ARL13B regulates Sonic hedgehog signaling from outside primary cilia
Figures
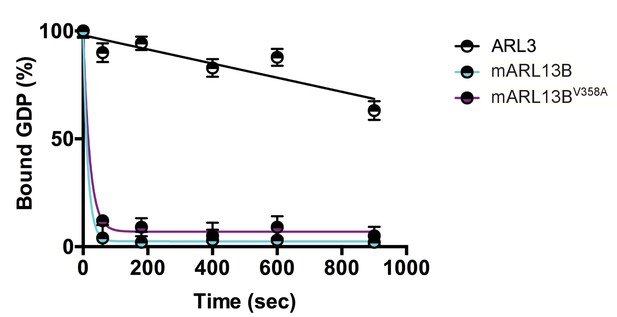
ARL3 GEF activity is retained in the ARL13BV358A mutant.
Time course of the release of pre-bound [3H]GDP from purified, recombinant ARL3 in the absence (ARL3) or presence of mouse wild type ARL13B or ARL13BV358A (mARL13B) are shown. See Methods for details. Error bars ± standard deviation.
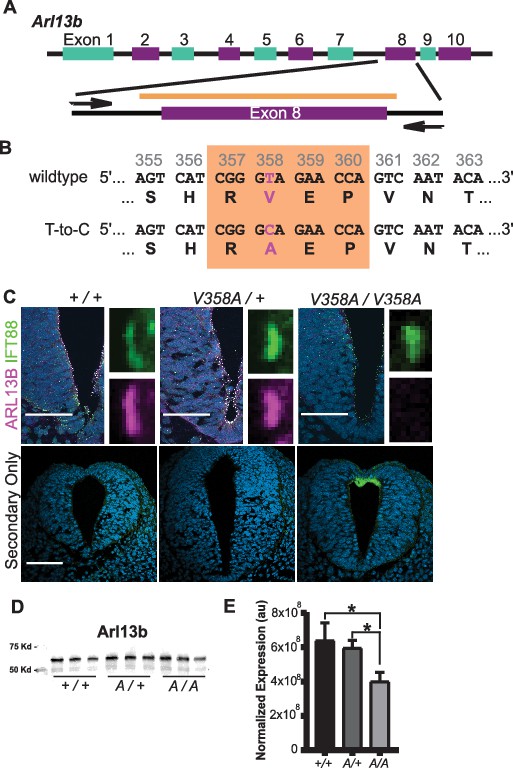
Generation of the Arl13bV358A/V358A mouse.
(A) Schematic of Arl13b gene and donor oligo (orange bar) at exon 8 used to generate the V358A causing point mutation. Arrows indicate primers used for allele validation. (B) Arl13b DNA and relevant amino acid sequence with the RVEP sequence in the orange box and the T-to-C mutation highlighted in pink. (C) Confocal images of cilia marker IFT88 (green) and ARL13B (magenta; NeuroMab) staining in neural tube of E10.5 somite-matched embryos. ARL13B-positive cilia are visible in Arl13b+/+ and Arl13bV358A/+, but not in Arl1bV358A/V358A embryos. (See Figure 2—figure supplement 1 for images of neural tube cilia under saturating conditions.) At least 5 embryos per genotype across five litters were examined. Scale bars are 50 μm. (D) ARL13B western blot of E10.5 whole embryo lysates, wild type (+/+), Arl13bV358A/+ (A/+) and Arl13bV358A/V358A (A/A) and (E) quantification presented as average intensity normalized to total protein ± standard deviation. Representative blot of whole embryo lysates (n = 3 embryos per genotype with technical duplicate of each). *p<0.05, one-way ANOVA and Tukey’s multiple comparison.
-
Figure 2—source data 1
ARL13B western blot quantification.
