β3-Adrenoceptor redistribution impairs NO/cGMP/PDE2 signalling in failing cardiomyocytes
Figures
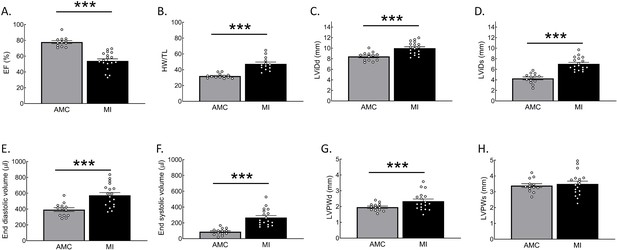
Histograms of echocardiography and biometric data in rat age matched control (AMC) hearts and hearts with myocardial infarction (MI).
(A) Ejection Fraction, (B) Heart weight (HW) corrected to tibia length (TL), (C) left ventricular diastolic internal dimension (LViDd), (D) left ventricular systolic internal dimension (LViDs), (E) end-diastolic volume, (F) end-systolic volume, (G) end-diastolic left ventricular posterior wall thickness (LVPWd), (H) end-systolic left ventricular posterior wall thickness (LVPWs). Statistical significance was analysed via two-sided T-test. ***p<0.001.
-
Figure 1—source data 1
Echocardiography and biometric data for respective treatment groups.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig1-data1-v2.xlsx
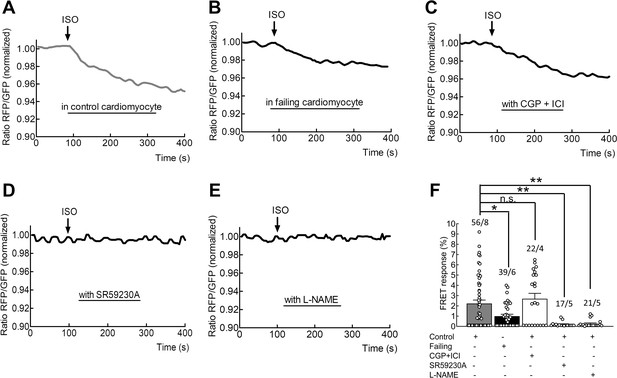
Measurements of β3-AR-dependent cGMP responses in adult cardiomyocytes.
Representative FRET tracings of a control (A) or a failing cardiomyocyte (B) treated with isoproterenol (100 nmol/L). FRET responses of control cardiomyocytes pre-treated for 5 min with either with the β1-AR and β2-AR inhibitors CGP20712A (100 nmol/L) and ICI118,551 (50 nmol/L) (C), β3-AR inhibitor SR59230A (100 nmol/L) (D) or for 10 min with the nitric oxide synthase blocker L-NAME (300 µmol/L) (E), before the application of isoproterenol (100 nmol/L). (F) Quantification of whole cell cGMP-FRET responses from protocols described in A-E). Error bars represent standard error of the mean. Numbers of cells/hearts are shown above the bars. Statistical significance was calculated via Mann Whitney U-test for independent treatments versus control followed by Bonferroni correction: *p<0.05; **p<0.01.
