TMEM16B regulates anxiety-related behavior and GABAergic neuronal signaling in the central lateral amygdala
Figures
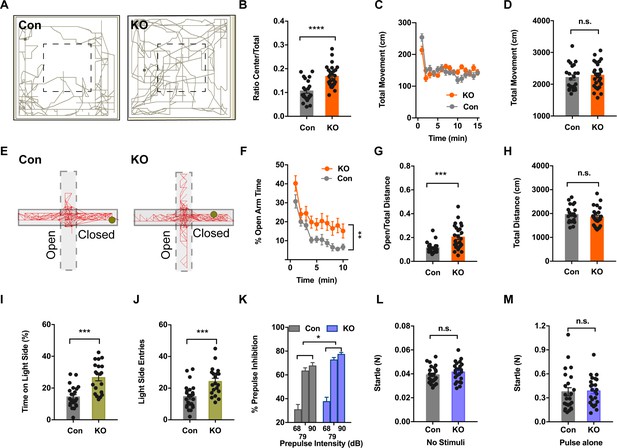
Ano2 Mutant Mice Display Reduced Anxiety-related Behavior and Impaired Pre-pulse Inhibition.
(A) Representative traces of Ano2 KO mice and control (Con) littermates in open field. (B–D) Ano2 mutant mice display reduced anxiety-related behavior in the open field test, shown as time spent at the center normalized by total time (B). Overall exploratory behavior in the open-field test, shown as distance traveled per minute as function of time (C) and total distance traveled (D), is comparable between mutant and control mice. Two-tail unpaired t test in (B), t = 4.744, df = 46.7, p<0.0001. Two-way ANOVA in (C), F (1,47)=0.04778, p=0.8279. Two-tail unpaired t test in (D), t = 0.5721, df = 47, p=0.5700, n = 26 for KO and n = 23 for control (number of mice tested) for (B–D). (E) Representative elevated plus maze (EPM) tracks of control and Ano2 KO mice. (F–H) Ano2 mutant mice display reduced anxiety-related behavior in the EPM test, plotted as time spent in the open arms as function of time (F) and distance traveled in open arms normalized by the total distance traveled (G), with the total distance traveled in the elevated plus maze (H) as control. Two-way ANOVA in (F), F (1, 47)=9.067, p=0.0042. Mann Whitney test in (G), U = 120, p=0.0002. Two-tail unpaired t test in (H), t = 0.9944 df=46, p=0.3252, n = 26 for KO and n = 23 for control (number of mice tested) for F–H. (I and J) Ano2 mutant mice show reduced anxiety-related behavior in the light-dark box test, shown as the percentage of total time spent in the bright compartment (I) and number of entries to the bright compartments (J) over a 10 min period. Two-tail unpaired t test in (I), t = 4.804, df = 32.94, p<0.0001. Two-tail unpaired t test in (J), t = 3.79, df = 37.77, p=0.0005, n = 20 for KO and n = 24 for control for I and J. (K–M) Ano2 mutant mice show reduced pre-pulse inhibition (PPI) of startle responses induced by pre-pulses at 68 dB, 79 dB and 90 dB(K). Startle responses to no stimuli (L) and startle response to 120 dB stimuli without pre-pulse (M) are comparable between mutant and control mice. Two-way ANOVA in (K). F (1, 46)=4.612, p=0.0370. Two-tail unpaired t test in (L), t = 1.119, df = 46, p=0.2688. Two-tail unpaired t test in (M), t = 0.1889, df = 46, p=0.8510, n = 24 for KO and n = 24 for control (number of mice tested) for K–M. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
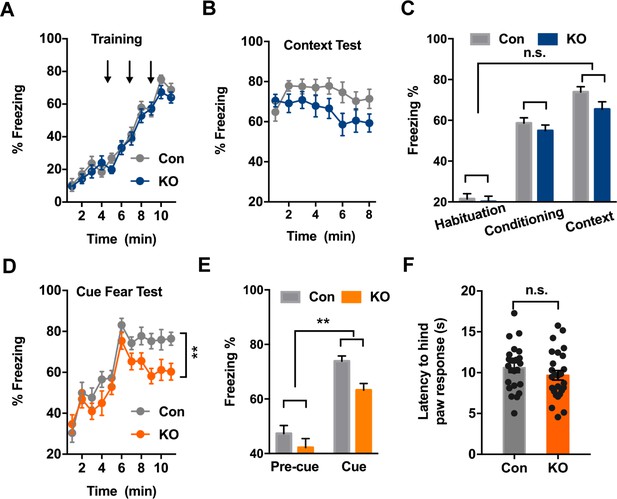
Ano2 Knockout Mice Exhibit Reduced Context-independent Cued Fear Expression.
