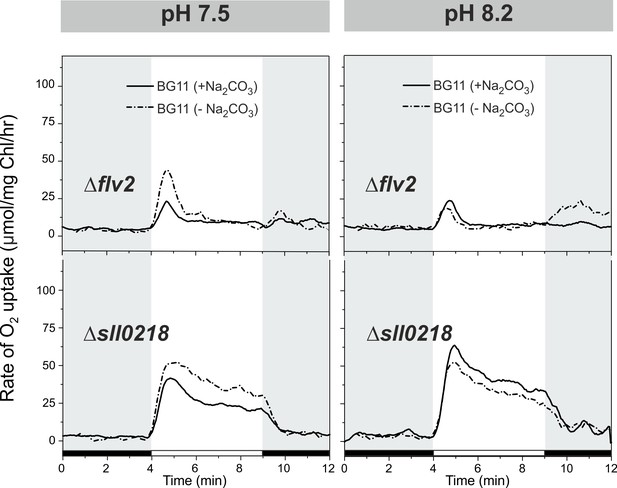
O2 photoreduction rates of the ∆flv2 and ∆sll0218 mutants grown at LC pH 7.5 and 8.2 with and without Na2CO3.
Pre-cultures were grown under HC for 3 days at pH 7.5 or pH 8.2 in BG-11 media with or without Na2CO3. For O2 photoreduction experiments, cells were shifted to LC at OD750≈0.2 and grown for 4 days.
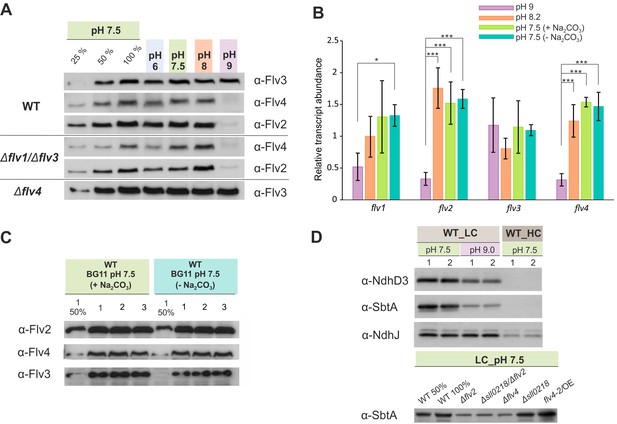
The effect of the pH of growth medium on the protein and transcript accumulation.
(A, B) The effect of the pH and (B, C) sodium carbonate in the growth medium (A, C) on the protein and (B) transcript levels of FDP. (D) Protein immunoblots demonstrating the accumulation of bicarbonate transporter (SbtA) and NDH-1 subunits (NdhD3 and NdhJ) in the cells grown at different pH and CO2 concentration. Cells were pre-grown at different pH levels (+Na2CO3) under HC for 3 days, harvested, resuspended in fresh BG-11 (pH maintained), adjusted to OD750≈0.2 and shifted to LC for 4 days. At pH 7.5, the cells were grown at LC in the presence (+ Na2CO3, at final concentration of 0.189 mM) or in the absence (- Na2CO3) of sodium carbonate (B, C). Transcript abundance is presented as mean ± SD, n = 2–4 biological replicates, asterisks indicate a statistically significant difference to the WT (*p<0.05; ***p<0.001) (Figure 3—source data 1). Numbers 1–3 indicate different biological replicates. 25% and 50% correspond to 1:4, 1:2 diluted total protein sample, and 100% indicates undiluted total protein sample.
-
Figure 3—source data 1
Transcript abundance of flv1, flv2, flv3 and flv4 genes.
- https://doi.org/10.7554/eLife.45766.012
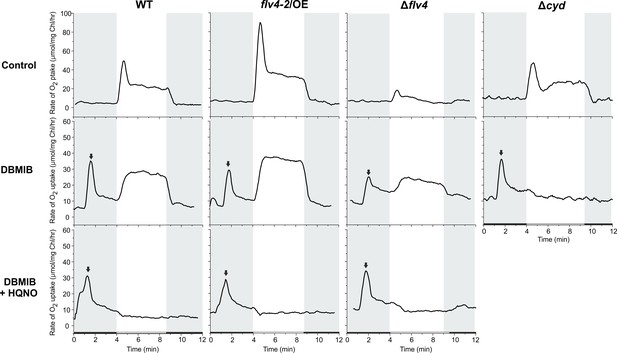
O2 uptake in the WT, flv4-2/OE, ∆flv4 and ∆cyd mutant.