-
Figure 2—source data 1
FRET microscopy data - 'whole-cell' analysis.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig2-data1-v2.xlsx
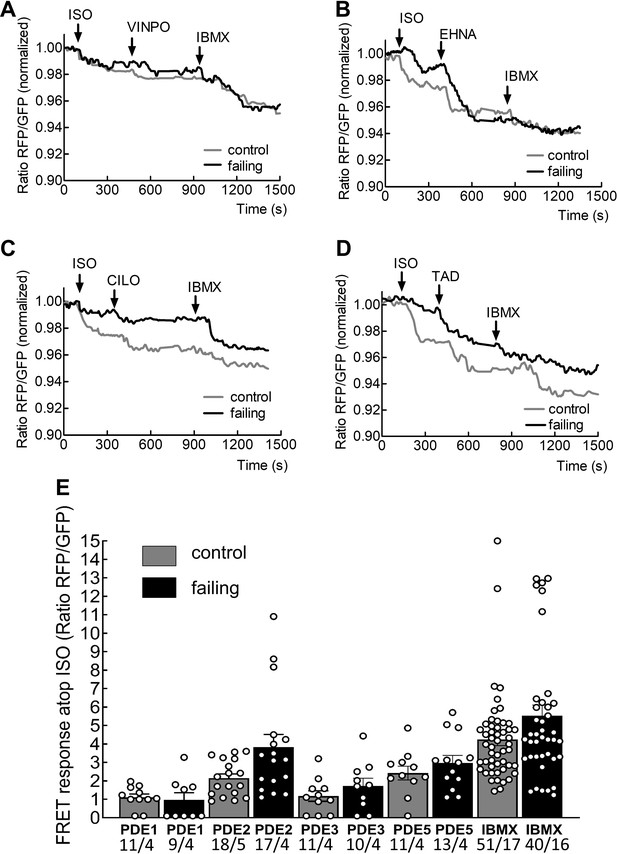
Investigation of phosphodiesterase regulation of β3-AR/cGMP in adult cardiomyocytes.
Representative FRET response curves of control (grey line) and failing (black line) cardiomyocytes following whole cell treatment with isoproterenol (100 nmol/L) followed by the PDE1 blocker vinpocetine (VINPO, 10 µmol/L) (A), the PDE2 inhibitor EHNA (10 µmol/L) (B), the PDE3 inhibitor cilostamide (CILO, 10 µmol/L) (C) and the PDE5 inhibitor tadalafil (TAD, 100 nmol /L) (D) followed by the non-specific PDE blocker IBMX (100 µmol/L). The scatter plot/histograms present whole cell cGMP-FRET responses evoked by PDE inhibition further to the isoproterenol responses in % from (A–D) (E) Error bars represent standard error of the mean. Numbers of cells/hearts are shown below the bars. Statistical significance was calculated via mixed ANOVA followed by χ2-test: No statistically significant differences between control and failing conditions for any PDE could be detected, only tendencies to increased responses for PDE2, PDE3 and PDE5 inhibitors.
-
Figure 3—source data 1
FRET microscopy data - 'whole cell' analysis PDEs.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig3-data1-v2.xlsx
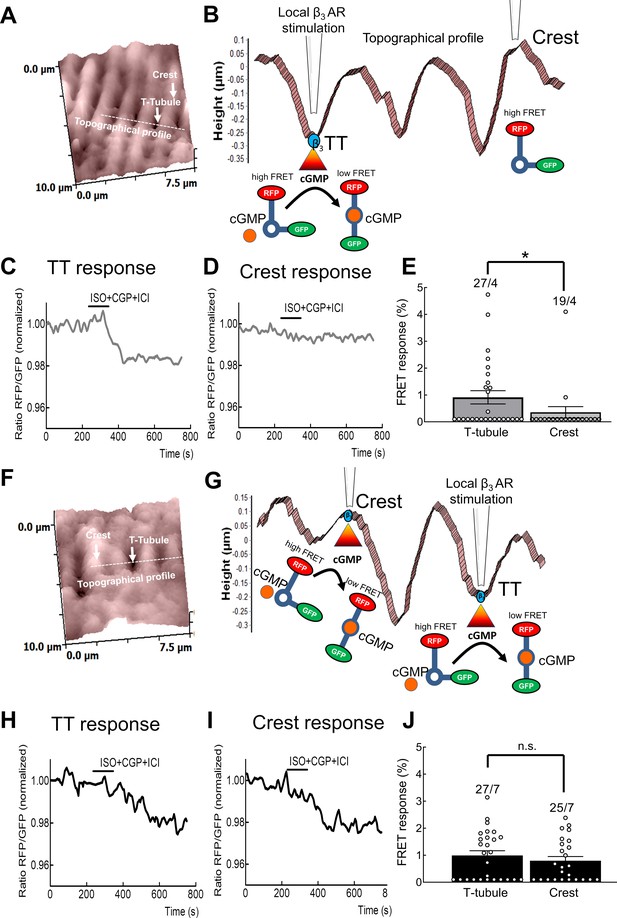
Identification of β3-AR/cGMP signal localization using scanning ion conductance microscopy (SICM) combined with Förster Resonance Energy Transfer (FRET).