(A) Ano2 KO and control mice exhibited comparable freezing levels during fear acquisition. After habituation for 5 min, freezing levels during fear training with three 30 s 80 dB tones that each was terminated at the same time as a 2 s, 0.45 mA foot shock, separated by 120 s interval for monitoring. Two-way ANOVA in (A), F (1, 46)=0.7443, p=0.3928. (B) Context-dependent fear recall tested 24 hr after fear conditioning. Mice were placed in the fear conditioning apparatus for 8 min. No shock was presented. Two-way ANOVA in (B), F (1, 46)=3.601, p=0.0640. (C) Ano2 KO and control mice exhibited comparable context-dependent fear expression, shown as averaged freezing levels during habituation, conditioning and context test. Two-way ANOVA in (C), F (1, 46)=2.173, p=0.1472. (D and E) The context-independent cue fear test took place 5–24 hr after the context-dependent test in (B). After a 5 min baseline (pre-cue), three 30 s 80 dB tone cues not accompanied with foot shock were presented with 120 s interval for monitoring (cue), in the absence of context cues. Two-way ANOVA in (D), F (1, 46)=9.151, p=0.004. Two-way ANOVA in (E), F (1, 46)=9.007, p=0.0043. TEMEM16 KO mice displayed lower levels of freezing than control mice during cued fear expression. (F) Pain threshold was comparable between control and Ano2 KO mice.Two tail unpaired t test in (F), t = 1.114, df = 46, p=0.2711, n = 24 for KO and n = 24 for control (number of mice tested for A–F). Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
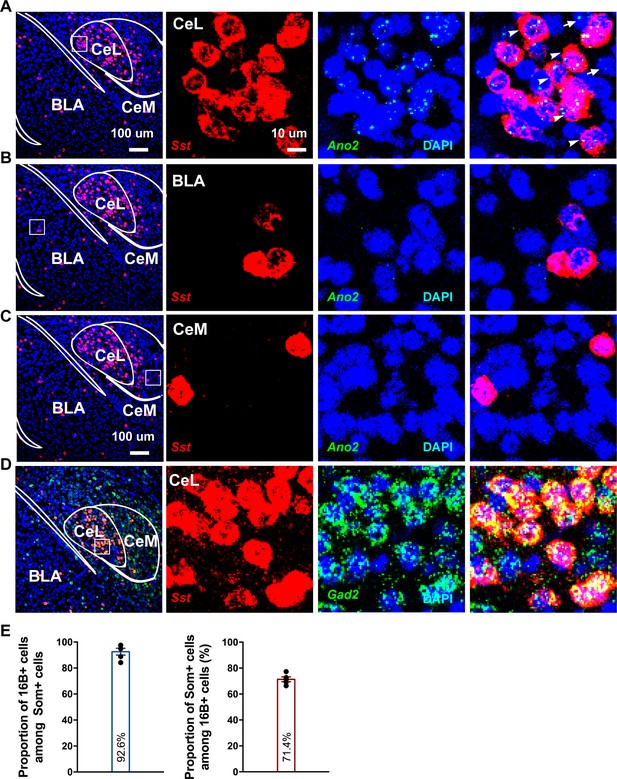
Ano2 mRNA Expression in Somatostatin-positive GABAergic Neurons in the Central Lateral Amygdala.
(A–C) RNA scope studies reveal that Ano2 is expressed in central lateral amygdala (CeL), but not in basal lateral amygdala (BLA) or central medial amygdala (CeM). Representative images of immunofluorescence staining for Sst (red) and RNA scope labeling of Ano2 mRNA (green). Cell nuclei are stained with DAPI (blue). (D) Representative images of double immunofluorescence staining for Gad2 (green) and Sst (red). Cell nuclei are stained with DAPI (blue). (E) Left, percentage of Sst-positive neurons that express Ano2 mRNA. Right, percentage of Ano2 mRNA-positive neurons that express Sst. n = 653 neurons from five mice.