The cells were grown at LC in BG-11 at pH 7.5. 25 µM DBMIB and 50 µM HQNO were added directly to the cuvette immediately prior to MIMS measurement. The arrow indicates the time when inhibitor was added to the sample. The ∆cyd mutant was previously described in Howitt and Vermaas (1998).
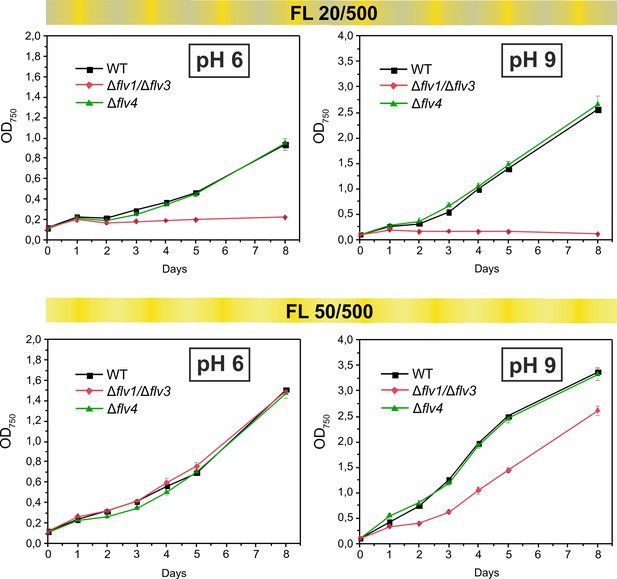
Growth curves of the different FDPs mutants under fluctuating light intensities.
Pre-cultures were grown in BG-11 medium under HC for 3 days illuminated with constant light of 50 µmol photons m−2 s−1. The cells pre-grown at pH 9 or pH 8.2 (for experimental culture at pH 6) were harvested, resuspended in fresh BG-11 (pH 9 or 6), adjusted to OD750 = 0.1 and shifted to LC. Experimental cultures were grown under FL 20/500 or 50/500 regime for 8 days. The experiment was conducted in two independent biological replicates and average values was plotted.
-
Figure 4—source data 1
Growth of the different FDPs mutants under fluctuating light intensities.
- https://doi.org/10.7554/eLife.45766.015
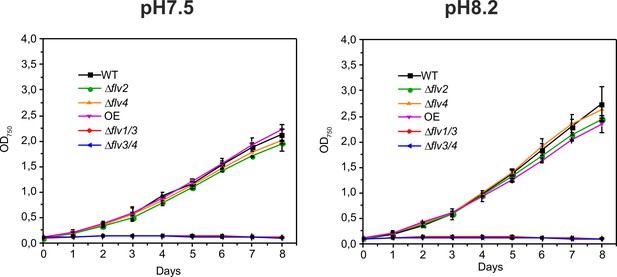
Growth curves of the different FDP mutants under fluctuating light intensities (FL20/500 - 20 μmol photons m−2s−1 background light is interrupted with 30 s of 500 μmol photons m−2s−1 light every 5 min).
Cells were grown in BG-11 (pH 7.5) in the absence of Na2CO3 and shifted from HC to LC at pH 7.5 or pH 8.2.
