Soluble PD-L1 generated by endogenous retroelement exaptation is a receptor antagonist
Figures
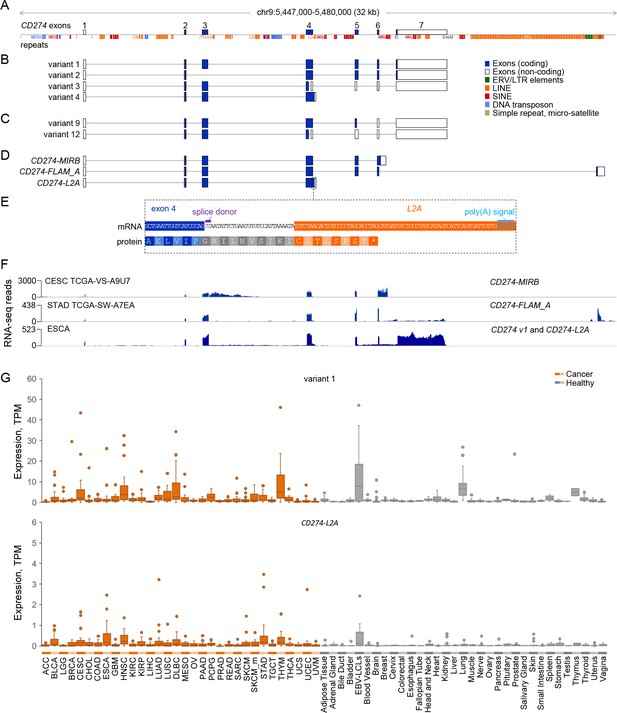
CD274 protein domains and splice variants.
(A) Depiction of reference genome EREs and other repeats in the genomic locus spanning the CD274 gene, relative to CD274 exons. (B) GENCODE or RefSeq annotated splice variants of the CD274 gene. Variant numbers correspond to the following NCBI Reference Sequence accessions: variant 1 (NM_014143), variant 2 (NM_001267706), variant 3 (NR_052005) and variant 4 (NM_001314029). (C) Recently-described novel variants encoding sPD-L1 (Zhou et al., 2017). (D) CD274 splice variants de novo assembled in this study and overlapping one or more EREs. (E) Inclusion of an L2A element as a terminal exon and polyadenylation site in splice variant CD274-L2A. The relative position of the intronic L2A element, as well as the novel C-terminal amino acid created from its exonisation are also indicated. (F) RNA-seq traces representative of each of the ERE-overlapping CD274 variants, CD274-MIRB, CD274-FLAM_A and CD274-L2A. For the low recurrence CD274-MIRB and CD274-FLAM_A variants, the samples with the highest expression are shown, whereas for the high recurrence CD274-L2A, a representative ESCA sample is shown. (G) Box plot of CD274 variant 1 and CD274-L2A expression (in TPMs) in the indicated cancer patient (n = 24 for each indication) and healthy control samples (n between 2 and 156).
-
Figure 1—source data 1
Expression of CD274 variant 1 and CD274-L2A in TCGA and GTEx samples.
- https://cdn.elifesciences.org/articles/50256/elife-50256-fig1-data1-v2.xlsx
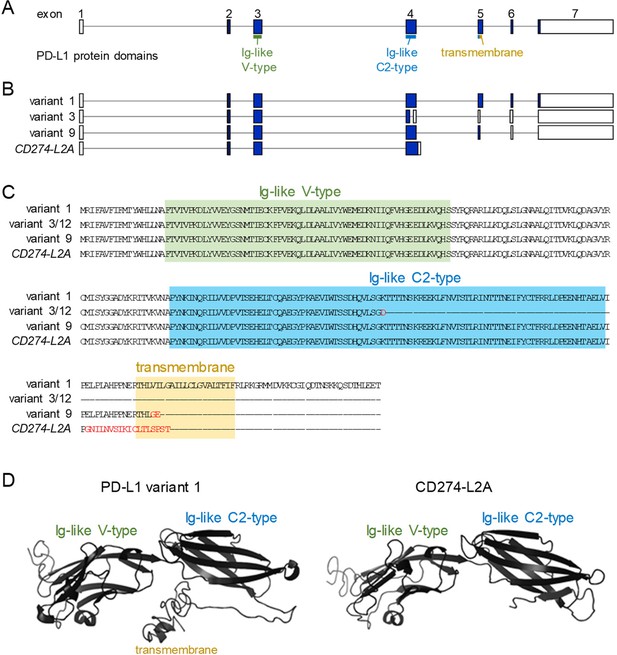
The CD274-L2A protein product retains receptor binding domains but not transmembrane domain.
(A) CD274 exons encoding receptor binding and transmembrane domains. (B) Transcript structure of the full-length CD274 variant 1 and variants 3/12, nine and CD274-L2A lacking the transmembrane domain. (C) Amino acid sequence of the same variants as in B. Amino acid residues differing from the index sequence are indicated in red. (D) Modelled structure of full-length CD274 variant one and truncated variant CD274-L2A, illustrating the receptor binding and transmembrane domains.