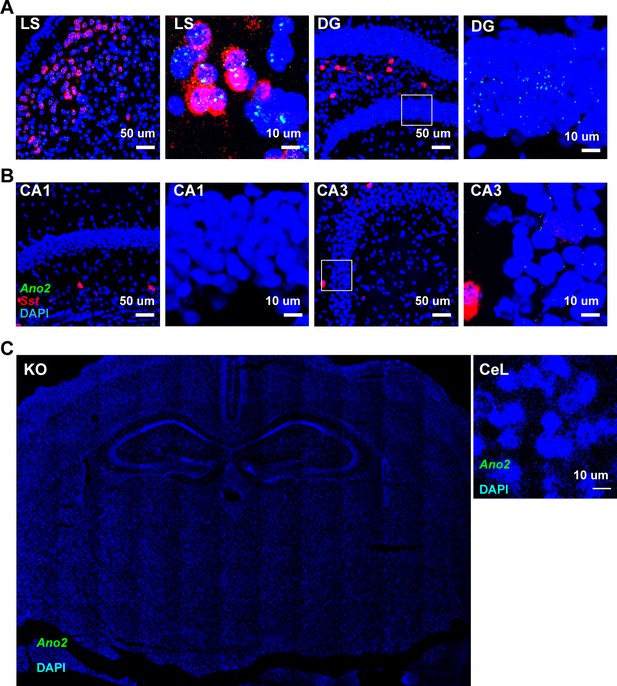
Ano2 mRNA Expression Patterns.
(A and B) RNA scope labeling of Ano2 mRNA in lateral septum, CA1, CA3 and DG. (C) No detectable signals are observed in samples from Ano2 KO mutant mice as specificity control.
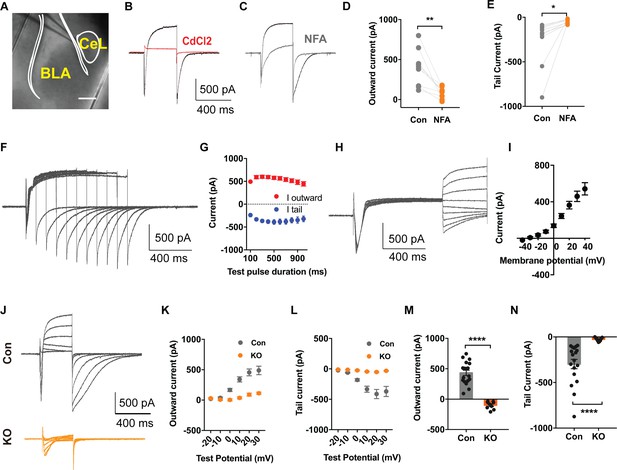
TMEM16B Mediated Ca2+-activated Cl- Currents in Somatostatin-positive Neurons of the Central Lateral Amygdala.
(A) Brain slice of Sst-IRES-Cre and Ai14 reporter mice with fluorescently marked somatostatin-positive (SOM+) neurons in the amygdala. Scale bar, 200 μm. (B and C) Depolarization of SOM+ neurons to +10 mV elicited a Ca2+ current superimposed with a large outward current that developed over time, followed with a tail current upon hyperpolarization to −80 mV. The outward current and tail current can be inhibited by 100 μM niflumic acid (NFA) (C), and it is completely abolished by 200 μM CdCl2, a blocker of voltage-gated Ca2+ channels, indicating that it is induced by Ca2+ influx (B). (D and E) NFA reduced the outward current and tail current. Paired t test in (D), t = 4.392, df = 8, p=0.0023. Paired t test in (E), t = 2.55, df = 8, p=0.0342, n = 9 cells for each group from three mice per group for D and E. (F and G) Summary plot of the peak amplitude of the outward current and tail current as a function of the duration of the test pulse to +10 mV. n = 7 cells from three mice. (H and I) Measurements of reversal potential indicating that the current induced by Ca2+ influx in SOM+ neurons arises from Ca2+-activated Cl- channels (CaCCs). Membrane potential was depolarized to +10 mV to activate Ca2+ channels and then held from −40 mV to +40 mV in 10 mV increments for reversal potential measurement, followed with repolarization to −80 mV. n = 15 cells from four mice. (J–L) Genetic ablation of Ano2 completely eliminated the outward current and tail current in SOM+ neurons, indicating that TMEM16B CaCC mediates the Ca2+-sensitive outward current and tail current in SOM+ neurons. Summary plot of the outward current (K) and the tail current (L) at various membrane potentials. Membrane potential was depolarized from −20 mV to +30 mV in 10 mV increments to activate Ca2+ channels, and then repolarized to −80 mV. (M and N) Comparison of the amplitudes of the outward current and tail current of SOM+ neurons from Ano2 KO mice and control mice. Mann Whitney test in (M), U = 0, p<0.0001. Mann Whitney test in (N), U = 0, p<0.0001. Cells were held at −80 mV. n = 18 cells from 5 KO and n = 9 cells from four control for K, L, M and N. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
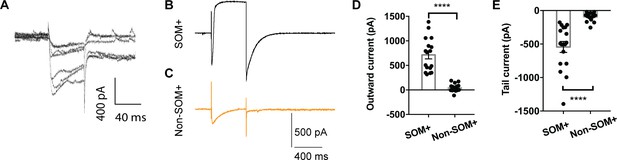
No detectable CaCC in non-SOM+ neurons.