- https://cdn.elifesciences.org/articles/50434/elife-50434-fig2-data1-v2.xlsx
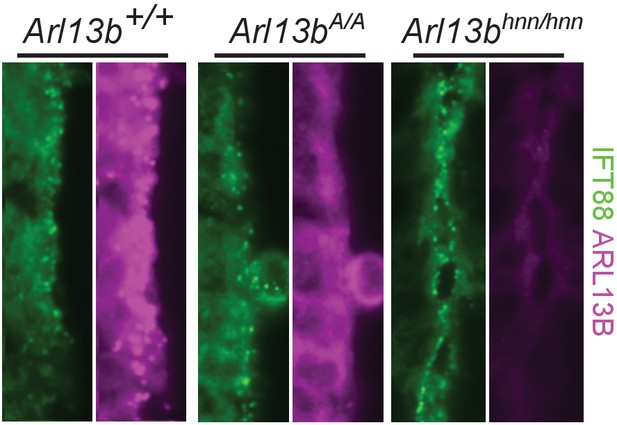
Overexposure of Arl13bV358A/V358A cilia in E10.5 neural tube reveals no clear ARL13BV358A presence in cilia.
Overexposure of cilia in E10.5 Arl13b+/+, Arl13bV358A/V358A, and Arl13bhnn/hnn embryonic neural tube, stained for IFT88 and ARL13B (NeuroMab). The ARL13B channel was overexposed for four seconds instead of one second.
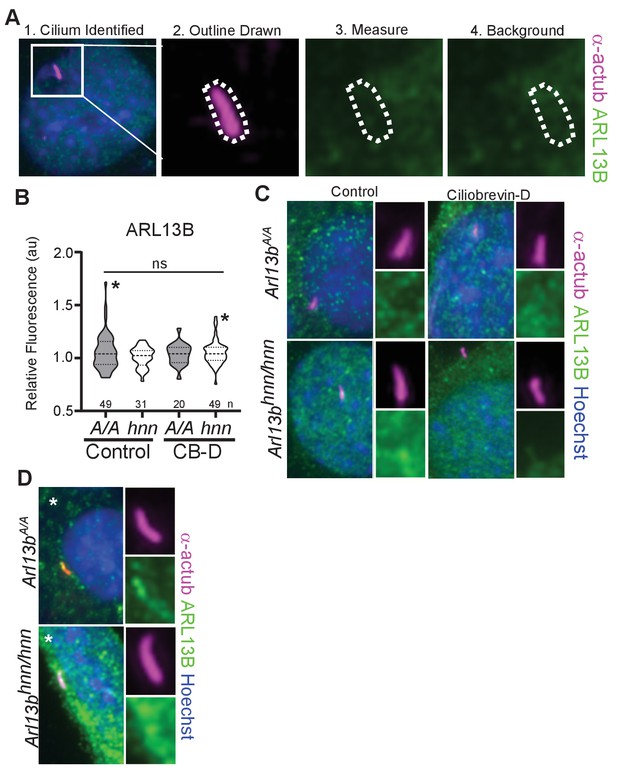
Overexposure of Arl13bV358A/V358A cilia in MEFs reveals no clear ARL13BV358A presence in cilia.
(A) We identified acetylated α-tubulin positive cilia and used the marker to outline the area of the cilium. We quantified ARL13B immunofluorescence detected by Caspary lab polyclonal anti-ARL13B antibody (Caspary et al., 2007) and used the same trace to measure background levels in a different area of the cell body. (B) Arl13bV358A/V358A (A/A) and Arl13bhnn/hnn (hnn) MEFs treated with control or ciliobrevin-D 0.5% FBS Media. Samples were overexposed five times longer than normal, saturating the detector in the ARL13B channel. We measured no difference in ciliary ARL13B immunofluorescence between control and ciliobrevin-D treated Arl13bV358A/V358A and Arl13bhnn/hnn MEFs. Data presented as violin plots and analyzed by one-way ANOVA. Respective number of cilia listed below each plot. Cilia that had ARL13B immunofluorescent readings above background (*) are shown in panel D. (C) Representative images of over exposed Arl13bV358A/V358A and Arl13bhnn/hnn MEFs in control and ciliobrevin-D treatment conditions. (D) Representative images of cilia with ARL13B levels above background, marked by * in panel B.