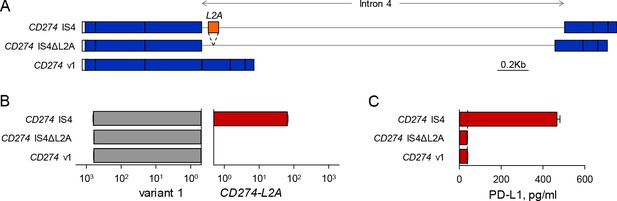
The L2A element in intron 4 of the CD274 gene is essential for sPD-L1 production.
(A) Minigenes comprising the full-length CD274 cDNA with an intact intron 4 (CD274 IS4), or an intron 4 lacking the L2A element (CD274 IS4ΔL2A) or CD274 variant 1 cDNA (CD274 v1), were expressed in HEK293T cells. (B) Expression of CD274 variant 1 and CD274-L2A, measured by qRT-PCR using variant-specific primers in HEK293T cells expressing the indicated minigenes. Mean (± SEM) expression normalized to HPRT from three independent experiments is shown. (C) Quantification of soluble PD-L1 by ELISA in the supernatants of HEK293T expressing the indicated minigenes. The dashed line represents background measurements.
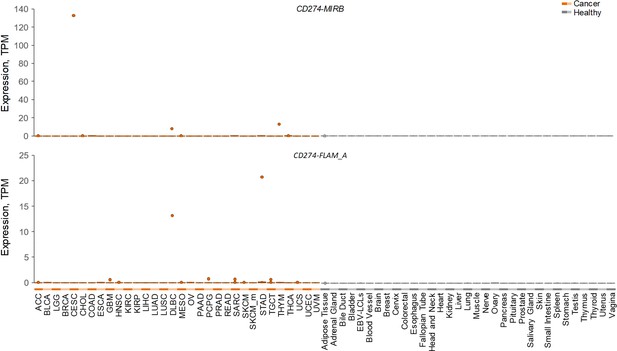
Expression of CD274-MIRB and CD274-FLAM_A variants.
Box plots of CD274-MIRB (top) and CD274-FLAM_A (bottom) expression in cancer patient samples (listed by TCGA abbreviations) and in healthy control samples from the indicated tissues.
-
Figure 1—figure supplement 3—source data 1
Expression of CD274-MIRB and CD274-FLAM_A in TCGA and GTEx samples.
- https://cdn.elifesciences.org/articles/50256/elife-50256-fig1-figsupp3-data1-v2.xlsx
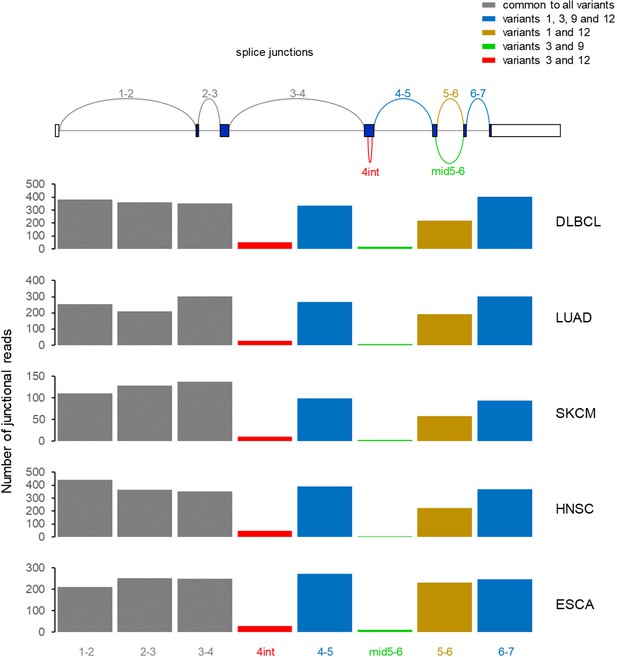
Splice junction analysis of CD274 variant expression.
Annotated splice junctions in the CD274 gene (top) were numbered according to the linked exons (e.g. 1–2); 4int refers to splicing internal to exon 4, specific to variants 3 and 12; mid5-6 refers to splicing with donor internal to exon five and acceptor at the start of exon 6, specific to variants 3 and 9. Plotted are the numbers of junctional reads corresponding to the annotated splice junctions in aggregate bam files from the indicated TCGA cancer types.
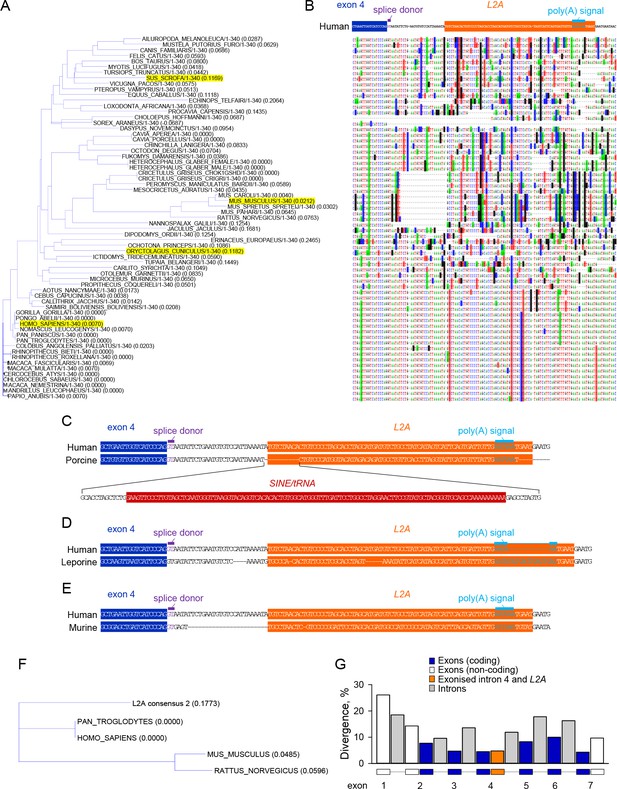
Evolutionary conservation of CD274-L2A genomic features in hominids.
