Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation
Figures
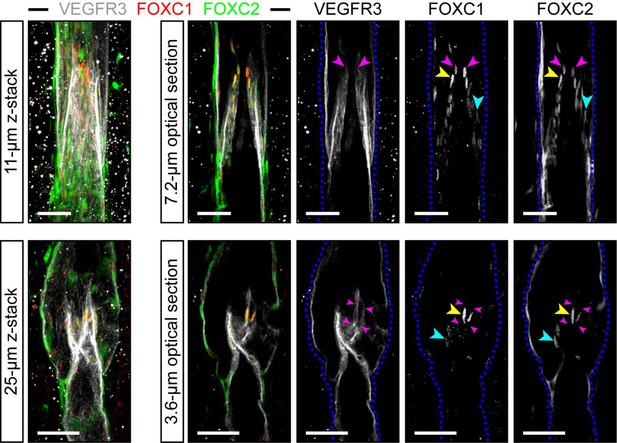
FOXC1 is highly expressed in a subset of LECs at the free edge of lymphatic valve leaflets.
Representative images of maximum intensity projections (left) and optical sections (right) from mesentery collecting vessels of a 4 week old C57Bl6 mouse immunostained with VEGFR3 (white), FOXC1 (red), and FOXC2 (green). Purple arrowheads denote the position of valve leaflet free-edges. Yellow arrowheads denote FOXC1HIGH/FOXC2HIGH LECs located near the leaflet free-edge. Blue arrowheads denote FOXC2-positive LECs in valve leaflets with only weakly expressed FOXC1. Dashed blue lines on the single-channel images outline the vessel borders. Scale bars are 50 μm.
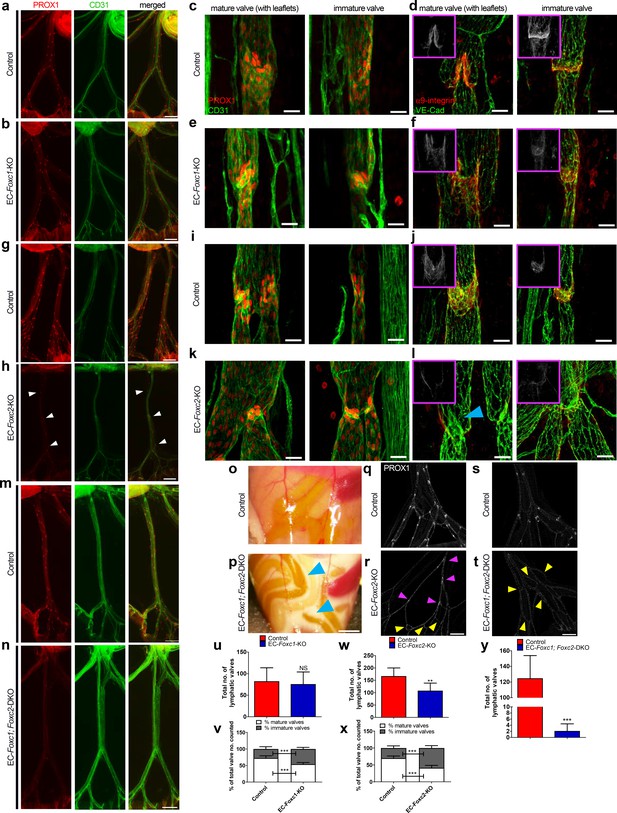
Compound endothelial-specific Foxc1; Foxc2 mutants present severe chylous ascites and are nearly absent of PROX1-high expressing lymphatic valves.
(a, b, g, h, m, n) Representative images of PROX1 and CD31 immunostained lymphatic collecting vessels in P6 littermate control (a, g, m) and EC-Foxc1-KO (b), EC-Foxc2-KO (h), and EC-Foxc1; Foxc2-DKO (n) individuals. White arrowheads denote PROX1-high valves. Scale bars are 500 μm. (c – f, i – l) Representative images of mature and immature lymphatic valves immunostained with PROX1 and CD31 or α9-integrin and VE-Cadherin in P6 littermate control (c, d, i, j) and EC-Foxc1-KO (e, f) or EC-Foxc2-KO individuals (k, l). Pink inserts denote single channel α9-integrin (white) images. Blue arrowhead denotes VE-Caderhin-positive intraluminal valve leaflet with markedly reduced α9-integrin expression in l. Scale bars are 25 μm. (o, p) Appearance of chylous ascites in the peritoneal cavity of a P6 EC-Foxc1; Foxc2-DKO mouse (p) compared to littermate control (o). Blue arrow heads indicate chylous effusion; scale bar equals 1 mm. (q – t) Representative images of PROX1 immunostained lymphatic collecting vessels in P6 littermate control (q, s) and EC-Foxc2-KO (r) or EC-Foxc1; Foxc2-DKO (t) individuals show degeneration of lymphatic valves in Foxc2 mutants and regression of collecting vessels into a primitive lymphatic architecture in EC-specific Foxc1 and Foxc2 mutants. Pink arrowheads denote degenerating PROX1-high expressing valve regions. Yellow arrowheads highlight looping and interconnections between branches of collecting vessels. Scale bar is equal to 200 μm. (u) Quantification of total lymphatic valve number (identified by PROX1-high expression) in lymphatic collecting vessels of P6 Control and EC-Foxc1-KO individuals. N = 7 for Control and N = 7 for EC-Foxc1-KO individuals, four collecting vessels analyzed per individual. (v) Percentage of mature and immature lymphatic valves normalized to total valves counted in P6 Control and EC-Foxc1-KO individuals. (w) Quantification of total lymphatic valve number in lymphatic collecting vessels of P6 Control and EC-Foxc2-KO individuals. N = 9 for Control and N = 9 for EC-Foxc2-KO individuals, four collecting vessels analyzed per individual. (x) Percentage of mature and immature lymphatic valves normalized to total valves counted in P6 Control and EC-Foxc2-KO individuals. (y) Quantification of total lymphatic valve number in lymphatic collecting vessels of P6 Control and EC-Foxc1; Foxc2-DKO individuals. N = 6 for Control and N = 6 for EC-Foxc1; Foxc2-DKO individuals, four collecting vessels analyzed per control individual and all lymphatic collecting vessels assessed per mutant individual. Data are presented as mean (± SD) and analyzed using Student’s t-test. NS denotes no significance. ** denotes p<0.01. *** denotes p<0.001.
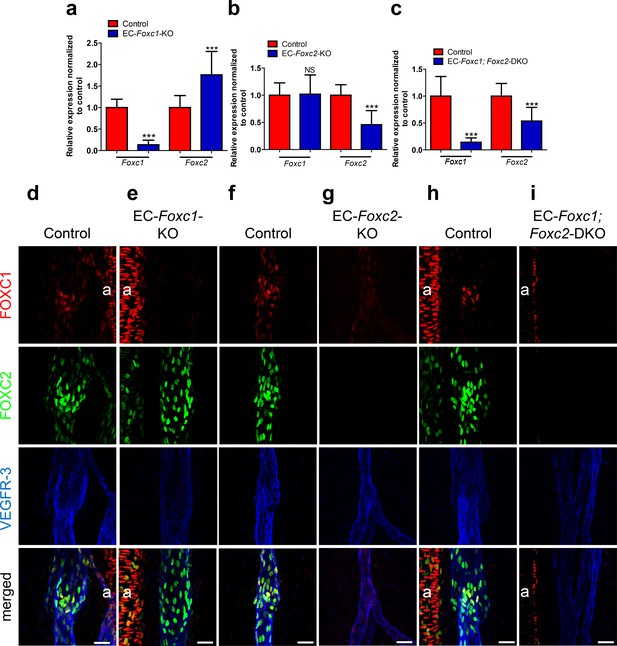
Expression of Foxc1 and Foxc2 mRNA and FOXC1 and FOXC2 protein levels in endothelial-specific knockout models.
qPCR analysis was performed on RNA extracted from CD31+ cardiac cells of P6 EC-Foxc1-KO (a), EC-Foxc2-KO (b), and EC-Foxc1; Foxc2-DKO (c) individuals and littermate controls. Data are shown as mean relative expression (± SD) normalized to WT littermate controls and analyzed using Student’s t-test. N = 11 for Foxc1 Control and N = 12 for EC-Foxc1-KO individuals, N = 14 for Foxc2 Control and N = 14 for EC-Foxc2-KO individuals, N = 12 for Foxc1; Foxc2 Control and N = 11 for EC-Foxc1; Foxc2-DKO individuals. *** denotes p<0.001. NS denotes no significance. (d – i) Representative images of FOXC1, FOXC2, and VEGFR-3 immunostained lymphatic collecting vessels from P6 littermate control (d, f, h) and EC-Foxc1-KO (e), EC-Foxc2-KO (g), and EC-Foxc1; Foxc2-DKO (i) individuals. a, arterioles covered by FOXC1-positive smooth muscle cells. Scale bars are 25 µm.