(A) Calcium current in SOM+ neurons induced by 60 ms voltage clamp steps from −40 mV to +10 mV in 10 mV increments. (B and C) Depolarization of SOM+ neurons (B) and non-SOM+ neurons (C) to +10 mV elicited a Ca2+ current superimposed with a large outward current that developed over time, followed with a tail current upon hyperpolarization to −80 mV. (D and E) Comparison of the amplitudes of the outward current and tail current of SOM+ neurons and non-SOM+ neurons. Mann Whitney test in (D), U = 0, p<0.0001. Mann Whitney test in (E), U = 0, p<0.0001. Cells were held at −80 mV. n = 17 cells for SOM+ neurons and n = 16 cells for non-SOM+ neurons from four mice. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
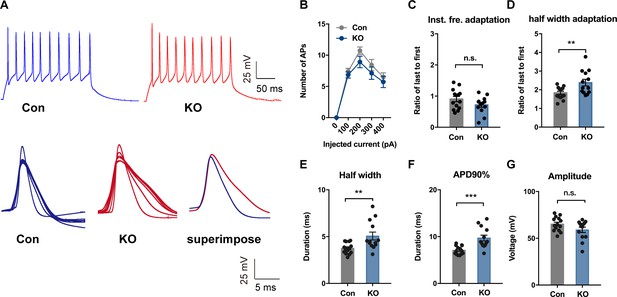
TMEM16B-CaCC Regulates Action Potential Waveform of Somatostatin-positive Neurons in the Central Lateral Amygdala.
(A) upper panel, representative traces of action potentials in SOM+ neurons from control and Ano2 KO mice in response to 100 pA current pulse lasting for 500 ms. Lower panel, overlay of action potentials induced by the current pulse of neurons with (left) or without (middle) Ano2, with superimposition of the averaged spikes from neurons with or without Ano2 shown on the right. (B) Number of spikes induced by step current injection in SOM+ neurons from control and Ano2 KO mice. Two-way ANOVA in (B), F (1, 49)=1.804, p=0.1855. n = 25 cells for KO and 26 cells for control, from seven mice each. (C) Ratio of the last to the first instantaneous firing frequency, from control and Ano2 KO brain slices. Two tail Unpaired t test, t = 1.635, df = 25, p=0.1146. n = 13 cells for KO and 14 cells for control, from three mice each. (D) Ratio of the last to the first half width, from control and Ano2 KO brain slices. Mann Whitney u test in (D), U = 41, p=0.0145. n = 13 cells for KO and 14 cells for control, from three mice each. (E) Half width of action potentials in SOM+ neurons with or without Ano2. Mann Whitney test, U = 38, p=0.0091. n = 13 cells for KO and 14 cells for control, from three mice each. (F) The averaged action potential duration at 90% repolarization (APD90%) in SOM+ neurons from control and Ano2 KO mice. Mann Whitney u test in (F), U = 21, p=0.0003. n = 13 cells for KO and 14 cells for control, from three mice each. (G) Average amplitude of action potential in SOM+ neurons with or without Ano2. Two tail Unpaired t test, t = 1.771, df = 25, p=0.0888. n = 13 cells for KO and 14 cells for control, from three mice each. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
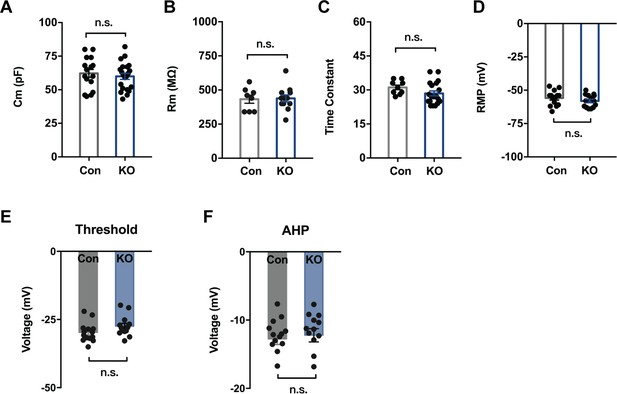
SOM+ Neurons Lacking Ano2 Have Normal Resting Potential, Membrane Capacitance, Input Resistance, Time Constant, Voltage threshold and Afterhyperpolarization.