(A–B) Genomic alignment of the indicated portion of the CD274 gene using nucleotide sequences from 65 eutherian mammals. A large SINE insertion in the porcine Cd274 gene and a smaller insertion in the leporine Cd274 gene were removed to aid the visual representation of alignment (both these species are highlighted in A. Base substitutions are indicated by highlighting and absence of highlighting denotes base conservation. (C) Comparison of the human and porcine genes, illustrating the SINE/tRNA insertion in the latter. (D) Comparison of the human and leporine genes, illustrating an insertion in the polyadenylation site of the latter. (E) Comparison of the human and murine genes, illustrating a 24-nucleotide deletion in the latter and mutations at the splice and polyadenylation sites. (F) Alignment tree depicting the distance of the consensus L2A element sequence from the respective human, chimpanzee, murine and rat elements. (G) Sequence divergence of coding and non-coding exons, introns and of the 100 nucleotides covering the exonised part of intron 4 and embedded L2A element in CD274 genomic sequences from 10 primate species. The individual segments of the CD274 gene compared were scaled to the same width.
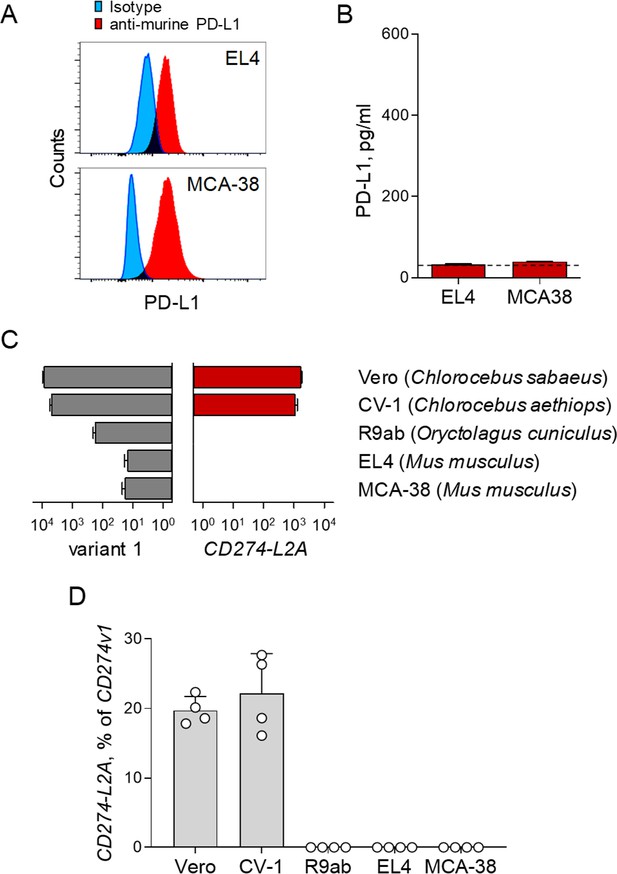
Expression of CD274-L2A and production of sPD-L1 in mammalian species.
(A) Flow cytometric detection of cell surface PD-L1 in murine EL4 and MCA-38 cells. (B) Quantification of soluble murine PD-L1 by ELISA in the supernatants murine EL4 and MCA-38 cells. The dashed line represents background measurements (C) Expression of CD274 variant 1 and CD274-L2A, measured by qRT-PCR using species-specific and variant-specific primers in the indicated cell lines of green monkey (Chlorocebus sp.), rabbit (Oryctolagus cuniculus) or mouse (Mus musculus) origin. Mean (± SEM) expression normalized to HPRT from two independent experiments, with replicates each, is shown. (D) Expression of CD274-L2A, as a percentage of CD274 variant 1, in the same cell lines as in C.