Representative SICM surface scan of a 10 × 10 µm area of a healthy (A) and a failing cardiomyocyte (F). White arrows indicate T-tubule or crest structures and a dotted white line indicates the areas selected for the topographical profiles presented in (B) and (G). Representative topographical profiles of healthy (B) and failing cardiomyocytes (G). Images present schematics of local β3-AR stimulation with Isoproterenol (50 µmol/L) either inside a T-tubule opening or on the cell crest via the SICM nanopipette. Representative FRET response curves during perfusion with the β1-AR blocker CGP20712A (100 nmol/L) and the β2-AR blocker ICI118551 (50 nmol/L) and local stimulation inside the T-tubule (C) and the crest of a control cardiomyocyte (D) or the T-tubule (H) and crest (I) of a failing cardiomyocyte. Scatter plots presenting the localised cGMP-FRET responses of control (E) and failing cardiomyocytes (J). Error bars represent standard error of the mean. Numbers of cells/hearts are shown above the bars. *p<0.05, n.s. – not significant by Mann-Whitney U test.
-
Figure 4—source data 1
FRET microscopy data - SICM/FRET.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig4-data1-v2.xlsx
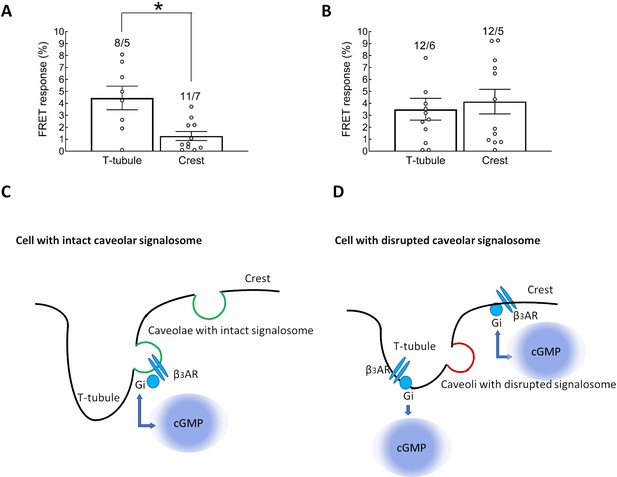
The effect of Caveolin-3 displacement on β3AR/cGMP localization measured by SICM/FRET.
Control cardiomyocytes (A) treated with scrambled peptide and (B) with C3SD peptide targeting caveolin-3 scaffolding domains. In scrambled peptide treated control cells, the β3-AR-induced cGMP signal is significantly stronger in T-tubules than in de-tubulated crest membrane areas. In cardiomyocytes treated with the C3SD peptide, which disrupts caveolar signalosomes the β3-AR derived cGMP signal in de-tubulated crest areas is even stronger than when stimulated in T-tubules. Cardiomyocyte membrane structures with (C, in green) and without (D, in red) intact caveolar signalosomes. (C) Confined β3-AR/cGMP signalling is facilitated by intact caveolar signalosome localization inside T-tubules, which generates regulated cGMP pools. (D) Dissociation of caveolae signalosomes that is through the C3SD peptide competing with the caveolae scaffolding domain and the associated increase in β3-ARs in the crest leads to unconfined cGMP pools. Error bars represent standard error mean. Numbers of cells/hearts are shown above the bars. Statistical significance was analyzed by mixed ANOVA followed by χ2-test: *p<0.05, n.s. – not significant.