(A–D) Passive membrane property is measured in SOM+ neurons from control and Ano2 KO mice, including capacitance (A), input resistance (B), membrane time constant (C), resting membrane potential (D), Voltage threshold (E) and Afterhyperpolarization (AHP) (F). Significance for multiple comparisons: Two tail unpaired t test in (A, B, C, D). (A) t = 0.613, df = 35, p=0.5438. n = 21 cells from 4 KO mice and n = 16 cells from three control mice. (B) t = 0.1486, df = 18, p=0.8835. n = 12 cells from 4 KO mice and n = 8 cells from three control mice. (C) t = 1.487, df = 26, p=0.1491. n = 19 cells from 4 KO mice and n = 9 cells from three control mice. (D) t = 1.129, df = 26, p=0.2694. n = 14 cells from 4 KO mice and n = 14 cells from three control mice. (E) Two tail Unpaired t test, t = 1.583, df = 25, p=0.1261. (F) Two tail Unpaired t test, t = 0.4115, df = 25, p=0.6842. n = 13 cells for KO and 14 cells for control, from four mice each, for E – F. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
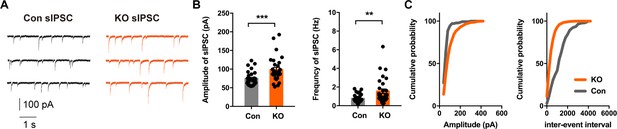
Altered Inhibitory Neurotransmission in the Central Lateral Amygdala of Ano2 KO mice.
(A) Representative traces of sIPSCs from SOM+ neurons of Ano2 KO and control mice. (B) The SOM+ neurons of Ano2 KO mice showed increased sIPSC frequency and sIPSC amplitude. Mann Whitney test in (B), U = 215, p=0.0004 for amplitude of sIPSC. U = 245, p=0.0021 for frequency of sIPSC. n = 31 cells for KO and n = 29 cells for control, from 10 mice each. (C) Representative cumulative probability plot of sIPSC frequency and amplitude of SOM+ neurons in CeL from Ano2 KO and control mice. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
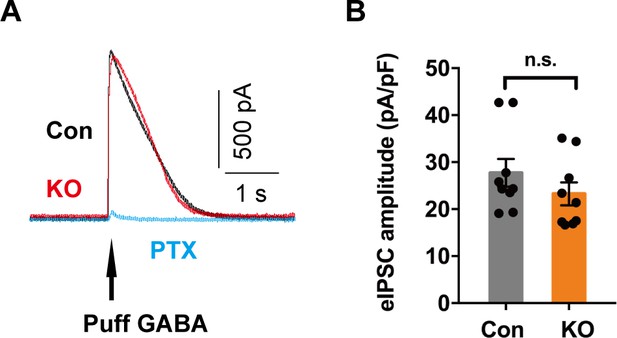
Similar eIPSC amplitudes of SOM+ neurons in control and Ano2 knockout mice.
(A) Representative IPSC induced by 100 μm GABA puff from control (indicated as black) and KO (red) mice (puff duration 50 ms, 4–6 psi). The GABA induced IPSC is blocked by picrotoxin (PTX), a GABAA receptor antagonist. (B) Summary analysis of IPSC amplitude from control and Ano2 knockout neurons. Two tail Unpaired t test, t = 1.161, df = 16, p=0.2628. n = 9 cells for each genotype from three mice each. Data are presented as mean ± SEM. n.s., no significant difference.