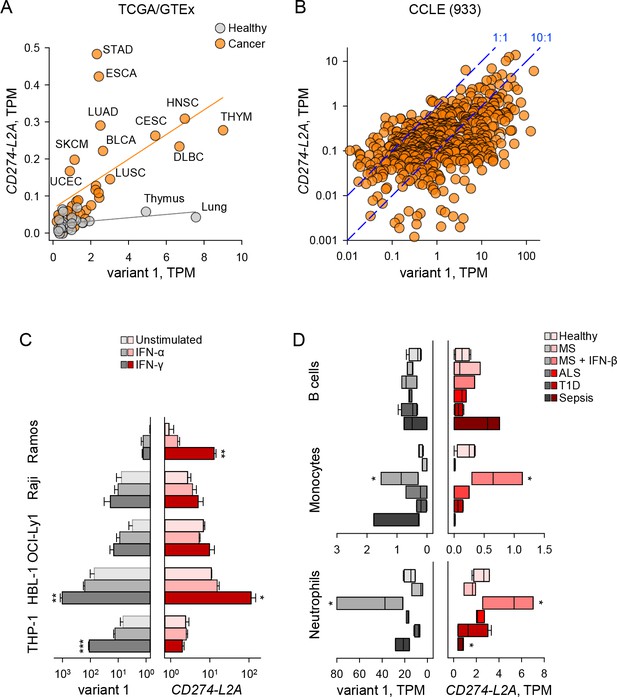
CD274-L2A and CD274v1 expression are decoupled under certain stimuli.
(A) CD274 variant 1 and CD274-L2A expression across TCGA tumour and GTEx healthy samples. Average TPM is shown per tissue type, with linear regression performed separately for tumour and healthy samples. (B) CD274 variant 1 and CD274-L2A expression (in TPMs) across the CCLE dataset. Dashed lines denote a 10:1 and 1:1 ratio of CD274v1:CD274-L2A, respectively. (C) Expression of CD274 variant 1 and CD274-L2A, measured by qRT-PCR using variant-specific primers in five leukocyte cell lines. Cells were stimulated with IFN-α or IFN-γ for 48 hr or were left untreated. Mean (± SEM) expression normalized to HPRT from three independent experiments is shown. (D) Expression of CD274 variant 1 and CD274-L2A (in TPMs), calculated using RNA-seq data (SRP045500) from B cells, monocytes and neutrophils isolated from peripheral blood of healthy individuals of patients with Sepsis, ALS or T1D or from MS patients before and 24 hr after the first treatment with IFN-β.
-
Figure 3—source data 1
Expression of CD274 variants in the CCLE collection.
- https://cdn.elifesciences.org/articles/50256/elife-50256-fig3-data1-v2.xlsx
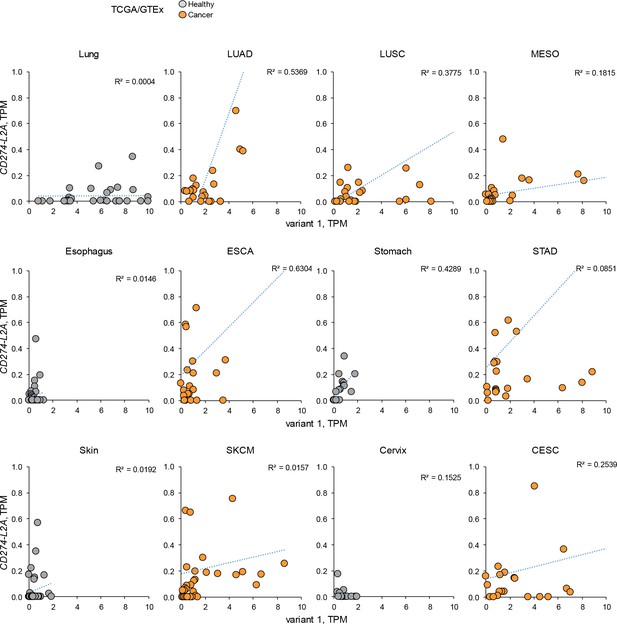
CD274-L2A and CD274v1 expression in individual cancer and healthy samples.
CD274 variant 1 and CD274-L2A expression across TCGA tumour and GTEx healthy samples. Each symbol represents an individual sample. Dashed blue lines represent linear regressions for each cancer type or healthy tissue.
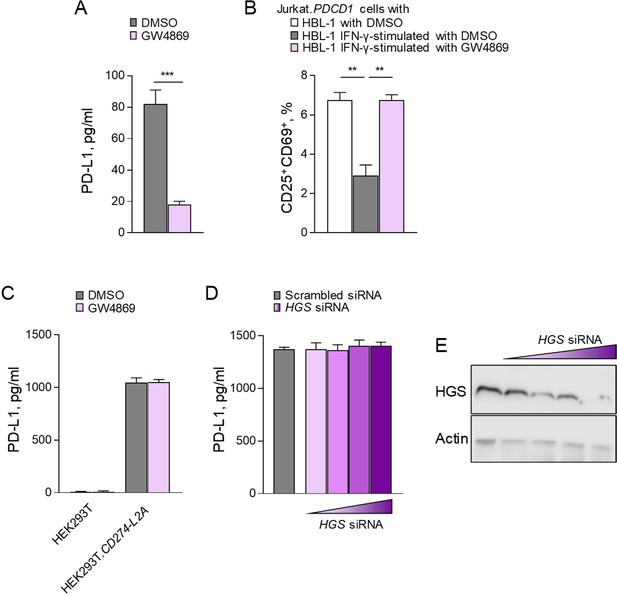
CD274-L2A-derived soluble PD-L1 is exosome independent.