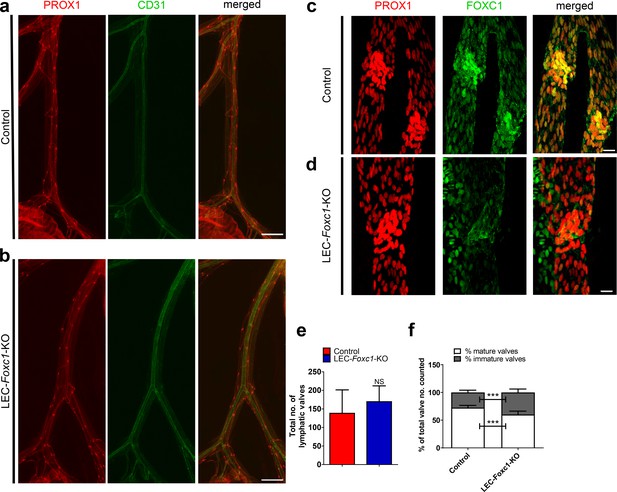
LEC-specific knockout of Foxc1 recapitulates phenotype observed in Cdh5-CreERT2; Foxc1fl/fl knockout mice.
(a, b) Representative images of PROX1 and CD31 immunostained lymphatic collecting vessels in P6 Control (a) and LEC-Foxc1-KO (b) individuals administered 100 μg of Tamoxifen from P1-5. Scale bars are 500 μm. (c, d) Representative images of PROX1 and FOXC1 immunostained lymphatic collecting vessels in P6 Control (c) and LEC-Foxc1-KO (d) individuals administered 100 μg of Tamoxifen from P1-5. Scale bars are 20 μm. (e) Quantification of total lymphatic valve number in lymphatic collecting vessels of P6 Control and LEC-Foxc1-KO individuals. N = 7 for Control and N = 8 for LEC-Foxc1-KO individuals, four collecting vessels analyzed per individual. (f) Percentage of mature and immature lymphatic valves normalized to total valves counted in P6 Control and LEC-Foxc1-KO individuals. Data are presented as mean (± SD) and analyzed using Student’s t-test. *** denotes p<0.001. NS denotes no significance.
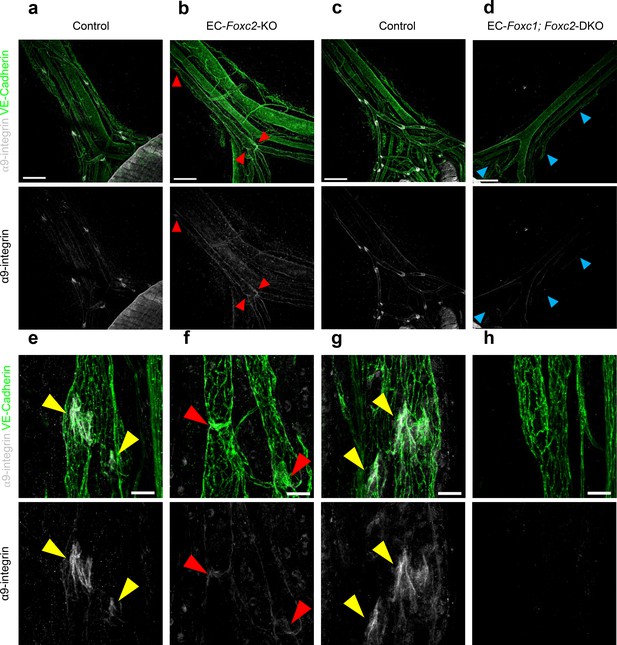
α9-integrin expression is absent in lymphatic collecting vessels of EC-specific Foxc1; Foxc2 mutant mice.
Representative, low and high-power images of P6 mesenteries immunostained with antibodies targeted towards α9-integrin and VE-Cadherin in Foxc2 Control (a, e), EC-Foxc2-KO (b, f), Foxc1; Foxc2 Control (c, g), and EC-Foxc1; Foxc2-DKO (d, h) individuals respectively. Yellow arrowheads highlight α9-integrin- positive lymphatic valves. Red arrowheads highlight α9-integrin- positive regressing lymphatic valves in Foxc2 mutants. Blue arrowheads denote lymphatic collecting vessels absent of α9-integrin positive valve expression in compound Foxc1; Foxc2 mutants. Scale bars are 200 µm in panels a – d and 25 μm in panels e – h.
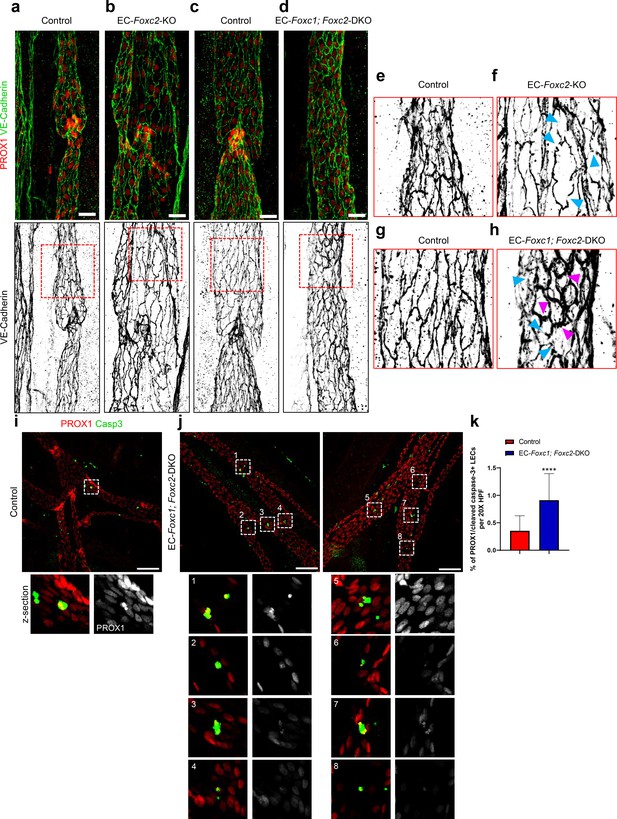
Cell elongation and junctional integrity is markedly impaired in compound EC-specific Foxc1; Foxc2 mutants, accompanied by increased apoptosis in lymphatic collecting vessels.