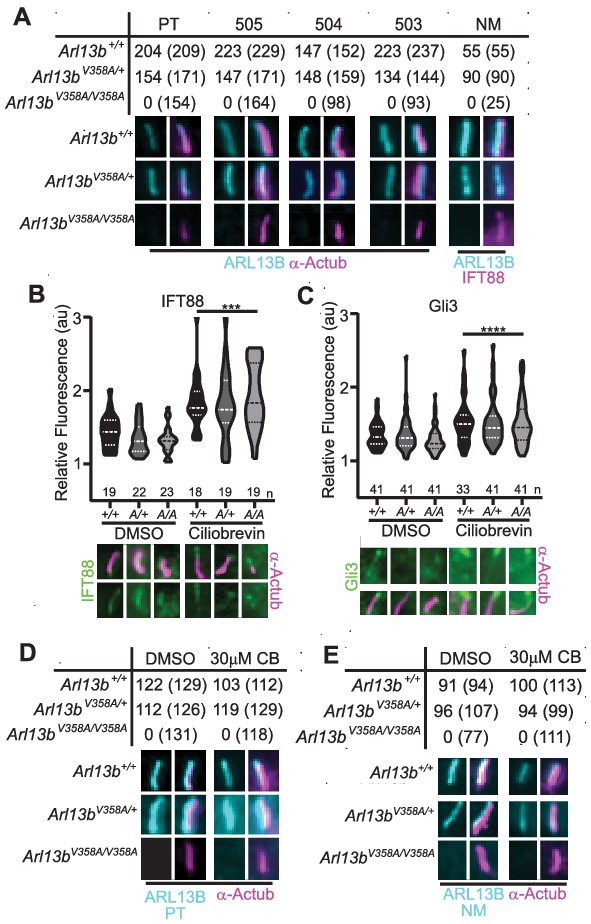
ARL13BV358A is undetectable in cilia and cannot be enriched by inhibition of retrograde transport.
(A) Antibodies against ciliary markers acetylated α-tubulin or IFT88 (magenta) and ARL13B (cyan) in Arl13bV358A/V358A MEFs. Representative images show staining for five indicated ARL13B antibodies: (PT) polyclonal rabbit antibody against full-length human ARL13B from ProteinTech, (503, 504, 505) polyclonal rabbit sera from three distinct rabbits raised against C-terminus of mouse ARL13B (amino acids 208–427) (Caspary et al., 2007), and (NM) monoclonal mouse antibody against C terminus of mouse ARL13B from NeuroMab. Arl13b+/+ and Arl13bV358A/+ show ciliary ARL13B staining. Table lists ARL13B-positive cilia and the total number of cilia identified by acetylated α-tubulin or IFT88 antibody in parentheses. Cilia appear shorter in Arl13bV358A/V358A cells (see Figure 6). (B) IFT88 and (C) Gli3 (green) is enriched in the tips of cilia in Arl13b+/+ (+/+), Arl13bV358A/+ (A/+) and Arl13bV358A/V358A (A/A) cells following ciliobrevin-D treatment. Violin plots depict relative fluorescence of IFT88 and Gli3 at cilia tip to cell body with number of cilia measured (n) listed beneath each plot. (D and E) Table lists ARL13B (cyan) positive cilia (rabbit anti-ARL13B; ProteinTech or mouse anti-ARL13B; NeuroMab) and the total number of cilia (acetylated α-tubulin: magenta) examined in control (DMSO) and ciliobrevin-D treated (30 μM CB) cell lines. Representative images show staining for cilia and ARL13B. Staining of IFT88 and Gli3 analyzed by two-way ANOVA and Sidak’s multiple comparisons. (***p<0.001, ****p<0.0001).
-
Figure 3—source data 1
Analysis of ARL13B in MEFs.
- https://cdn.elifesciences.org/articles/50434/elife-50434-fig3-data1-v2.xlsx
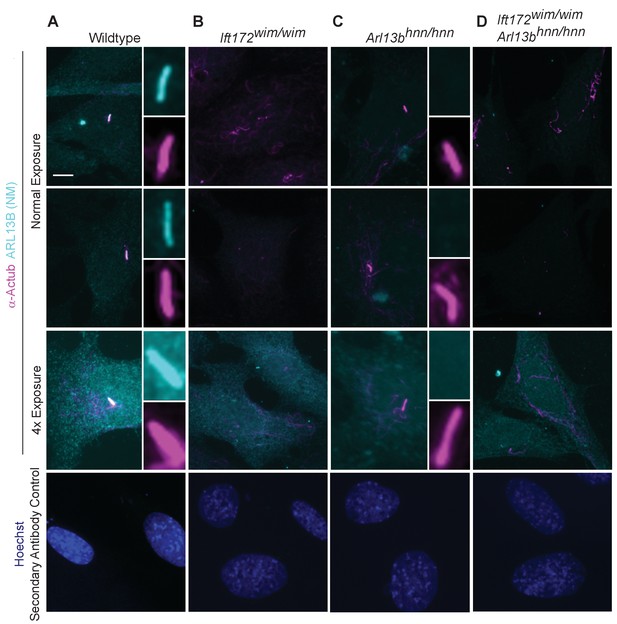
Endogenous ARL13B is undetectable in the cell body of cilia mutant Ift172wim/wim cells.