(A) Quantification of soluble PD-L1 by ELISA in the supernatant of HBL-1 cells treated with exosome inhibitor GW4869 or DMSO as denoted. Mean (± SEM) concentration from three independent experiments are shown. (B) Activated (CD25+CD69+) Jurkat.PDCD1 cells in the presence of conditioned media from parental HBL-1 cells or IFN-γ-stimulated HBL-1 cells treated with GW4869 or DMSO. Mean (± SEM) proportion from three independent experiments are shown. (C) Quantification of soluble PD-L1 by ELISA in the supernatants of HEK293T or HEK293T.CD274-L2A cells treated with GW4869 or DMSO as denoted. Mean (± SEM) concentration from three independent experiments are shown. (D) Quantification of soluble PD-L1 by ELISA in the supernatants of HEK293T. CD274-L2A cells following treatment with siRNA targeting HGS or scrambled sequence. HGS knockdown was assessed by immunoblot. Mean (± SEM) concentration from three independent experiments are shown, with representative immunoblots shown.
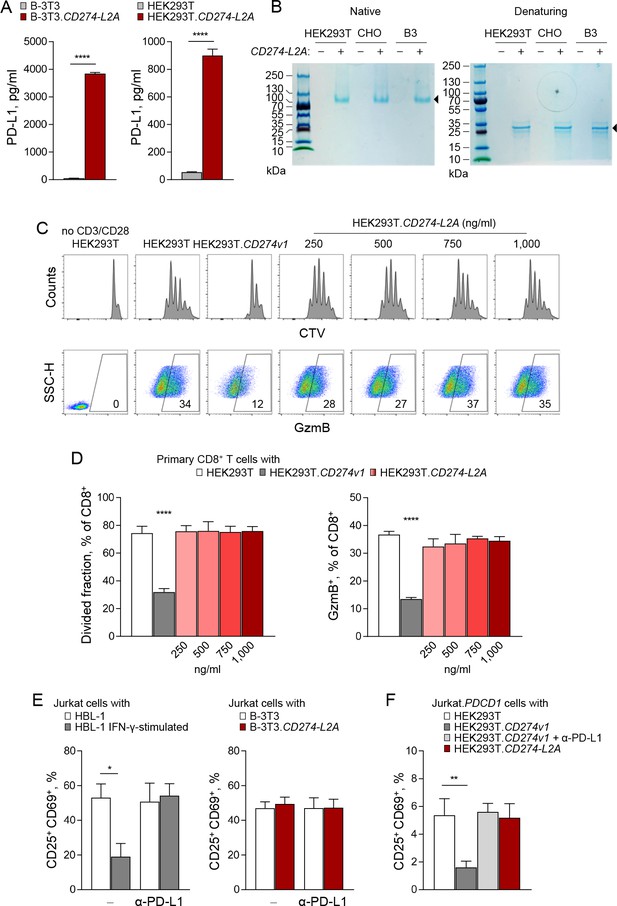
CD274-L2A-derived soluble PD-L1 is not immunosuppressive.
(A) Quantification of soluble PD-L1 by ELISA in supernatants of B-3T3 and HEK293T cells retrovirally transduced with CD274-L2A. Mean (± SEM) concentration from three independent experiments are shown. (B) Coomassie Brilliant Blue stain, under native or reducing (β-ME/SDS) PAGE conditions, of serum-free supernatants from HEK293T, CHO and B3 cells transfected or not with CD274-L2A. (C–D) Primary CD8+ T cells were labelled with CTV and stimulated with CD3- and CD28-coated beads for 72 hr, alone or co-cultured with HEK293T, HEK293T.CD274v1 or HEK293T.CD274-L2A cells transfected with the indicated amount of plasmid DNA. T cells were stained for intracellular GzmB at the end of the culture period. Representative histograms and scatter plots are shown in C; quantification of CTVlo and GzmB+ cells of three healthy donors according to the amount of transfected plasmid DNA is shown in D. (E) Percentage of activated (CD25+CD69+) Jurkat cells in the presence of conditioned media from parental HBL-1 cells, IFN-γ-stimulated HBL-1 cells, parental B-3T3 cells or B-3T3.CD274-L2A transduced cells. Cells were stimulated with CD3- and CD28-coated beads for 24 hr, with 10 µg/mL of a PD-L1-blocking antibody added where indicated. Mean (± SEM) proportion from three independent experiments are shown. (F) Percentage of activated (CD25+CD69+) Jurkat.PDCD1 cells after co-cultured with parental HEK293T, HEK293T.CD274v1, or HEK293T.CD274-L2A cells. Cells were stimulated with CD3- and CD28-coated beads for 24 hr, with 10 µg/mL of a PD-L1-blocking antibody added where indicated. Mean (± SEM) proportion from three independent experiments are shown.
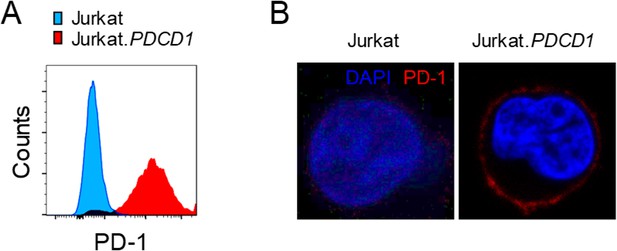
Generation of Jurkat.PDCD1 cells.