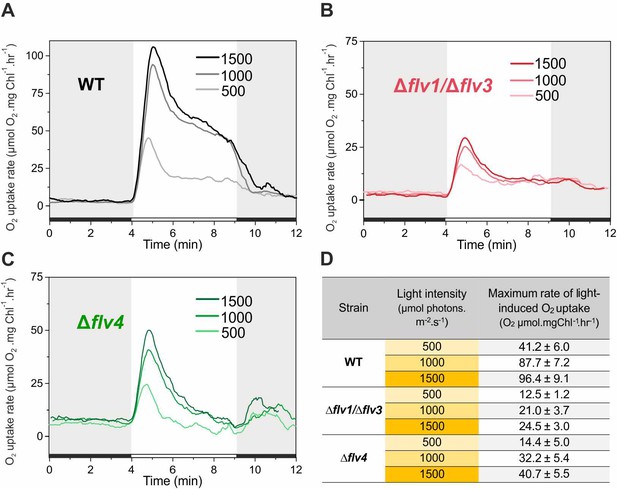
Rates of O2 reduction in response to increasing light intensity in WT, ∆flv1/∆flv3 and ∆flv4 mutant cells (A, B, C, respectively).
O2 reduction rate was recorded in darkness (gray background) and under illumination with actinic white light intensities of 500, 1000 and 1500 µmol photons m−2 s−1 (white background). In order to create comparable conditions for MIMS measurements, all cells were supplemented with 1.5 mM NaHCO3 prior to the measurements. Pre-cultures were grown in BG-11 medium (pH 7.5) under 3% CO2 (HC) for 3 days and then shifted to LC (atmospheric 0.04% CO2 in air) at OD750 = 0.2 and pH 7.5 for 4 days. For MIMS measurements, cells were harvested and resuspended in fresh BG-11 medium at a Chl a concentration of 10 µg mL−1. (D) Maximum rate of light-induced O2 uptake (O2 µmol mgChl a−1 hr−1) of WT, ∆flv1/∆flv3 and ∆flv4 mutant cells at different light intensities applied. The experiment was conducted in three independent biological replicates and a representative plot is shown (Figure 5—source data 1).
-
Figure 5—source data 1
Rates of O2 reduction in response to increasing light intensity in WT, ∆flv1/∆flv3 and ∆flv4 mutant cells.
- https://doi.org/10.7554/eLife.45766.019
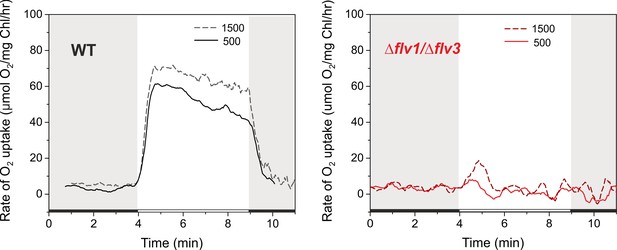
Rates of O2 reduction in response to increasing light intensity in WT and ∆flv1/∆flv3 mutant cells grown under 3% CO2 (HC).
O2 reduction rate was recorded in darkness (gray background) and under illumination with actinic white light intensities of 500 and 1500 µmol photons m−2 s−1 (white background). Cells were grown under 3% CO2 (BG-11, pH 8.2), harvested and resuspended in fresh BG-11 at Chla 10 ug/ml for MIMS measurements.
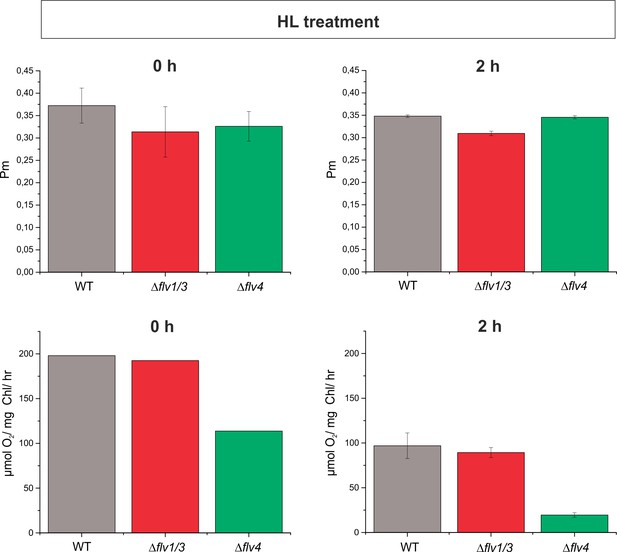
The maximum oxidisable amount of P700 (Pm) and PSII activity of the WT, ∆flv1/∆flv3 and ∆flv4 mutant cells.