Immunofluorescent detection of ARL13B (cyan; NeuroMab) and cilia marker acetylated α-tubulin (magenta) in wildtype, Arl13bhnn/hnn, Ift172wim/wim, and Ift172wim/wim Arl13bhnn/hnn cell lines. (A) In wildtype cells, ARL13B is detectable in the cilium under non-saturating conditions, these parameters are kept constant across all samples. (B) In Ift172wim/wim non-ciliated cells, ARL13B protein is confined to the cell body, but is nearly undetectable by standard immunofluorescence. (C–D) In Arl13bhnn/hnn and Ift172wim/wim Arl13bhnn/hnn cells, null for Arl13b, any signal is due primary antibody background. At an exposure rate 4x above normal the ARL13B signal saturates the detector in wildtype cells. In Ift172wim/wim cells, overexposure reveals no ARL13B positive stain that is above the background detected in Arl13bhnn/hnn and Ift172wim/wim Arl13bhnn/hnn cells. Images taken at 40x. Scale bar is 5 μm. Experiments repeated in duplicate and examined 4–6 fields of view per condition.
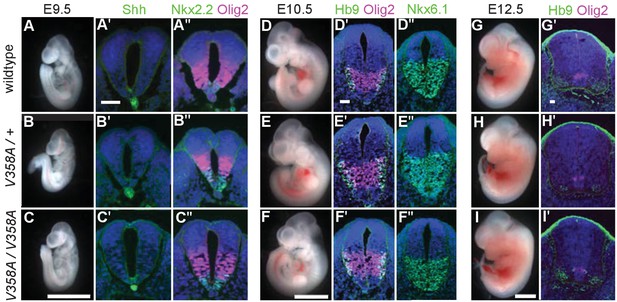
ARL13BV358A mediates normal Shh signaling and neural tube patterning.
(A–I) Whole embryo and neural tube sections of somite-matched littermates at E9.5, E10.5, and E12.5 stained with indicated markers of cell fate. Whole embryo scale bars: 2 mm. (A’-I’ and A’-F’) Shh-dependent neural tube patterning at three separate time points. Cell fate markers are listed above each image. All neural tube scale bars are 50 μm. Neural tube patterning was examined in five embryos for each embryonic stage. E, embryonic day.