(A) Surface expression of PD-1 on sorted PDCD1-transduced Jurkat cells (red) and parental Jurkat cells (blue). Representative histograms are shown. (B) Immunostaining of Jurkat.PDCD1 and parental Jurkat cells for PD-1 (red) and DAPI (blue). Representative images are shown.
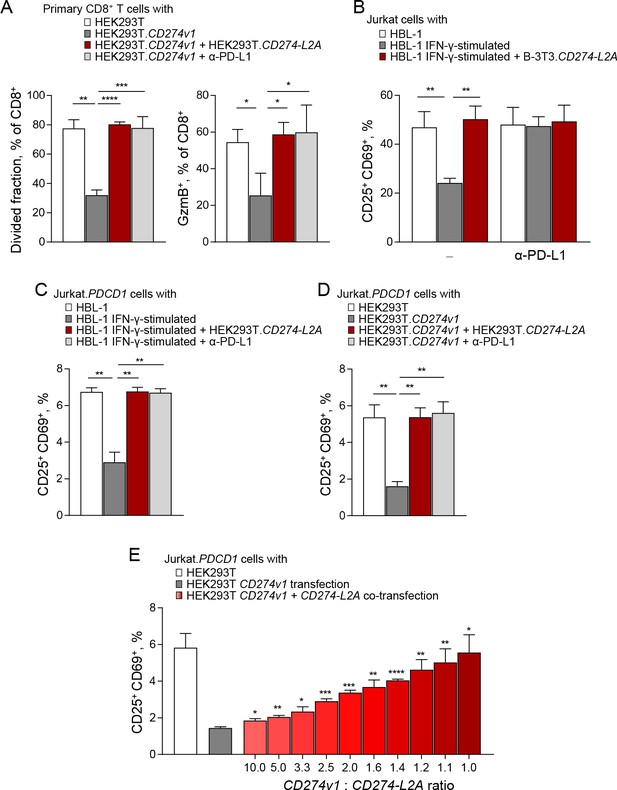
CD274-L2A-derived soluble PD-L1 acts as a receptor antagonist in the presence of transmembrane PD-L1.
(A) Primary CD8+ T cells were labelled with Cell Trace Violet (CTV) and stimulated with CD3- and CD28-coated beads for 72 hr. T cells were co-cultured with parental HEK293T or HEK293T.CD274v1 transfected cells in the presence of conditioned media from HEK293T.CD274-L2A transduced cells or a PD-L1-blocking antibody. Mean (± SEM) proportion of CTVlo and GzmB+ cells of three healthy donors is shown. (B) Percentage of activated (CD25+CD69+) Jurkat cells in the presence of conditioned media from parental HBL-1 cells, IFN-γ-stimulated HBL-1 cells, and transduced B-3T3.CD274-L2A cells. Cells were stimulated with CD3- and CD28-coated beads for 24 hr, with 10 µg/mL of a PD-L1-blocking antibody added where indicated. Mean (± SEM) proportion from three independent experiments are shown. (C) Percentage of activated (CD25+CD69+) Jurkat.PDCD1 cells in the presence of conditioned media from parental HBL-1 cells, IFN-γ-stimulated HBL-1 cells, and transduced HEK293T.CD274-L2A cells. Cells were stimulated with CD3- and CD28-coated beads for 24 hr, with 10 µg/mL of a PD-L1-blocking antibody added where indicated. Mean (± SEM) proportion from three independent experiments are shown. (D) Percentage of activated (CD25+CD69+) Jurkat.PDCD1 cells following co-culture with parental HEK293T or HEK293T.CD274v1 transfected cells in the presence of conditioned media from HEK293T.CD274-L2A or a PD-L1-blocking antibody. Cells were stimulated with CD3- and CD28-coated beads for 24 hr. Mean (± SEM) proportion from three independent experiments are shown. (E) Percentage of activated (CD25+CD69+) Jurkat.PDCD1 cells following co-culture with HEK293T cells transfected with varying ratios of CD274v1 and CD274-L2A as shown. The concentration of the CD274v1 plasmid is kept constant across all conditions at 1000 ng. Cells were stimulated with CD3- and CD28-coated beads for 24 hr. Mean (± SEM) proportion from three independent experiments are shown.
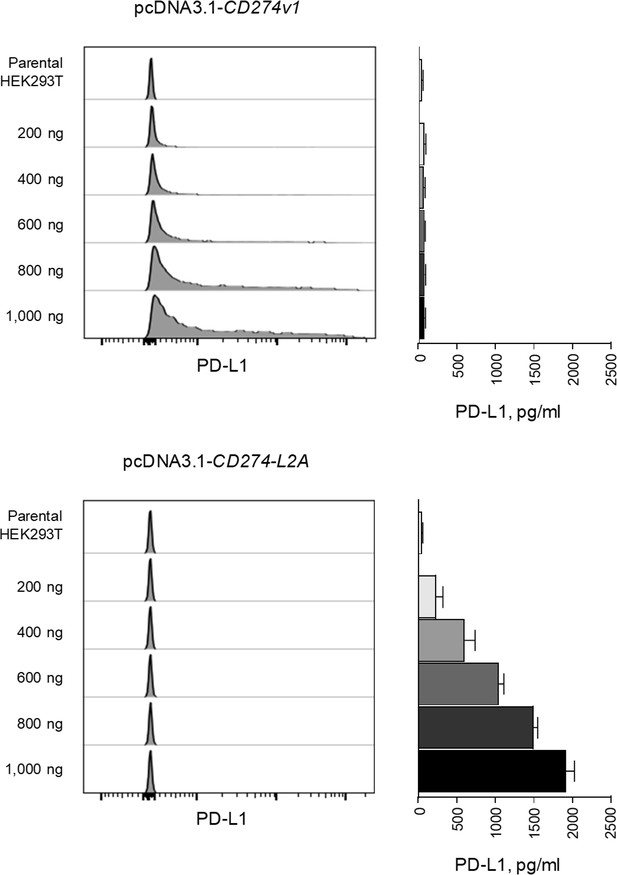
CD274v1 and CD274-L2A predominantly produce transmembrane and soluble PD-L1 respectively.