Cells were grown in BG-11 (pH 7.5) and shifted from HC to LC for 4 d and illuminated with 50 µmol photons m−2s−1. Prior to the HL treatment, Chl a concentration was set to 10 µgmL-1. Measurements were made in dark-adapted samples after 0 hr and 2 hr of HL treatment (1500 µmol photons m−2 s−1). The PSII oxygen evolving activity was in measured in the presence of 0.5 mM DMBQ under 1000 µmol photons m−2s−1 white illumination. Pm was determined under far red illumination by applying a strong white pulse (5000 µmol photons m−2 s−1). Data are represented as mean of 2 biological replicates (± SD).
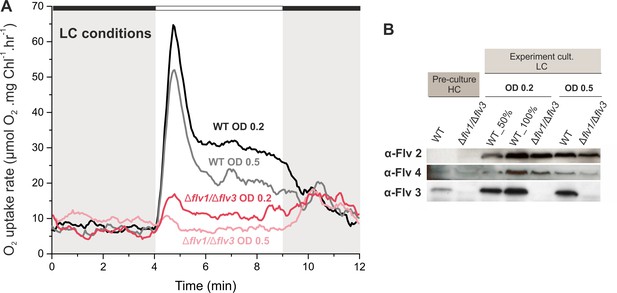
Effect of inoculum size on the O2 photoreduction and accumulation of FDPs in the WT and Δflv1/Δflv3 mutant cells.
(A) Rates of O2 uptake measured by MIMS during darkness (gray background) and under illumination with actinic white light at an intensity of 500 µmol photos m−2s−1 (white background). In order to create comparable conditions for MIMS measurements, all cells were supplemented with 1.5 mM NaHCO3 prior to the measurements. (B) Protein immunoblots showing the relative accumulation of different FDPs in the WT and Δflv1/Δflv3 mutant cells. Pre-cultures were grown in BG-11 (pH 8.2) under HC until late logarithmic phase (OD750≈2.5), then harvested and inoculated in fresh BG-11 under LC at OD750 = 0.2 for 4 days or OD750 = 0.5 for 3 days. The experiment was conducted in three independent biological replicates and a representative plot is shown in (A). WT_50% corresponds to 1:2 diluted total protein sample and 100% to undiluted total protein sample.
-
Figure 6—source data 1
Rates of O2 reduction of WT, ∆flv1/∆flv3 and ∆flv4 mutant cells grown at different inoculum size.
- https://doi.org/10.7554/eLife.45766.021
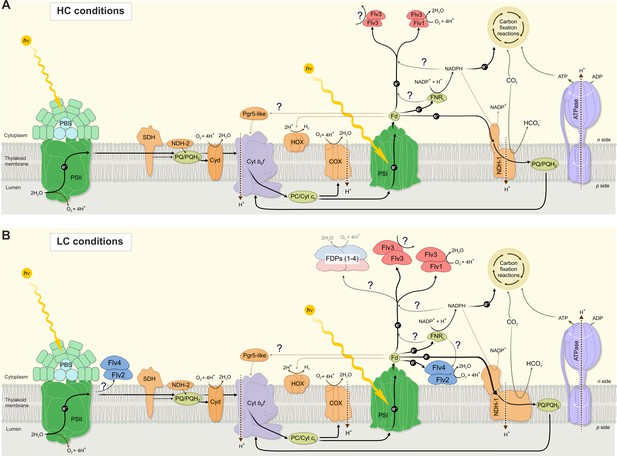
A schematic drawing of photosynthetic light reactions and alternative electron transport routes.
(A) A steady-state Mehler-like reaction in HC is carried out by the low-abundant, yet catalytically efficient Flv1/Flv3 heterodimer. The Flv3/Flv3 homooligomer is involved in photoprotection as an electron valve with unknown acceptor or as a component of a signaling/regulating network (Mustila et al., 2016). (B) In LC-grown cells the two pairs of FDP heterodimers are involved in the Mehler-like reaction: Flv1/Flv3 mainly drives rapid and transient O2 photoreduction and Flv2/Flv4 operates relatively slowly and provides a steady-state background O2 photoreduction. The soluble Flv1/Flv3 heterodimers function as an immediate acceptor of electrons presumable from reduced Fed, whereas association of Flv2/Flv4 with the thylakoid membrane (and/or Flv1/Flv3) is controlled by pmf and Mg2+. Several oligomeric forms of FDPs are hypothesized to exist, including a heterotetramer comprising different FDP protein compositions. The higher abundance of total NDH-1 complexes and FDPs oligomers in LC conditions, compared to HC conditions, is represented by larger size of the protein complexes.