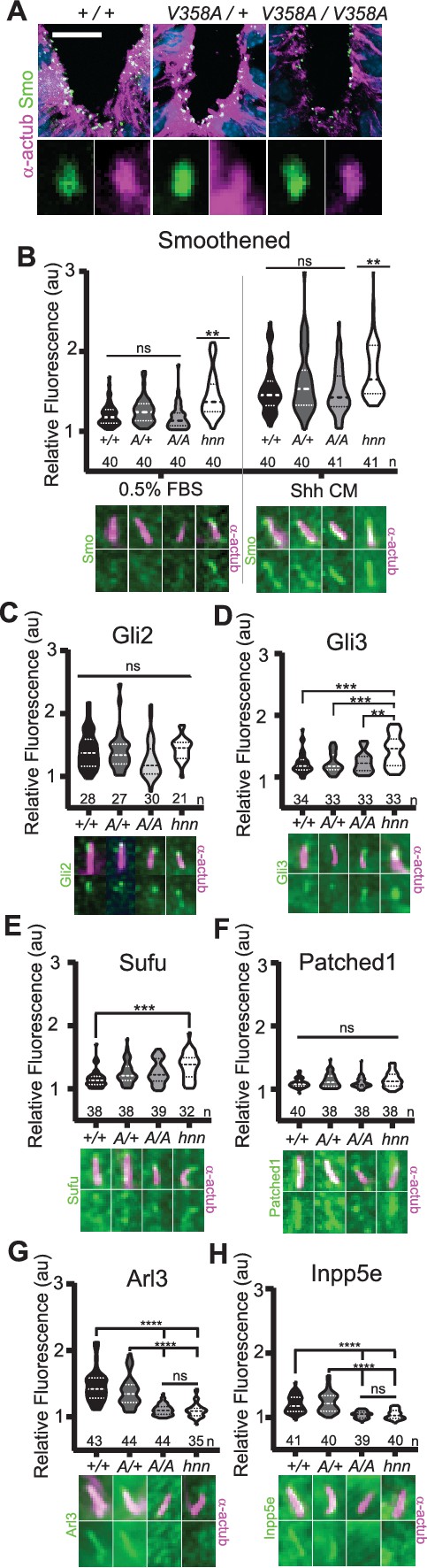
ARL13BV358A mediates normal ciliary enrichment of Shh components, but not ARL3 or INPP5E.
(A) Smo (green) enrichment in ventral neural tube cilia (acetylated α-tubulin: magenta) is normal in E10.5 embryos. Images are confocal projections. Scale bar is 25 μm. (B-H, Top) Quantification of average fluorescence intensity in the tip of the cilium (Gli2, Gli3, and Sufu) or the entire cilium (Ptch1, Smo, ARL3, and INPP5E) relative to background level. Violin plots depict relative fluorescent intensity per cilium with number of cilia examined below each plot. (B-H, Bottom) Representative images for each condition and cell type with the cilia marker acetylated α-tubulin (magenta) and indicated protein (green). Arl13b+/+ (+/+), Arl13bV358A/+ (A/+), Arl13bV358A/V358A (A/A) and Arl13bhnn/hnn (hnn). Data analyzed by one-way ANOVA and Tukey’s multiple comparisons, except Smo data analyzed by two-way ANOVA and 16 comparisons, corrected p<0.003 deemed significant. (**p<0.01, ***p<0.001, ****p<0.0001).
-
Figure 5—source data 1
Immunofluorescence of cilia proteins.
- https://cdn.elifesciences.org/articles/50434/elife-50434-fig5-data1-v2.xlsx
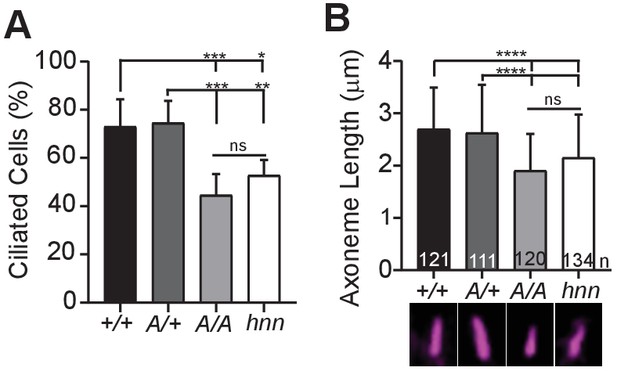
ARL13BV358A results in decreased ciliation rates and short cilia.
(A) Quantification of ciliation rates in all cell types; Arl13b+/+ (+/+), Arl13bV358A/+ (A/+), Arl13bV358A/V358A (A/A) and Arl13bhnn/hnn (hnn). Fewer Arl13bV358A/V358A and Arl13bhnn/hnn MEFs form cilia compared to Arl13b+/+ or Arl13bV358A/+ cells. (B) Quantification of axoneme length as labeled by acetylated α-tubulin (magenta) in indicated MEFs. Data are presented as mean (μm)± standard deviation with the number of cilia measured per genotype depicted at the base of each bar. Data analyzed by one-way ANOVA and Tukey’s multiple comparisons. (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
-
Figure 6—source data 1
Analysis of ciliated MEFs.