(a – d) Representative images of lymphatic collecting vessels immunostained with antibodies targeted to PROX1 and VE-Cadherin in a P6 EC-Foxc2-KO (b) and littermate control (a), and EC-Foxc1; Foxc2-DKO (d) and littermate control (c) individuals respectively. Scale bars are 25 μm. (e – h) Representative images of VE-Cadherin immunostaining in magnified fields denoted by boxed regions in (a – d) show discontinuous cell-cell junctions (blue arrowheads) observed in both EC-Foxc2-KO and EC-Foxc1; Foxc2-DKO mice and the presence of overlapping cell junctions (pink arrowheads) upon combined inactivation of Foxc1 and Foxc2 (h). (i, j) Representative images of PROX1 and cleaved caspase-3 antibody immunostained lymphatic collecting vessels from P6 littermate control (i), and EC-Foxc1; Foxc2-DKO (j) individuals. Boxed regions denote corresponding magnified z-section images from confocal maximum intensity projections represented in i) and j). Scale bars are 100 μm. (k) Quantitative analysis of the percentage of PROX1/cleaved caspase 3-positive LECs in 20X high-powered fields from mesentery collecting vessels of P6 Foxc1; Foxc2 Control and EC-Foxc1; Foxc2-DKO individuals. N = 27 fields from six individuals for Foxc1; Foxc2 Control and N = 34 fields from seven individuals for EC-Foxc1; Foxc2-DKO individuals. Data are presented as mean (± SD) and analyzed using Student’s t-test. **** denotes p<0.0001.
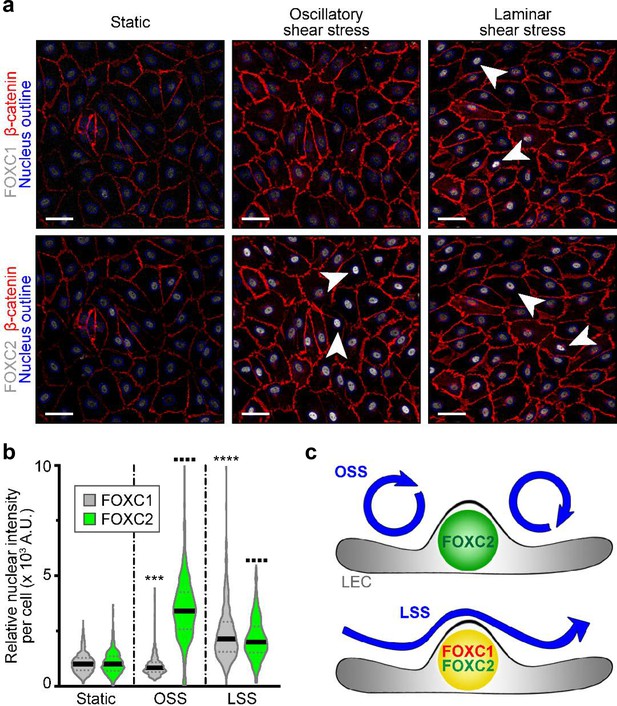
FOXC1 expression is induced by unidirectional laminar shear stress, but not by reciprocating shear stress, on the contrary to FOXC2.
(a) Representative images of cultured LECs under static, OSS, or LSS show increased expression of FOXC1 when subjected to 24 hr to LSS, whereas FOXC2 is induced by both OSS and LSS. Immunostaining for β-catenin (red), FOXC1 (white, top panels), and FOXC2 (white, bottom panels). Nuclei are outlined with dashed blue lines. Arrowheads denote cells with strong nuclear expression of FOXC1 or FOXC2. Scale bars are 10 μm. (b) Corresponding quantification of FOXC1 or FOXC2 nuclear intensity per cell (100–200 cells were quantified per condition). Data are presented as violin plots with median values indicated by solid lines and are representative of 3 independent experiments. P-values were obtained using mixed-effects analysis. ***p<0.001, ****p<0.0001 to Static FOXC1 and ▪▪▪▪ p<0.0001 to Static FOXC2 . (c) Scheme summarizing the observed regulation of FOXC1 and FOXC2 by flow-mediated shear stress: cells under OSS have high levels of FOXC2, whereas cells under LSS have high levels of both FOXC1 and FOXC2.
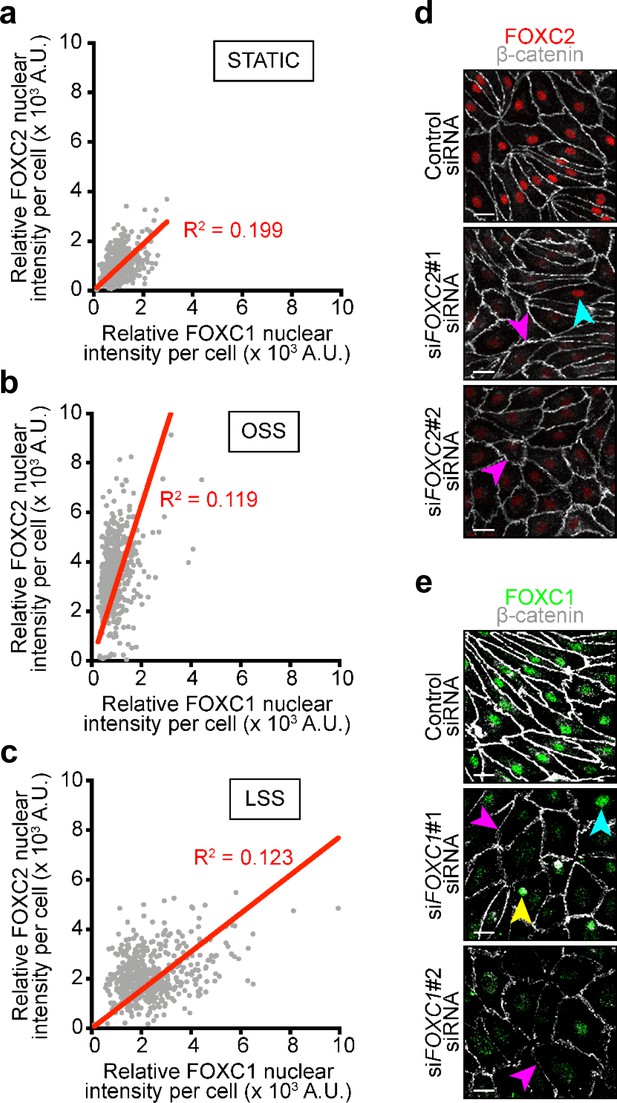
Absence of correlation of FOXC1 and FOXC2 expression in relation to mechanical stress, and validation of FOXC1 and FOXC2 antibodies and siRNA used in this study.
(a–c) Relative FOXC1 and FOXC2 nuclear intensity was quantified per each cell and a linear regression analysis was applied with FOXC1 intensity on the x-axis and FOXC2 intensity on the y-axis for static (a), OSS (b), and LSS (c) assuming interaction with 0 for both axes. The correlation coefficient (R2) is indicated on each graph in association with the linear regression and shows absence of correlation. (d–e) Representative images of confluent LECs 2 days after transfection with control siRNA (upper) or two different target-specific siRNA (middle and lower) showing efficient knockdown in each case. Cells were immunostained for β-catenin (white) and FOXC2 (red) in (d) or FOXC1 (green) in (e). Scale bars are 30 µm.