Quantification of surface and soluble PD-L1 on HEK293T cells transfected with CD274v1 or CD274-L2A. Surface expression was quantified by flow cytometry on transfected cells 24 hr post-transfection. Soluble PD-L1 was quantified by ELISA in cell supernatant of transfected cells 24 hr post-transfection. Histograms or mean concentration (± SEM) from three independent experiments are shown.
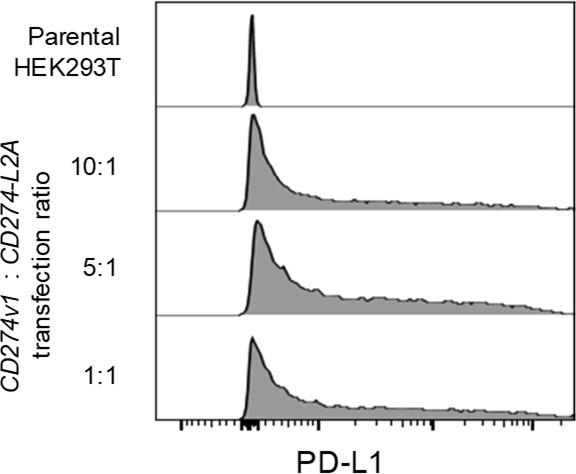
Surface PD-L1 is not lost upon co-transfection with CD274-L2A.
Surface expression of PD-L1 on HEK293T cells transfected with CD274v1 and CD274-L2A. The concentration of the CD274v1 plasmid is kept constant across all conditions at 1000 ng. Transfected HEK293T cells were stained following co-culture with Jurkat.PDCD1 cells. Histograms representative of three independent experiments are shown.
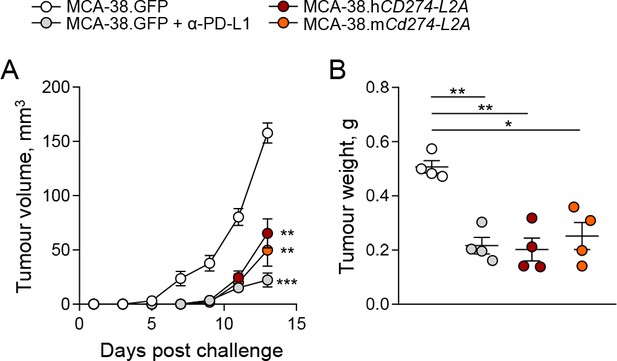
CD274-L2a-derived soluble PD-L1 delays in vivo tumour growth.
(A–B) Tumour growth following subcutaneous inoculation of 1 × 106 MCA-38 cells expressing human sPD-L1 (MCA-38.hCD274-L2A), a constructed murine sPD-L1 variant (MCA-38.mCd274-L2A) or GFP. One group of recipient mice was treated with anti-PD-L1 antibodies. Mean tumour volumes (± SEM) throughout the experiment (A) and tumour weights at endpoint (B) of 4 mice per group are plotted from one representative of two experiments.
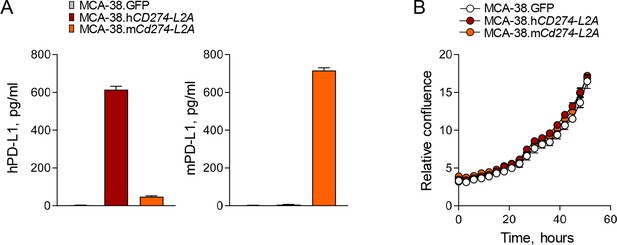
Characterisation of sPD-L1-producing MCA-38 cells.