-
Figure 4—figure supplement 1—source data 1
SICM/FRET data TAT-C3SD Treatment.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig4-figsupp1-data1-v2.xlsx
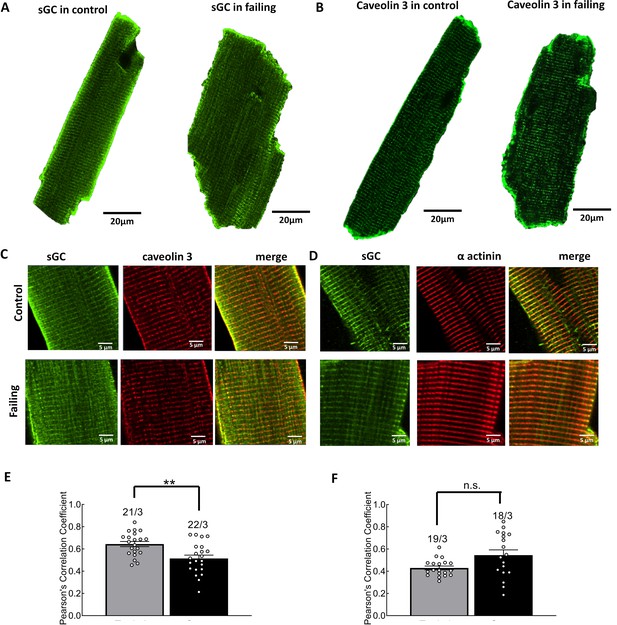
Investigation of sGC and caveolin-3 localization in control and failing cardiomyocytes.
Representative, confocal images of sGCα1 (A) and caveolin-3(B) in control and failing cardiomyocytes. Magnified representations of double staining of sGCα1(C) with caveolin-3 and of sGCα1 (D) with α-actinin. Quantification of sGC (E) and caveolin-3 and of sGC (F) and α-actinin co-localization in control and failing cells. Error bars represent standard error of the mean. Numbers of cells/hearts are shown above the bars. Statistical significance was analyzed via mixed ANOVA followed by χ2-test; **p<0.01, n.s. – not significant.
-
Figure 5—source data 1
Immunostaining data sGC and Cav3 colocalization control.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig5-data1-v2.xlsx
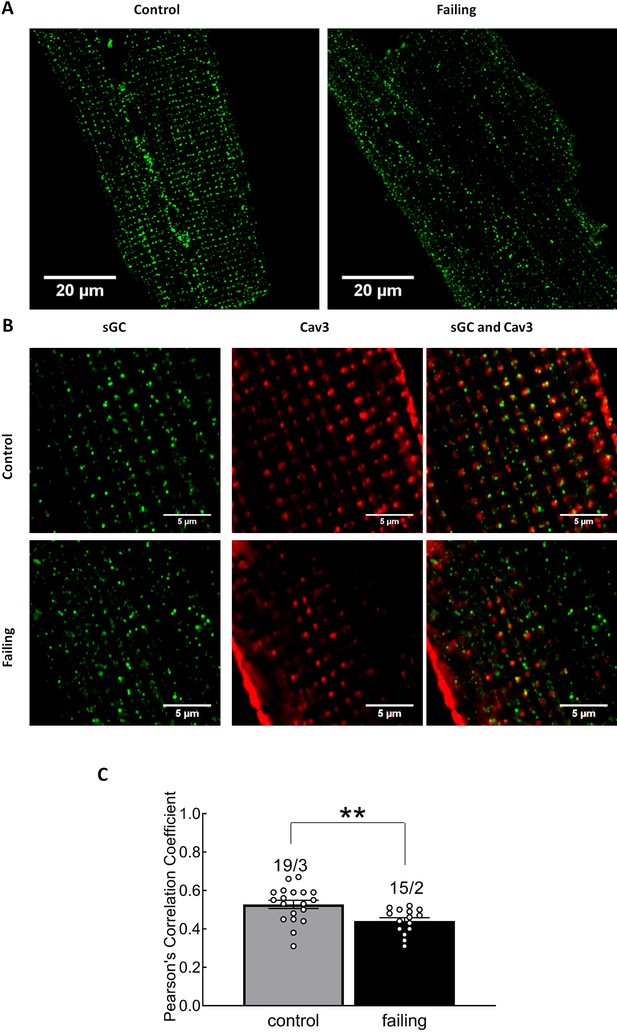
Investigation of sGC and caveolin-3 localization in control and failing cardiomyocytes.
Representative, confocal images of sGCβ1 in control and failing cardiomyocytes (A) including higher magnifications (B). Quantification of co-localization in control and failing cells is in (C). Error bars represent standard error of the mean. Numbers of cells/hearts are shown above the bars.(Statistical significance was analyzed by mixed ANOVA followed by χ2-test. **p<0.01.