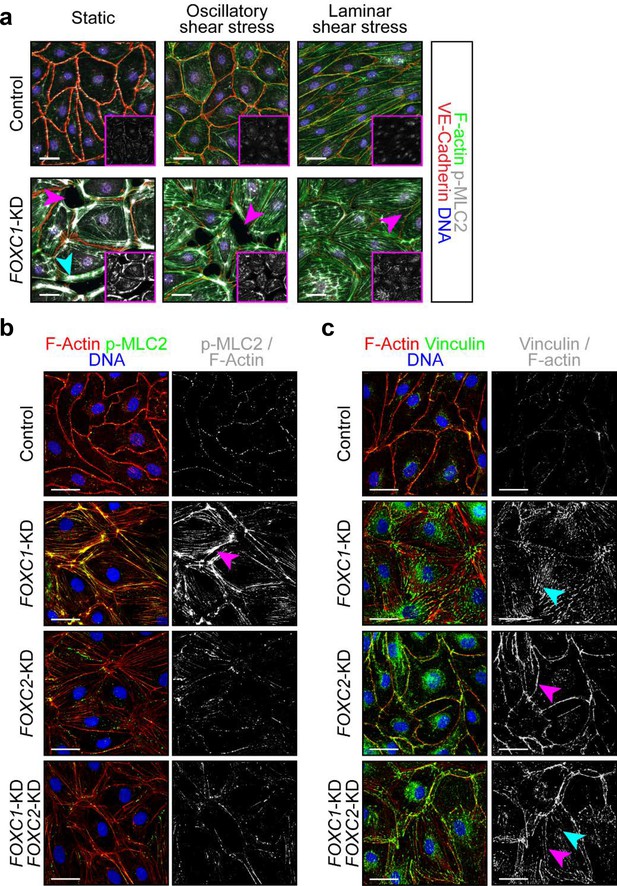
FOXC1 regulates actin cytoskeletal organization and cell-matrix adhesion.
(a) Representative images of Control and FOXC1-KD LECs cultured under static, OSS, or LSS show reduced viability and higher number of contractile stress fibers (cyan arrowhead). Immunostaining for VE-Cadherin (red), F-actin (green), p-MLC2 (white), and DNA (blue). Pink arrowheads denote areas devoid of cells in the endothelial monolayer. Pink inserts denote single-channel p-MLC2 (white) images. Scale bars are 30 μm. (b, c) Representative images of Control, FOXC1-KD, FOXC2-KD, and combined FOXC1-KD/FOXC2-KD LECs show higher number of contractile stress fibers (b, pink arrowhead) and focal adhesions (c, cyan arrowhead) upon FOXC1 knockdown. In comparison, FOXC2 knockdown rather induced focal adherens junctions (c, pink arrowhead). (b) Immunostaining for F-actin (red), p-MLC2 (green), and DNA (blue). Images on the right show a mask applied to visualize only double F-actin+/p-MLC2+ (white) stress fibers. Scale bars are 30 μm. (c) Immunostaining for F-actin (red), vinculin (green), and DNA (blue). Images on the right show a mask applied to visualize only double F-actin+/vinculin+ (white) adhesion sites. Scale bars are 30 μm.
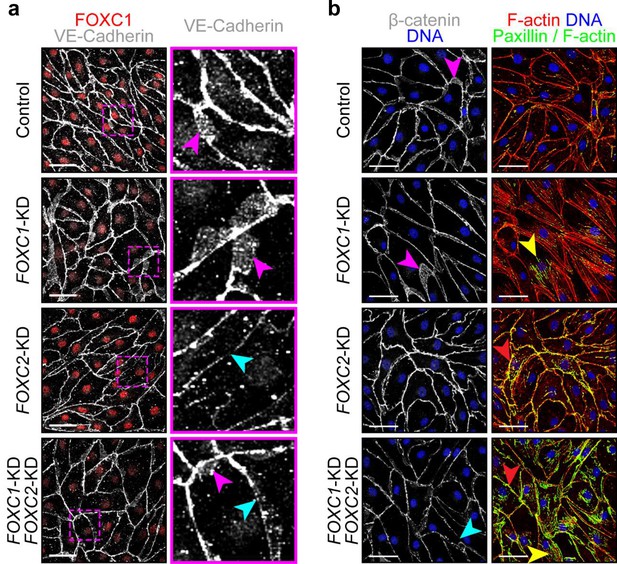
FOXC1 regulates intercellular junctions, actin cytoskeletal organization and cell-matrix adhesion.
(a) Representative images of Control, FOXC1-KD, FOXC2-KD, and combined FOXC1-KD/FOXC2-KD LECs show overlapping junctions (pink arrowhead) upon FOXC1 knockdown but disrupted junctions (cyan arrowhead) upon FOXC2 knockdown. Immunostaining for VE-Cadherin (white) and FOXC1 (red). Images on the right show a 4x zoom of the area delineated by the pink box. Scale bars are 50 μm. (b) Representative images of Control, FOXC1-KD, FOXC2-KD, and combined FOXC1-KD/FOXC2-KD LECs show overlapping junctions (pink arrowhead) upon FOXC1 knockdown but disrupted junctions (cyan arrowhead) upon combined FOXC1 and FOXC2 knockdown. Immunostaining for β-catenin (white) and DNA (blue). Images on the right show immunostaining for F-actin (red), DNA (blue) and a mask applied to visualize only double F-actin+/paxillin+ (green) adhesion sites. Yellow arrowheads denote increased focal adhesions. Red arrowheads denote increased focal adherens junctions at the cell cortex. Scale bars are 30 μm.
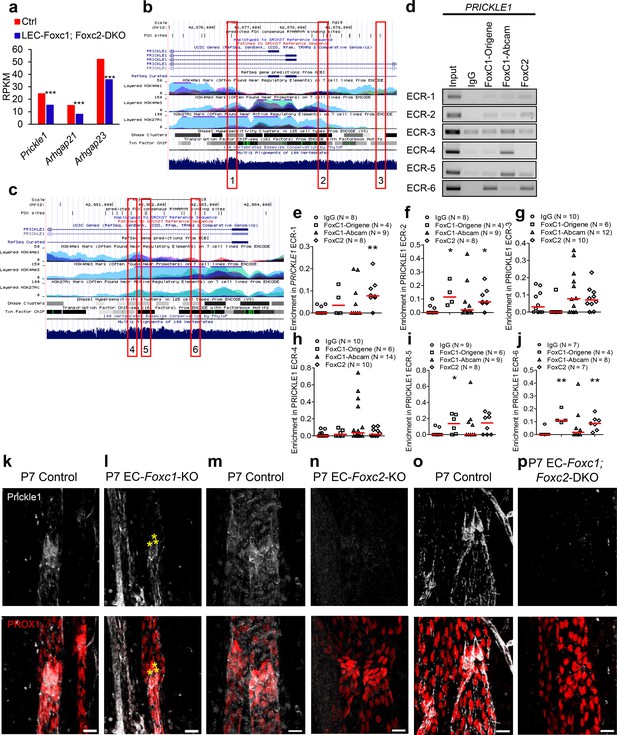
FOXC1 and FOXC2 mediate lymphatic expression of the PCP signaling component and RHOA signaling regulator Prickle1.
(a) Reduced expression of Prickle1, Arhgap21, and Arhgap23 in Foxc1/c2-compound mutant LECs isolated from the dorsal skin at E15.5. Graph shows RPKM values from RNA-seq analysis. *** denotes p<0.001. (b, c) Putative FOX-binding sites in regions of the human PRICKLE1 locus as viewed on the UCSC genome browser (http://genome.ucsc.edu; Kent et al., 2002). Vertical lines on the ‘FOX sites’ track indicate putative FOX binding sites predicted using HOMER (see methods). Red boxes indicate evolutionary conserved regions (ECRs) containing FOX-binding sites between human and mouse genomes that are conserved and aligned. (d) ChIP showing specific binding of FOXC1 and FOXC2 to the consensus FOX-binding sites within ECRs 1, 2, 3, 4, 5, and six in PRICKLE1 in HDLECs. (e–j) ChIP assays were performed using antibodies against Foxc1, Foxc2, and normal goat IgG. The binding of FOXCs to candidate ECRs identified in b and c in the PRICKLE1 locus was determined with regular PCR and expressed as relative folds of input whose band intensity was normalized to 1. Data are presented as a scatter plot with median indicated in red. * denotes p<0.05, ** denotes p<0.01 as determined by Mann-Whitney two-tailed test. (k–p) Representative images of lymphatic valve regions in mesenteric collecting vessels immunostained with antibodies targeted to Prickle1 and PROX1 in P7 Foxc1 Control (k), EC-Foxc1-KO (l), Foxc2 Control (m), EC-Foxc2-KO, (n) Foxc1; Foxc2 control and (o) EC-Foxc1; Foxc2-DKO (p) mice show reduced Prickle1 expression in the valve leaflets and lymphangion of Foxc2 and compound Foxc1; Foxc2 mutants compared to littermate controls whereas Prickle1 is reduced primarily in leaflet-free-edge LECs, denoted by yellow asterisks, of Foxc1 mutants. Scale bars are 20 μm.