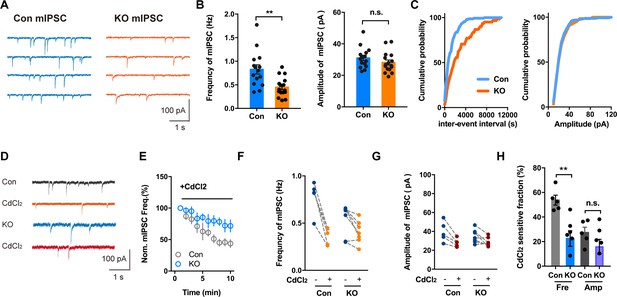
Voltage-activated Ca2+ Channel Involvement in mIPSC in Brain Slices With or Without Ano2.
(A) Representative traces of mIPSCs from SOM+ neurons of Ano2 KO and control mice. (B) The SOM+ neurons of Ano2 KO mice showed a decrease of mIPSC frequency, and normal mIPSC amplitude. Mann Whitney test in (B, left panel), U = 42.5, p=0.0016 for frequency of mIPSC. Two tail unpaired t test in (B, right panel), t = 1.231, df = 29, p=0.2283 for amplitude of mIPSC. n = 16 cells for KO and n = 15 cells for control, from five mice each. (C) Representative cumulative probability plot of mIPSC frequency and amplitude of SOM+ neurons in CeL from Ano2 KO and control mice. (D) Representative traces of mIPSCs from Ano2 KO and control brain slices in basal solution with or without Cd2+. (E) Cd2+ treatment reduced the mIPSC frequency of SOM+ neurons in the central lateral amygdala from KO mice and control littermates, as shown in the plot of normalized mIPSC frequency as function of time. Data are normalized to the baseline of mIPSC recorded in the first 3 min before Cd2+ application. (F) The effects of Cd2+ treatment on the mIPSC frequency in SOM+ neurons from control and KO mice. (G) The effects of Cd2+ treatment on the mIPSC amplitude in SOM+ neurons from control and KO mice. (H) The Cd2+-sensitive fraction of mIPSCs in SOM+ neurons from central lateral amygdala of KO mice and control littermates. Two tail Unpaired t test, t = 3.765, df = 10, p=0.0037 for Cd2+ sensitive mIPSC frequency. t = 1.424, df = 10, p=0.1848 for Cd2+ sensitive mIPSC amplitude. n = 7 cells from KO and n = 5 cells from control, three mice each. Data are presented as mean ± SEM. *p<0.05, **p<0.01; ***p<0.001; ****p<0.0001; n.s., no significant difference.
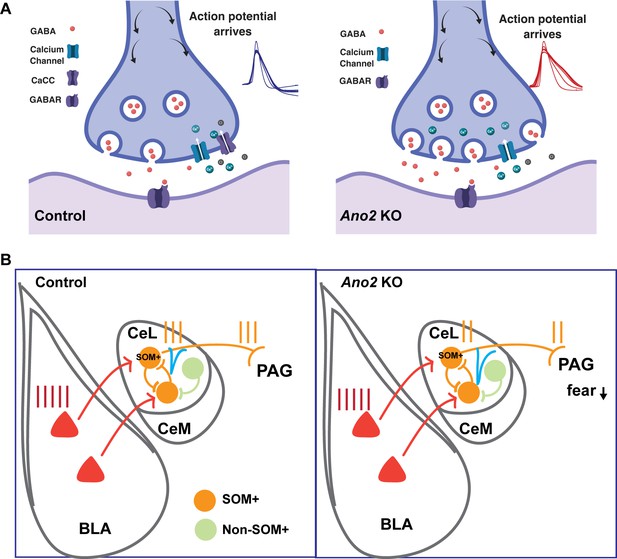
Mechanistic model for how presynaptic loss of function of TMEM16B, a Ca2+-activated Cl- channel, in central lateral amygdala (CeL) SOM+ GABAergic neurons mediates the electrophysiological and behavioral phenotypes.
(A) Loss of TMEM16B mediated CaCC in SOM+ CeL neurons results in broadening of action potential, thereby increasing presynaptic calcium channel activity and GABA release evoked by spike firing. (B) Increased inhibition tone onto SOM+ CeL neurons could contribute to the reduction of fear and anxiety-related behaviors in Ano2 KO mice, by reducing the extent that SOM+ CeL neurons are excited by neurons in the basolateral complex of the amygdala (BLA) as well as reducing the extent that SOM+ CeL neurons exert inhibition of the periaqueductal gray (PAG).
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47106.014