- https://cdn.elifesciences.org/articles/50434/elife-50434-fig6-data1-v2.xlsx
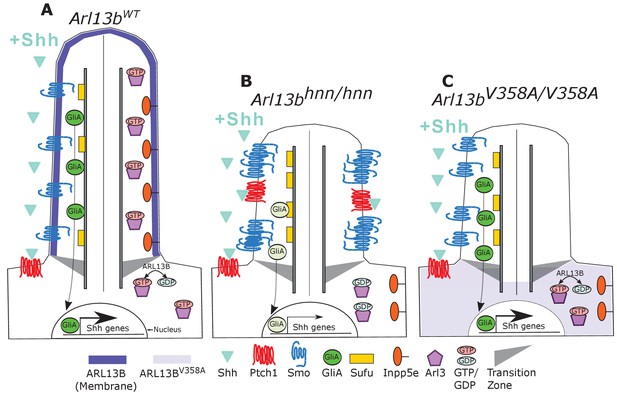
Model comparing complete loss of ARL13B function to ciliary loss of ARL13B function.
Wildtype (left), Arl13bhnn/hnn (middle), and Arl13bV358A/V358A (right) cilia represented as two halves. On the left half is the organization of Shh components in the presence of Shh ligand and on the right half is the organization of ARL13B interactors ARL3 and INPP5E. (A) ARL13B associates with the ciliary membrane. In the presence of Shh, Ptch1 is removed from cilia and Smo is visibly enriched in cilia. Smo is activated which promotes the processing of full-length Gli transcription factors into their activator forms (GliA), that are shuttled out of the cilium to promote Shh-dependent gene transcription. In addition, cilia proteins ARL3 and INPP5E are localized to the primary cilium. (B) In Arl13bhnn/hnn cells which are null for ARL13B, cilia are shorter than normal. Ciliary Ptch1 and Smo are visible, although Smo appears punctate instead of diffuse. In addition, loss of ARL13B decreases transcription of Shh-dependent genes due to lowered GliA. Arl13bhnn/hnn cilia also display a failure of INPP5E and ARL3 to localize to the cilium. Because ARL13B is the GEF for ARL3, we speculate in this schematic that ARL3 remains GDP bound in Arl13bhnn/hnn cells. (C) In Arl13bV358A/V358A cells, ARL13BV358A is not detectable in cilia and appears diffuse within the cell body. Arl13bV358A/V358A cilia, like Arl13bhnn/hnn cilia, are shorter than wildtype. We observe normal Shh-dependent ciliary Smo enrichment and normal Shh transcriptional output. However, ARL3 and INPP5E are absent from cilia, indicating that ciliary ARL13B is required for ciliary residence of these proteins.
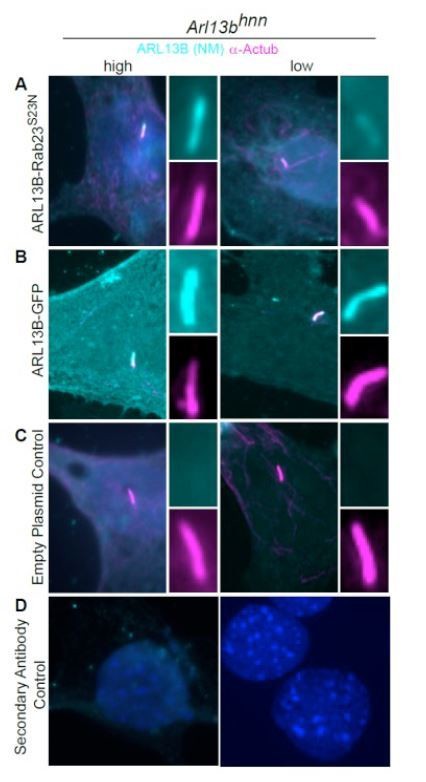
ARL13B-RAB23S23N fusion protein is present in the cilia of Arl13bhnn MEFs.
Arl13bhnn MEFs transfected with ARL13B-RAB23S23N, ARL13B-GFP, or control plasmid. (A) The ARL13BRAB23 S23N fusion protein is detectable in the cilium (acetylated a-tubulin, magenta) by ARL13B antibody (cyan, NeuroMab). (B) Wildtype ARL13B-GFP is present in the cilium in high- (left column) and low- (right column) expressing cells. (C) No ARL13B positive cilia were detected in control plasmid transfected cells. (D) Secondary only controls.