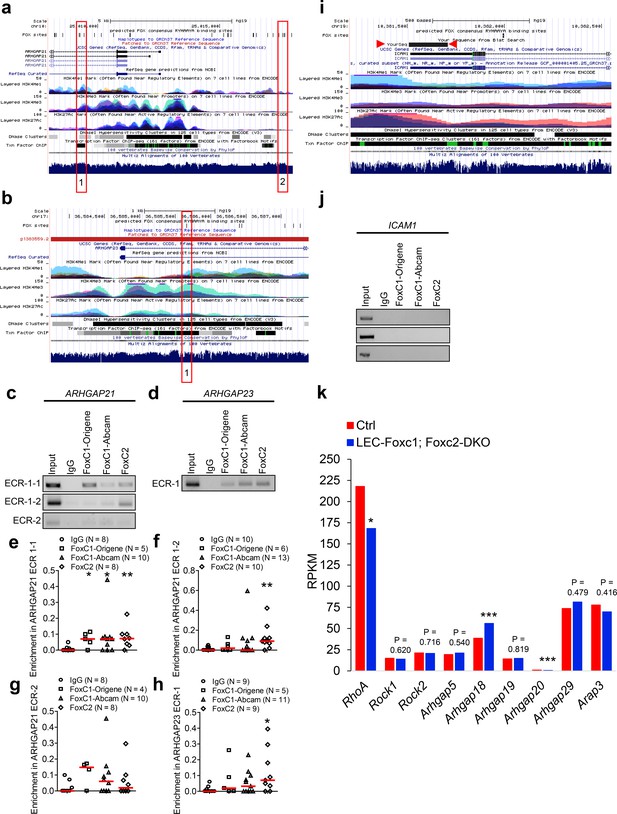
FOXC1 and FOXC2 mediate lymphatic expression of the RHO GTPase activating proteins Arhgap21 and Arhgap23.
(a, b) Putative FOX-binding sites in regions of the human ARHGAP21 (a) and ARHGAP23 (b) loci as viewed on the UCSC genome browser (http://genome.ucsc.edu; Kent et al., 2002). Vertical lines on the ‘FOX sites’ track indicate putative FOX binding sites predicted using HOMER (see methods). Red boxes indicate ECRs containing FOX-binding sites between human and mouse genomes that are conserved and aligned. (c, d) ChIP showing specific binding of FOXC1 and FOXC2 to the consensus FOX-binding sites within ECRs in ARHGAP21 (c) and ARHGAP23 (d) in HDLECs. (e–h) ChIP assays were performed using antibodies against Foxc1, Foxc2, and normal goat IgG. The binding of FOXCs to candidate ECRs in the ARHGAP21 (e–g) and ARHGAP23 (h) loci was determined with regular PCR and expressed as relative folds of input whose band intensity was normalized to 1. Data are presented as a scatter plot with median indicated in red. * denotes p<0.05, ** denotes p<0.01 as determined by Mann-Whitney two-tailed test.(i) Lack of FOX-binding sites in a region of the human ICAM1 locus as viewed on the UCSC genome browser. Red arrowheads denote a ChIP negative control PCR amplified region of the ICAM1 promoter, which was predicted to not bind FOX transcription factors. (j) ChIP assays were performed from three replicates using antibodies against Foxc1, Foxc2, and normal goat IgG. PCR was performed using primers to a negative control region of the ICAM1 promoter (i). (k) Expression of RhoA, Rock1, Rock2, Arhgap5, Arhgap18, Arhgap19, Arhgap20, Arhgap29, and Arap3 in littermate Control and Foxc1/c2-compound mutant LECs isolated from the dorsal skin at E15.5. Graph shows RPKM values from RNA-seq analysis. * denotes p<0.05, *** denotes p<0.001.
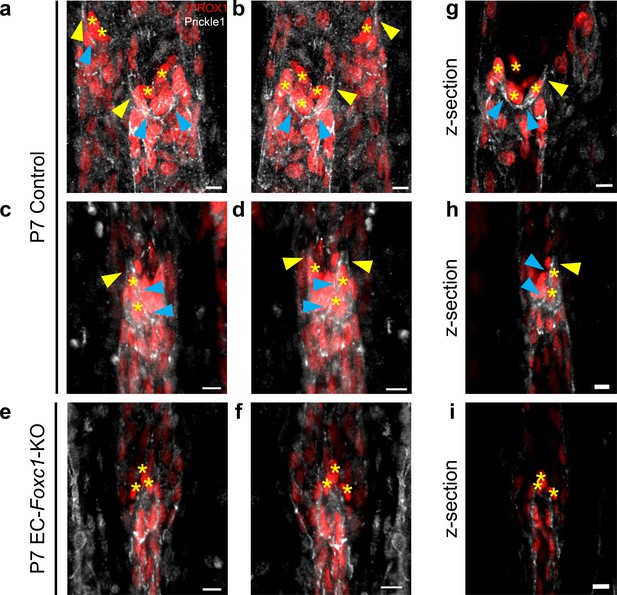
Prickle1 is more highly expressed in the leaflet-free-edge LECs of lymphatic valves.
(a–f) High magnification, representative images from two separate angles of three-dimensional reconstructions of PROX1 and Prickle1 immunostained collecting vessel generated from the same fields of P7 control individuals depicted in Figure 9C and Figure 9A as well as the P7 EC-Foxc1-KO individual depicted in Figure 9B. (g–i) Representative images of a single z-slice generated from the three-dimensional reconstructions depicted in panels (a–f). Yellow asterisks denote LECs located at the leaflet-free-edge. Blue arrowheads denote Prickle1 expression in leaflet-free-edge LECs. Yellow arrowheads denote areas of the valve buttress, which anchors leaflets to the vessel wall, where Prickle1 is expressed. Scale bars equal 10 μm.
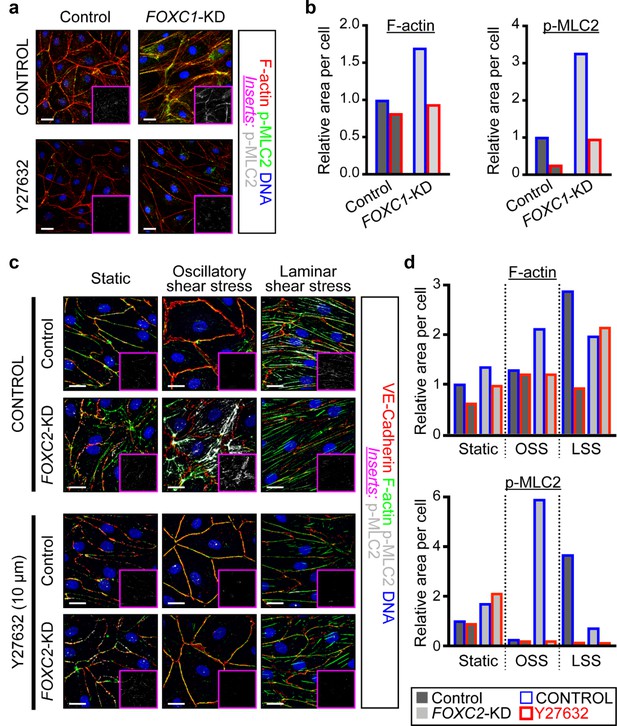
ROCK inhibition rescues hypercontractility of actin cytoskeleton in FOXC1-KD and FOXC2-KD LECs.