-
Figure 5—figure supplement 1—source data 1
Immunostaining data sGC and Cav3 colocalization failing cells.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig5-figsupp1-data1-v2.xlsx
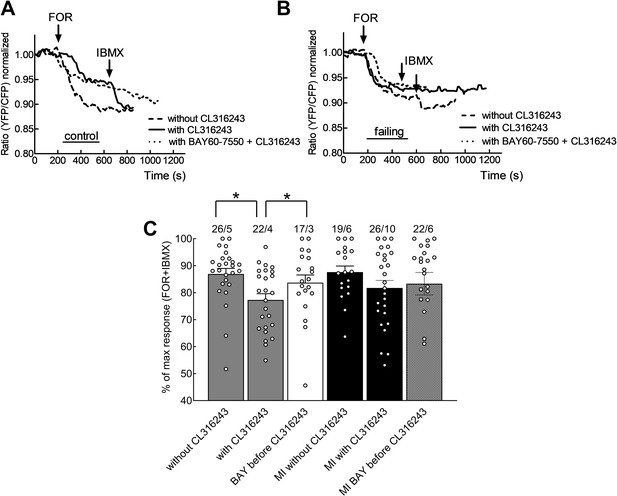
β3-AR signalling can affect cAMP levels via PDE2.
Representative FRET responses for control cardiomyocytes (A) or failing cardiomyocytes from myocardial infarction (MI) hearts (B) stimulated using forskolin (FOR, 10 µmol/L) applied with or without the β3-AR agonist CL316243 (1 µmol/L) and with or without pre-treatment with the PDE2 inhibitor BAY60-7550 (100 nmol/L). (C) Scatter plot/histogram presenting whole cell cAMP-FRET responses depicted as the percentage of the maximal possible cAMP FRET response (=Forskolin followed by IBMX). The measured Forskolin or IBMX responses were the respective maximal responses, equalling the lowest FRET ratio value, achieved after each stimulus. The effect of the β3-AR agonist on forskolin induced cAMP levels is no longer discernible after inhibition of PDE2 in control cardiomyocytes. This effect is no longer significant in failing cardiomyocytes. Error bars represent standard error mean. Statistical significance was calculated via mixed ANOVA followed by χ2-test* p<0.05.
-
Figure 6—source data 1
FRET microscopy data cAMP/cGMP crosstalk experiment.
- https://cdn.elifesciences.org/articles/52221/elife-52221-fig6-data1-v2.xlsx
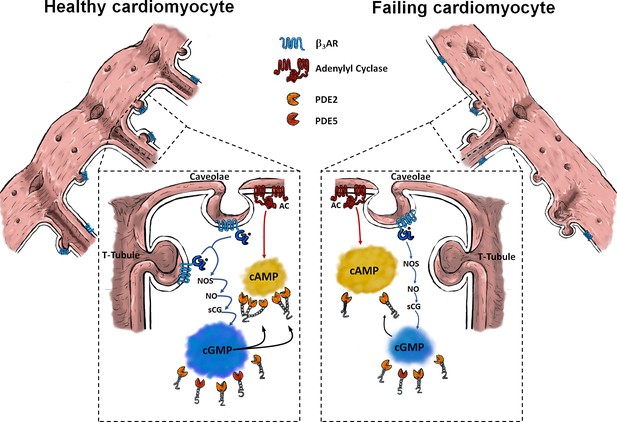
Schematic of β3-AR/cGMP signalling in healthy (left side) and failing (right side) cardiomyocytes.
In healthy cardiomyocytes, functional β3-ARs are associated with caveolar signalosomes and localized mostly in T-tubules. Via Gi/NOS/NO/sGC/cGMP signalling they can suppress strong cAMP responses by stimulating increased PDE2 dependent cAMP degradation through cGMP binding to GAF-B domain of PDE2. In heart failure, increased presence of β3-AR activity at non-tubular plasma membrane (Crest) and away from caveolin-3 associated membrane domains might disrupt receptor-associated cGMP signalosomes and lead to disrupted cGMP/cAMP-crosstalk.