Tables
Genotype of mice born to heterozygous and/or homozygous carrier parents.
Data analyzed by chi-squared test.
| MICE | Sex | wildtype | V358A/+ | V358A/V358A | |
|---|---|---|---|---|---|
| Het × Het Avg. Litter 7.3 | M (46.5%) | 3 | 11 | 6 | χ2 = 0.60 |
| F (53.5%) | 6 | 8 | 9 | ||
| % of Total | 20.9 | 44.2 | 34.9 | ||
| Het × Hom Avg. Litter 7.5 | M (60%) | - | 10 | 11 | χ2 = 0.69 |
| F (40%) | - | 6 | 8 | ||
| % of Total | - | 45.7 | 54.3 | ||
| Hom × Hom Avg. Litter 9.5 | M (47.4%) | - | - | 9 | |
| F (52.6%) | - | - | 10 | ||
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information | |
|---|---|---|---|---|---|
| Gene (M. musculus) | Arl13b | MGI Cat# 6115585, RRID:MGI:6115585 | |||
| Genetic reagent (M. musculus) | hennin | PMID:17488627 | MGI Cat# 3580673, RRID:MGI:3580673 | Arl13b null allele | |
| Genetic reagent (M. musculus) | em1Tc (V358A) | This paper | MGI: 6256969 | New CRISPR Point mutant | |
| Genetic reagent (M. musculus) | FBV/NJ | Jackson Laboratory | Stock #001800 MGI:2163709 | ||
| Cell lines (M. musculus) | Fibroblast (normal, embryonic) | This paper | Maintained in Caspary lab | ||
| Antibody | Anti-Shh (Mouse Monoclonal) | Developmental Studies Hybridoma Bank | DSHB Cat# 5E1, RRID:AB_528466 | 1:5 | |
| Antibody | Anti-Nkx2.2 (Mouse Monoclonal) | Developmental Studies Hybridoma Bank | DSHB Cat# 74.5A5, RRID:AB_531794 | 1:5 | |
| Antibody | Anti-Hb9 (Mouse Monoclonal) | Developmental Studies Hybridoma Bank | DSHB Cat# 81.5C10, RRID:AB_2145209 | 1:5 | |
| Antibody | Anti-Nkx6.1 (Mouse Monoclonal) | Developmental Studies Hybridoma Bank | DSHB Cat# F55A10, RRID:AB_532378 | 1:50 | |
| Antibody | Anti-acetylated a-tubulin (Mouse Monoclonal) | Millipore Sigma | Sigma-Aldrich Cat# T6793, RRID:AB_477585 | 1:2500 | |
| Antibody | Anti-Olig2 (Rabbit Polyclonal) | Millipore Sigma | Millipore Cat# AB9610, RRID:AB_570666 | 1:300 | |
| Antibody | Anti-Arl13b (Mouse Monoclonal) | NeuroMab | UC Davis/NIH NeuroMab Facility Cat# 73–287, RRID:AB_11000053 | 1:1000 | |
| Antibody | Anti-Arl13b (Rabbit Polyclonal | Protein Tech | Proteintech Cat# 17711–1-AP, RRID:AB_2060867 | 1:1000 | |
| Antibody | Anti-Arl13b (Rabbit Polyclonal | PMID:17488627 | 503 | 1:1000 | |
| Antibody | Anti-Arl13b (Rabbit Polyclonal | PMID:17488627 | 504 | 1:1000 | |
| Antibody | Anti-Arl13b (Rabbit Polyclonal | PMID:17488627 | 505 | 1:1000 | |
| Antibody | Anti-Smo (Rabbit Polyclonal) | K. Anderson | 1:1000 | ||
| Antibody | Anti-IFT88 (Rabbit Polyclonal) | B. Yoder | 1:1000 | ||
| Antibody | Anti-Arl3 (Rabbit Polyclonal) | PMID:8034651 | 1:1000 | ||
| Antibody | Anti-Inpp5e (Rabbit Polyclonal) | Protein Tech | Proteintech Cat# 17797–1-AP, RRID:AB_2167120 | 1:150 | |
| Antibody | Anti-Gli2 (Guinea Pig Polyclonal) | J. Eggenschwiler | 1:200 | ||