(a) Representative images of cultured LECs transfected with scramble control siRNA or FOXC1 siRNA and treated with vehicle control or Y-27632 (10 µM). Treatment with Y-27632 ROCK inhibitor shows rescue of cytoskeletal changes induced by FOXC1 inactivation. Immunostaining of F-actin (red), p-MLC2 (green), and DNA (blue). Pink inserts denote the single-channel p-MLC2 (white) images. Scale bars are 20 µm. (b) Quantification of relative F-actin (left) and p-MLC2 (right) area per Control (dark grey bars) and FOXC1-KD (light grey bars) cells treated with vehicle Control (blue outline) or Y-27632 (red outline). (c) Representative images of Control and FOXC2-KD LECs cultured under static, OSS, or LSS, treated with vehicle Control or Y-27632 (10 µM). Treatment with Y-27632 ROCK inhibitor shows rescue of cytoskeletal changes induced by FOXC2 inactivation that are most prominent under OSS. Immunostaining for VE-Cadherin (red), F-actin (green), p-MLC2 (white), and DNA (blue). Pink inserts denote the single-channel p-MLC2 (white) images. Scale bars are 20 µm. (d) Quantification of relative F-actin (top) and p-MLC2 (bottom) area per Control (dark grey bars) and FOXC1-KD (light grey bars) cells treated with vehicle Control (blue outline) or Y-27632 (red outline).
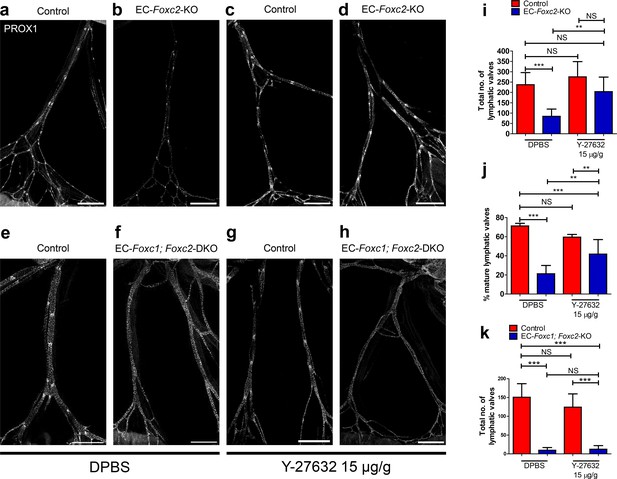
Inhibition of ROCK partially rescues postnatally induced valve degeneration in EC-specific Foxc2 mutant mice but not compound Foxc1; Foxc2 mutant mice.
(a–h) Representative images of lymphatic collecting vessels immunostained with PROX1 antibody in P7 littermate Control (a) and EC-Foxc2-KO (b), littermate Control (e) and EC-Foxc1; Foxc2-DKO (f) mice subcutaneously injected with DPBS vehicle or littermate Control (c) and EC-Foxc2-KO (d), littermate Control (g) and EC-Foxc1; Foxc2-DKO (h) mice subcutaneously injected with ROCK inhibitor Y-27632. Scale bars equal to 500 μm. (i, j) Quantification of total valve number (i) and percentage of mature valves (j) in littermate Control and EC-Foxc2-KO mice administered DPBS or Y-27632. N = 6 for Control DPBS, N = 6 for EC-Foxc2-KO DPBS, N = 9 for Control Y-27632, and N = 9 for EC-Foxc2-KO Y-27632. (k) Quantification of total valve number in littermate Control and EC-Foxc1; Foxc2-DKO mice administered DPBS or Y-27632. N = 5 for Control DPBS, N = 7 for EC-Foxc1; Foxc2-DKO DPBS, N = 8 for Control Y-27632, and N = 9 for EC-Foxc1; Foxc2-DKO Y-27632. P-values were obtained by One-way ANOVA with Tukey’s post test. Data are presented as mean (± SD). ** denotes p<0.01. *** denotes p<0.001. NS denotes no significance.
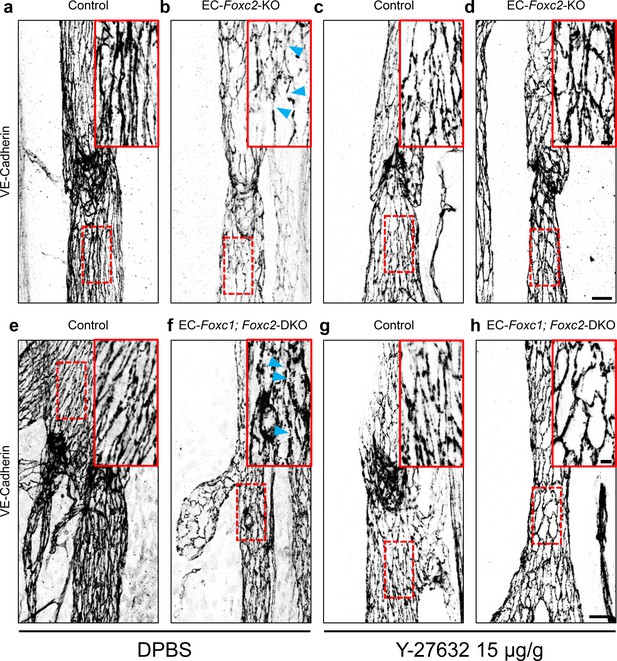
Inhibition of ROCK rescues discontinuous cell-cell junctions in EC-specific Foxc2 and compound Foxc1; Foxc2 mutant mice.
(a–h) High-magnification, representative images of lymphatic collecting vessels immunostained with VE-Cadherin antibody in P7 littermate Control (a) and EC-Foxc2-KO (b), littermate Control (e) and EC-Foxc1; Foxc2-DKO (f) mice subcutaneously injected with DPBS vehicle or littermate Control (c) and EC-Foxc2-KO (d), littermate Control (g) and EC-Foxc1; Foxc2-DKO (h) mice subcutaneously injected with ROCK inhibitor Y-27632. Scale bars are 20 µm. Red, dashed boxes highlight magnified regions shown inset. Scale bars equal to 5 μm. Gaps in LEC junctions are visible in EC-Foxc2-KO and EC-Foxc1; Foxc2-DKO mice administered DPBS (b, f) whereas inhibition of ROCK with Y-27632 is able to restore linear junctions in part (d, h). Blue arrowheads denote discontinuous LEC VE-Cadherin junctions.
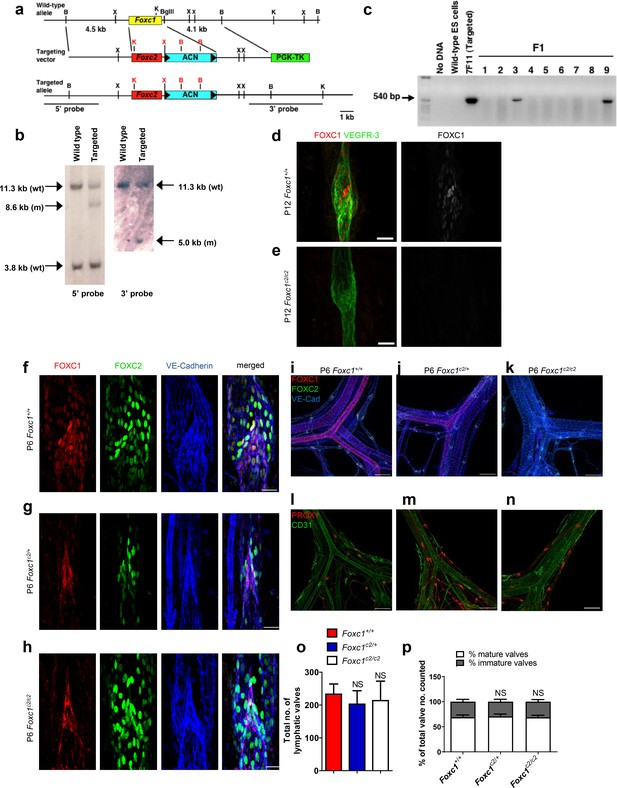
FOXC2 is able to functionally substitute for FOXC1 during lymphatic valve development.