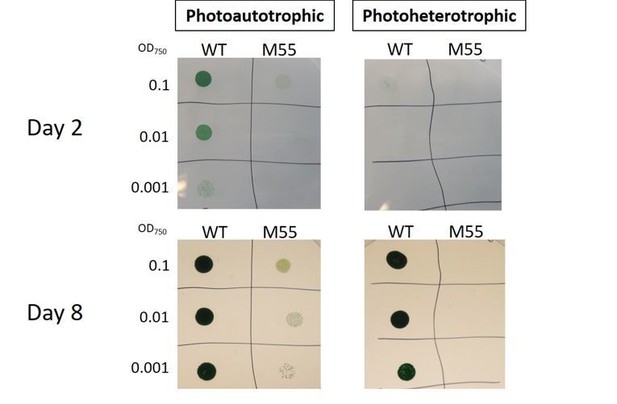
Photoheterotrophic growth of wild-type (WT) and M55 cells on BG-11 agar plates.
The WT and M55 mutant cells of Synechocystis were resuspended in BG-11 median at pH8.2. Three microliters of cell suspensions at OD750nm of 0.1 (top row), 0.01 (middle row), and 0.001 (bottom row) was spotted on agar plates. Five millimolars of Glucose and 10 μm DCMU were added to the plates for photoheterotrophic growth (right side) or were not added for photoautotrophic growth (left side). The plates were grown under ambient air for 8d at 50 μmol photons m-2 S-1.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Synechocystis sp. PCC 6803) | WT, Wild-type | Williams, 1988 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv2 | Zhang et al., 2012 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv4 | Zhang et al., 2012 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv1/∆flv3 | Allahverdiyeva et al., 2011 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆flv3/∆flv4 | Helman et al., 2003 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆sll0218‐flv2 | Helman et al., 2003 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | flv4-2/OE | Bersanini et al., 2014 | ||
| Genetic reagent (Synechocystis sp. PCC 6803) | ∆sll0218 | Bersanini et al., 2017 | ||
| Antibody | α-Flv2 (rabbit polyclonal) | AntiProt, against amino acids 521–535 of Synechocystis Flv2 | (1:500) | |
| Antibody | α-Flv3 (rabbit polyclonal) | AntiProt, against amino acids 377–391 of Synechocystis Flv3 | (1:2000) | |
| Antibody | α-Flv4 (rabbit polyclonal) | AntiProt, against amino acids 412–426 of Synechocystis Flv4 | (1:500) | |
| Antibody | α-NdhD3 (rabbit polyclonal) | Eurogentec, against amino acids 185 to 196 and 346 to 359 of Synechocystis NdhD3 | (1:1000) | |
| Antibody | α-SbtA | Kind gift from T. Ogawa, against amino acids 184 to 203 of Synechocystis SbtA | (1:5000) | |
| Antibody | α-NdhJ | Kind gift from J. Appel | (1:1000) | |
| Antibody | Secondary antibody, Amersham ECL Rabbit IgG, HRP-linked F(ab')₂ fragment (from donkey) | GE Healthcare | NA9340-1ML | (1:10000) |
| Commercial assay or kit | Amersham ECL Western Blotting Detection Reagent | GE Healthcare | RPN2209 | |
| Commercial assay or kit | iScript cDNA Synthesis Kit | BioRad, USA | Cat. #170–8891 | |
| Commercial assay or kit | iQ SYBR Green Supermix | BioRad, USA | Cat. #170–8882 | |
| Software, algorithm | qbase + software | Biogazelle, Zwijnaarde, Belgium - www.qbaseplus.com |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.45766.023