| Antibody | Anti-Gli3 (Goat Polyclonal) | R and D | R and D Systems Cat# AF3690, RRID:AB_2232499 | 1:200 | |
| Antibody | Anti-Ptch1 (Rabbit Polyclonal) | R. Rohatgi | 1:150 | ||
| Antibody | Anti-Sufu (Goat Polyclonal) | Santa Cruz | Santa Cruz Biotechnology Cat# sc-10933, RRID:AB_671172 | 1:100 | |
| Antibody | Alexa Fluor goat anti-mouse IgG2a 488 | ThermoFisher | Thermo Fisher Scientific Cat# A-21131, RRID:AB_2535771 | 1:300 | |
| Antibody | Alexa Fluor goat anti-mouse IgG1 488 | ThermoFisher | Thermo Fisher Scientific Cat# A-21121, RRID:AB_2535764 | 1:300 | |
| Antibody | Alexa Fluor goat anti-mouse Ig 488 | ThermoFisher | Molecular Probes Cat# A-11029, RRID:AB_138404 | 1:300 | |
| Antibody | Alexa Fluor goat anti-mouse IgG 568 | ThermoFisher | Thermo Fisher Scientific Cat# A-11031, RRID:AB_144696 | 1:300 | |
| Antibody | Alexa Fluor donkey anti-rabbit IgG 488 | ThermoFisher | Thermo Fisher Scientific Cat# A-21206, RRID:AB_2535792 | 1:300 | |
| Antibody | Alexa Fluor donkey anti-rabbit IgG 555 | ThermoFisher | Thermo Fisher Scientific Cat# A-31572, RRID:AB_162543 | 1:300 | |
| Antibody | Alexa Fluor goat anti-rabbit IgG 568 | ThermoFisher | Thermo Fisher Scientific Cat# A-11011, RRID:AB_143157 | 1:300 | |
| Antibody | Alexa Fluor goat anti-mouse IgG2b 568 | ThermoFisher | Thermo Fisher Scientific Cat# A-21147, RRID:AB_2535783 | 1:300 | |
| Antibody | Hoechst nuclear stain | Millipore Sigma | 94403 | 1:3000 | |
| Sequence-based reagent | CRISPR gRNA | Millpore Sigma, this paper | CCAGTCAATACAGACGAGTCTA | ||
| Sequence-based reagent | CRISPR donor oligo | Millpore Sigma, this paper | CCTATATTCTTCTAGAAAACAGTAAGAAGAAAACCAAGAAACTACGAATGAAAAGGAGTCATCGGGCAGAACCAGTGAATACAGACGAGTCTACTCCAAAGAGTCCCACGCCTCCCCAAC | ||
| Sequence-based reagent | F-231-Cac8I | This Paper | PCR Primer AAGAATGAAAAGGAGTCAGCG | ||
| Sequence-based reagent | REV-1 | This Paper | PCR PrimerTGAACCGCTAATGGGAAACT | ||
| Peptide, recombinant protein | Arl13bWT-GST | This Paper | Purified from cells | ||
| Peptide, recombinant protein | Arl13bV358A-GST | This Paper | Purified from cells | ||
| Peptide, recombinant protein | Arl3 (human) | PMID:11303027 | Purified from cells | ||
| Peptide, recombinant protein | Cas9 | Millipore Sigma | C120010 | 50 μg | |
| Peptide, recombinant protein | Cac8I | New England Biolabs | R0579L | Restriction enzyme | |
| Chemical compound, drug | Ciliobrevin-D | Millipore Sigma | 250401 | 30 μM | |
| Chemical compound, drug | [3H]GDP | PerkinElmer Life Sciences | NET966 | 3000 cpm/pmol | |
| Chemical compound, drug | GTPgS35 | PerkinElmer Life Sciences | NEG030H | ||
| Software, algorithm | ImageJ software | ImageJ (http://imagej.nih.gov/ij/) | ImageJ, RRID:SCR_003070 | ||
| Software, algorithm | GraphPad Prism software | GraphPad Prism (https://graphpad.com) | GraphPad Prism, RRID:SCR_002798 | Version 8.0.0 | |