(a) Schematic representation of the targeting vector and targeted allele. The entire protein coding region of Foxc1 is replaced with that of Foxc2. ACN, self-excision cassette including Cre driven by the testis-specific promoter. (b) Southern blot analysis to detect double-resistant ES cell colonies using 5’ and 3’ probes. (c) PCR genotyping of F1 heterozygotes to detect the Foxc1c2 allele. (d, e) Representative images of lymphatic valves in mesenteric collecting vessels immunostained with antibodies targeted to FOXC1 and VEGFR-3 from P12 P6 Foxc1+/+ (d) and Foxc1c2/c2 (e) mice. Scale bars are 25 µm. (f – h) Representative images of lymphatic valves in mesenteric collecting vessels immunostained with antibodies targeted to FOXC1, FOXC2, and VE-Cadherin from P6 Foxc1+/+ (f), Foxc1c2/+ (g), and Foxc1c2/c2 mice (h). Scale bars are 50 μm. (i – k) Representative images of the mesenteric vasculature immunostained with antibodies targeted to PROX1 and CD31 in P6 Foxc1+/+ (i), Foxc1c2/+ (j), and Foxc1c2/c2 mice (k). Scale bars are 200 μm. (l – n) Representative images of P6 mesenteric vasculature from P6 Foxc1+/+ (l), Foxc1c2/+ (m), and Foxc1c2/c2 mice (n) immunostained with antibodies targeted to FOXC1, FOXC2, and VE-Cadherin show gradual loss of FOXC1 expression in the blood and lymphatic vasculature and smooth muscle cells and conversely the increase of FOXC2 expression in blood vasculature and smooth muscle. Scale bars are 200 μm. (o) Quantification of total lymphatic valve number in lymphatic collecting vessels of P6 Foxc1+/+, Foxc1c2/+, and Foxc1c2/c2 individuals. N = 6 for Foxc1+/+, N = 8 for Foxc1c2/+, and N = 8 forFoxc1c2/c2 individuals. (p) Percentage of mature and immature lymphatic valves normalized to total valves counted in P6 Foxc1+/+, Foxc1c2/+, and Foxc1c2/c2 individuals. Data are presented as mean (± SD) and analyzed using Student’s t-test. NS denotes no significance.
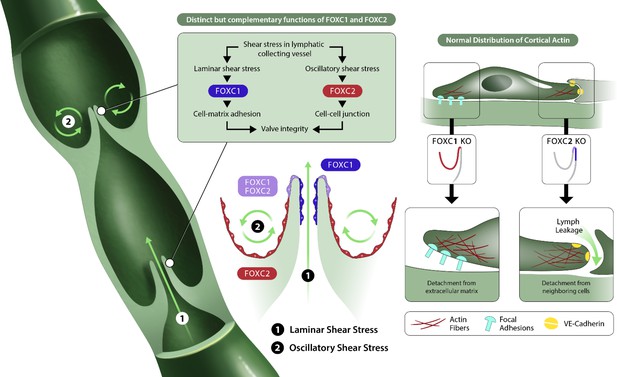
FOXC1 and FOXC2 maintain lymphatic valve integrity by regulating cytoskeletal organization in complementary roles.
Collecting vessels in the postnatal lymphatic vasculature are characterized by the presence of a high number of intraluminal bi-leaflet valves. These regions are exposed to disturbed flow in the valve sinuses (2) which strongly induces the expression of FOXC2. In contrast, the intraluminal side of valve leaflets is exposed to pulsatile laminar shear (1), which induces FOXC1 in addition to FOXC2. In the absence of FOXC1 and FOXC2, the cytoskeleton undergoes remodeling events in which actomyosin contractility is strongly induced with focal adhesion dynamics perturbed by loss of FOXC1 and intercellular junctions perturbed by loss of FOXC2, ultimately leading to valve degeneration.
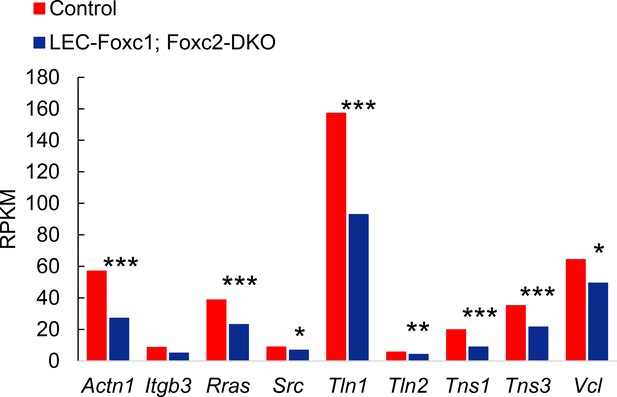
Differential expression of genes involved in regulation of focal adhesions in embryonic LECs isolated from the dorsal skin of LEC-specific Foxc1; Foxc2 mutant mice.
Expression of focal adhesion regulatory genes Actn1, Itgb3, Src, Tln1, Tln2, Tns1, Tns3, and Vcl in littermate Control and Foxc1/c2-compound mutant LECs isolated from the dorsal skin at E15.5. Graph shows RPKM values from RNA-seq analysis. * denotes p<0.05, ** denotes p<0.01, and *** denotes p<0.001.
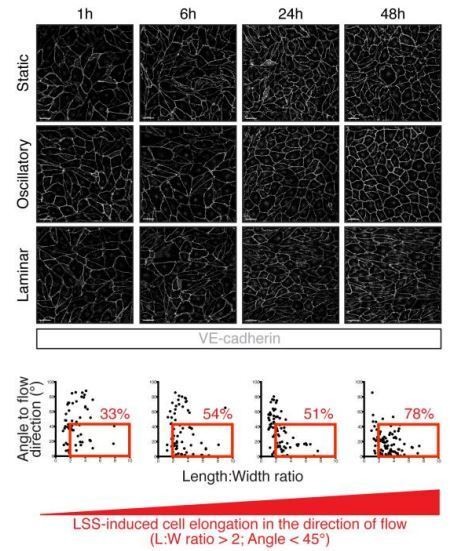
Kinetics of LEC elongation in the direction of flow under 4 dyn/cm2 laminar shear stress.
Confluent LECs were seeded and 24 hours later cultivated for 1 hour, 6 hours, 24 hours or 48 hours either under static conditions or oscillatory shear stress (4 dyn/cm2, 1/4 Hz oscillations) or unidirectional laminar shear stress (4 dyn/cm2). The upper part of the figure shows representative images of LECs stained for VE-cadherin (grey). Scale bar, 50 µm. The lower part of the figure shows graphs with individual cell measurement of cell shape (length:width ratio) and alignment to flow axis (horizontal) for each time point. LECs are defined as elongated when they have a length:width ratio greater than 2 are aligned to flow with an angle lower than 45°. These cells are highlighted in the red box and the percentage of total population is indicated in red on the figure.
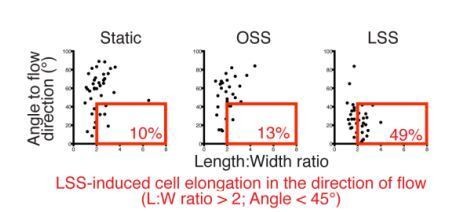
Quantification of cell elongation in the direction of flow corresponding to Figure 3.