(A) MCA-38 cells were transduced with retroviral vectors encoding human sPD-L1 (MCA-38.hCD274-L2A) or a chimeric murine sPD-L1 (exons 1–4) with the 18 C-terminal amino acids of human sPD-L1 (MCA-38.mCd274-L2A) or GFP (MCA-38.GFP). Concentrations of human (left) and murine (right) PD-L1 in the supernatants of parental MCA-38, MCA-38.hCD274-L2A and MCA-38.mCd274-L2A cells were determined by species-specific PD-L1 ELISAs. Mean (± SEM) concentration of three measurements are shown. (B) In vitro growth of MCA-38.GFP, MCA-38.hCD274-L2A and MCA-38.mCd274-L2A cells, measured with the IncuCyte S3 imaging system.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | C57BL/6J | The Jackson Laboratory | RRID: IMSR_JAX:000664 | |
| Cell line (Homo sapiens) | HEK293T | Cell Services Facility, Francis Crick Institute | RRID: CVCL_0063 | |
| Cell line (Homo sapiens) | Jurkat | Cell Services Facility, Francis Crick Institute | RRID: CVCL_0065 | |
| Cell line (Chlorocrbus sabaeus) | Vero | Cell Services Facility, Francis Crick Institute | RRID: CVCL_0059 | |
| Cell line (Chlorocebus aethiops) | CV-1 | Cell Services Facility, Francis Crick Institute | RRID: CVCL_0229 | |
| Cell line (Oryctolagus cuniculus) | R9ab | Cell Services Facility, Francis Crick Institute | RRID: CVCL_3782 | |
| Cell line (Cricetulus griseus) | CHO | Cell Services Facility, Francis Crick Institute | RRID: CVCL_0213 | |
| Cell line (Mus musculus) | B-3T3 | Cell Services Facility, Francis Crick Institute | RRID: CCL-163 | |
| Cell line (Mus musculus) | EL4 | Cell Services Facility, Francis Crick Institute | RRID: CVCL_0255 | |
| Cell line (Mus musculus) | MCA-38 | Cell Services Facility, Francis Crick Institute | RRID: CVCL_B288 | |
| Cell line (Mus musculus) | B3 | Cell Services Facility, Francis Crick Institute | RRID: CVCL_RP56 | |
| Antibody | Rat monoclonal anti-mouse PD-L1 (clone 10F.9G2) | Biolegend (FACS), BioXCell (in vivo) | Cat: 124315 (Biolegend) Cat: BE0101 (BioXCell) | FACS (1:200) In vivo injection (200 ug i.p.) |
| Antibody | Mouse monoclonal anti-human PD-L1 (clone 29E.2A3) | Biolegend (FACS) | Cat: 329706 | FACS (1:200) |
| Antibody | Mouse monoclonal anti-human PD-1 (clone EH12.2H7) | Biolegend (FACS) | Cat: 329908 | FACS (1:200) |
| Antibody | Mouse monoclonal anti-human CD25 (clone BC96) | Biolegend (FACS) | Cat: 302642 | FACS (1:200) |
| Antibody | Mouse monoclonal anti-human CD69 (clone FN50) | Biolegend (FACS) | Cat: 310906 | FACS (1:200) |
| Antibody | Mouse monoclonal anti-human CD8 (clone SK1) | Biolegend (FACS) | Cat: 344710 | FACS (1:200) |
| Antibody | Mouse monoclonal anti-human Granzyme B (clone QA16A02) | Biolegend (FACS) | Cat: 372204 | FACS (1:200) |
| Antibody | Mouse monoclonal anti-human HGS (clone C-7) | Santa Cruz (WB) | Cat: sc-271455 | WB (1:1000) |
| Antibody | HRP-conjugated mouse monoclonal anti-human Actin (clone AC-15) | Abcam (WB) | Cat: ab49900 | WB (1:25000) |
| Chemical compound, drug | GW4869 | Sigma Aldrich | Cat: D1692 | |
| Transfected construct (Homo sapiens, Mus musculus) | pRV-IRES-GFP (lentiviral vector) | This paper | Lentiviral construct expressing GFP; used as empty vector control | |
| Transfected construct (Homo sapiens, Mus musculus) | pRV-CD274-L2A-IRES-GFP (lentiviral vector) | This paper | Lentiviral construct expressing human CD274-L2A | |
| Transfected construct (Mus musculus) | pRV-mCd274-L2A-IRES-GFP (lentiviral vector) | This paper | Lentiviral construct expressing murine-human chimeric Cd274-L2A | |
| Transfected construct (Homo sapiens) | pRV-PDCD1-IRES-GFP (lentiviral vector) | This paper | Lentiviral construct expressing human PDCD1 (PD-1) | |
| Recombinant DNA reagent | pcDNA3.1-CD274 (plasmid) | This paper | Mammalian expression plasmid encoding human CD274 | |
| Recombinant DNA reagent | pcDNA3.1-CD274-L2A (plasmid) | This paper | Mammalian expression plasmid encoding human CD274-L2A | |
| Recombinant DNA reagent | pcDNA3.1-CD274 IS4 (plasmid) | This paper | Mammalian expression plasmid encoding human CD274 with intron 4 | |
| Recombinant DNA reagent | pcDNA3.1-CD274 IS4ΔL2A (plasmid) | This paper | Mammalian expression plasmid encoding human CD274 with intron four with L2A sequence deleted | |
| Commercial assay or kit | Human PD-L1 ELISA kit | Abcam | Cat: ab214565 | |
| Commercial assay or kit | Mouse PD-L1 ELISA kit | Biomatik | Cat: EKU06803 | |
| Commercial assay or kit | RNeasy Mini RNA extraction kit | Qiagen | Cat: 74104 | |
| Commercial assay or kit | High Capacity cDNA Reverse Transcription kit | Applied Biosystems | Cat: 4368814 |