Graphs show individual cell measurement of cell shape (length:width ratio) and alignment to flow axis (horizontal) for each time point. LECs are defined as elongated when they have a length:width ratio greater than 2 are aligned to flow with an angle lower than 45°. These cells are highlighted in the red box and the percentage of total population is indicated in red on the figure.
Videos
3D-visualization of VE-Cadherin expression in a mesentery lymphatic collecting vessel of a Foxc2fl/fl mouse.
Three-dimensional reconstruction of a P6 Foxc2fl/fl control mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against VE-Cadherin (green).
3D-visualization of VE-Cadherin expression in a mesentery lymphatic collecting vessel of an EC-Foxc2-KO mouse.
Three-dimensional reconstruction of a P6 EC-Foxc2-KO mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against VE-Cadherin (green). Blue arrows denote regions of discontinuous cell-cell junctions.
3D-visualization of VE-Cadherin expression in a mesentery lymphatic collecting vessel of a Foxc1fl/fl; Foxc2fl/fl mouse.
Three-dimensional reconstruction of a P6 Foxc1fl/fl; Foxc2fl/fl control mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against VE-Cadherin (green).
3D-visualization of VE-Cadherin expression in a mesentery lymphatic collecting vessel of an EC-Foxc1; Foxc2-KO mouse.
Three-dimensional reconstruction of a P6 EC-Foxc1; Foxc2-KO mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against VE-Cadherin (green). Blue arrows denote regions of discontinuous cell-cell junctions. Pink arrows denote regions of overlapping cell-cell junctions.
3D-visualization of Prickle1 expression in lymphatic valve leaflet free-edges in a mesentery collecting vessel of a Foxc1fl/fl mouse.
Three-dimensional reconstruction of a P7 Foxc1fl/fl control mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against Prox1 (red) and Prickle1 (white). Note Pricke1 is more highly expressed within LECs at the free-edges of valve leaflets and at the valve buttress.
3D-visualization of Prickle1 expression in lymphatic valve leaflet free-edges in a mesentery collecting vessel of a Foxc2fl/fl mouse.
Three-dimensional reconstruction of a P7 Foxc2fl/fl control mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against Prox1 (red) and Prickle1 (white). Note Pricke1 is more highly expressed within LECs at the free-edges of valve leaflets and at the valve buttress of both Prox1-hi valve regions.
3D-visualization of reduced Prickle1 expression in lymphatic valve leaflet free-edges in a mesentery collecting vessel of an EC-Foxc1-KO mouse.
Three-dimensional reconstruction of a P7 EC-Foxc1-KO mouse administered tamoxifen from P1-P5, using Imaris ‘Surpass’ function. Mesenteric collecting vessels were immunostained with antibodies against Prox1 (red) and Prickle1 (white). Note the reduction of Prickle1 expression within LECs at the leaflet free-edge compared to Videos 1 and 2, whereas Prickle1 expression is retained in the valve sinus regions.
Tables
Antibodies and Dyes.
| Antigen | Reactivity | Host Species | Origin |
|---|---|---|---|
| Primary antibodies | |||
| Active caspase 3 | Human/Mouse | Rabbit | R and D AF835 |
| α9-integrin | Mouse | Goat | R and D AF3827 |
| CD31 | Mouse | Rat | BD Pharmingen 553370 |
| Foxc1 | Human/Mouse/Rat | Rabbit | Cell Signaling 8758S |
| Foxc2 | Mouse | Sheep | R and D AF6989 |
| Foxc2 | Human/mouse | Rat | Kind gift from Dr. N Miura (Miura et al., 1997, Genomics) |
| Paxillin | Human/mouse | Mouse | BD Transduction Clone 349–610051 |
| Phospho-MLC2 (Thr18/Ser19) | Human | Rabbit | Cell Signaling #36745 |
| Prox1 | Human | Goat | R and D AF2727 |
| Prickle1 | Human | Rabbit | Thermo Fisher PA5-51570 |
| VE-Cadherin | Mouse | Rat | BD Pharmingen 555289 |
| VE-Cadherin | Human/Mouse | Goat | R and D AF1002 |
| Vegfr3 | Mouse | Goat | R and D AF743 |
| Vinculin | Human | Mouse | Sigma – Clone hVIN-1 V9131 |
| Secondary Antibodies | |||
| Alexa 405-conjugated | Rat | Donkey | Abcam ab175670 |
| Alexa 488-conjugated | Rabbit/Rat/Sheep Goat/Mouse | Donkey | Thermo Fisher |
| Alexa 555-conjugated | Goat/Mouse/Rabbit Rat | Donkey | Thermo Fisher |
| Alexa 568-conjugated | Goat/Rat | Goat/Donkey | Thermo Fisher |
| Alexa 647-conjugated | Goat/Mouse/Rabbit Rat | Donkey | Thermo Fisher |
| Dyes | |||
| Hoechst 33342 | - | - | Thermo Fisher |
| Alexa 488-conjugated phalloidin | - | - | Thermo Fisher |
| DAPI-containing Prolong Gold antifade reagent | - | - | Thermo Fisher |
Primers used for qPCR analysis.
| Gene | Forward | Reverse |
|---|---|---|
| Foxc1 | TTCTTGCGTTCAGAGACTCG | TCTTACAGGTGAGAGGCAAGG |
| Foxc2 | AAAGCGCCCCTCTCTCAG | TCAAACTGAGCTGCGGATAA |
| Ppia | CAAATGCTGGACCAAACACA | TGCCATCCAGCCATTCAGTC |
| 18S | GAAACTGCGAATGGCTCATTAAA | CCACAGTTATCCAAGTAGGAGAGGA |
List of siRNAs.
| Gene | Species | Company | Reference (Sequence) |
|---|---|---|---|
| Control | Human | QIAGEN | AllStars Neg. Control siRNA-1027281 |
| FOXC1 | Human | Origene | SR320173 #1 -rGrArUrArArArArCrArCrUrArGrArArGrUrUrArCrCrUrATT #2 - rCrUrArGrUrCrCrArUrGrUrCrArArArUrUrUrUrArCrUrAAA |
| FOXC2 | Human | Thermo Scientific (Dharmacon) | FISSH-000119 (AGGUGGUGAUCAAGAGCGAUU) FISSH-000321 (CAACGUGCGGGAGAUGUUCUU) |
Primers used for ChIP analysis.
| Evolutionary Conserved Region (ECR) | Forward | Reverse |
|---|---|---|
| PRICKLE1 ECR-1 | ACACAAGGCGGTGCTCTAAT | CTTGTTTCAAATGGGTGCT |
| PRICKLE1 ECR-2 | GCAAATGGCACATTTAAGCA | TGGCTCCTTTTCTTTGCTGT |
| PRICKLE1 ECR-3 | AGGCAGACCCTTTTTGGAAT | GGAAGCTTGCAACTGTCTCC |
| PRICKLE1 ECR-4 | GCAAGTGTGCAAACCCTTAAC | CAGCTGGAGCCTGAAGAAAG |
| PRICKLE1 ECR-5 | CCACCAGACAGCAAGATGAA | TTGACCGTCCCCAACATTAT |
| PRICKLE1 ECR-6 | TGCCTTGTTCATGGTCTCAG | AAGAAAAACAAACGGCATCG |
| ARHGAP21 ECR-1 | GCTTGCTAGCCAAGGACAAG | CCTACCTGCAACCTGGTGAT |
| ARHGAP21 ECR-2 | ATCACCAGGTTGCAGGTAGG | GGCAGAACTGTAGGTTTACATTTAG |
| ARHGAP21 ECR-3 | TGTGGAAGGCCATTCTATGA | GTTTTGCAAAGGCTTCAACC |
| ARHGAP23 ECR-1 | CCTCCCTGCTCCTAAGTTGA | CCAAGTCTTTCAGCCCTGTC |





