Self-restoration of cardiac excitation rhythm by anti-arrhythmic ion channel gating
Figures
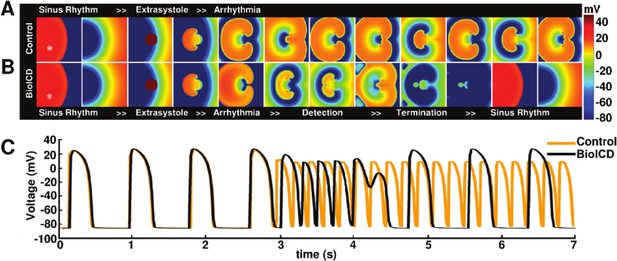
Anti-arrhythmic action of the BioICD channel (Model I, see below).
(A) Development of a sustained tachyarrhythmia, induced via an extrasystole, in a simulated monolayer of normal human ventricular tissue (TNNP model; ten Tusscher and Panfilov, 2006). (B) Auto-detection and termination of the tachyarrhythmia, followed by restoration of sinus rhythm in the same monolayer upon expression of the BioICD channel. (C) Voltage traces recorded from representative cardiomyocytes (white asterisks) in the simulation domains (orange: Control, black: BioICD). Time frames in A-B are chosen to represent the relevant stages in arrhythmia progression, detection and termination and therefore do not correspond linearly with the voltage traces in C.
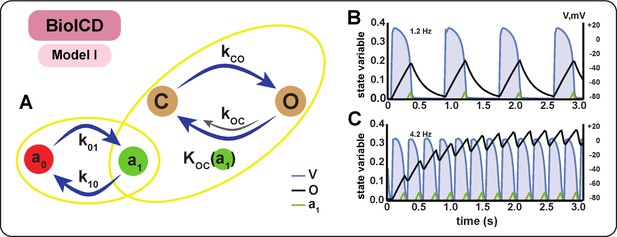
Schematic diagram and behaviour of BioICD channel Model I.
(A) Markov diagram for BioICD channel Model I. (B) and (C) Traces of voltage () and state variables and , at 1.2 Hz and 4.2 Hz, respectively. : open state of the frequency-sensing channel subunit, : closed state of the frequency-sensing channel subunit, : inactive state of the catalytic channel deactivation agent, : active state of the catalytic channel deactivation agent. A detailed mathematical description of , , and is provided in the Materials and methods section.
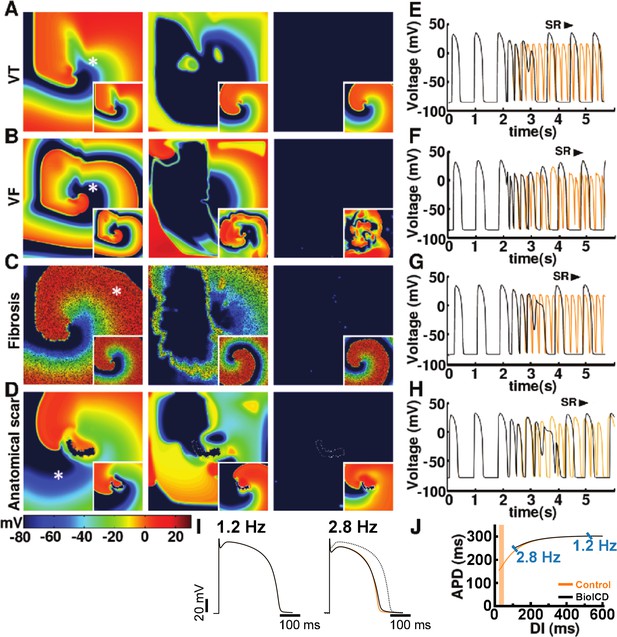
Self-restoration of excitation rhythm in human ventricular monolayers with reentrant tachyarrhythmias (TNNP model [ten Tusscher and Panfilov, 2006]) for four different pathological substrates.
(A) A substrate for VT. (B) A substrate for VF. (C) A substrate with diffuse fibrosis. (D) A substrate with an anatomical scar. In each panel, successive frames (from left to right) show the voltage distribution in the monolayers at subsequent relevant time points. The left column shows established reentrant tachyarrhythmias. The middle column shows, for the different arrhythmia conditions, stages of advanced repolarization in the arrhythmia termination process (large dark blue areas), while the right column shows the final tissue-wide repolarization (dark blue) stage, thereby allowing restoration of sinus rhythm (SR). The insets show the corresponding situation in the absence of BioICD channels. (E–H) Voltage time series extracted from representative cardiomyocytes (white asterisks) in the simulation domains of situations A-D, respectively. (I) The BioICD channel has no significant influence on the action potential (AP) at sinus rhythm (1.2 Hz, left AP traces), but slightly increases the AP duration in the tail (APD90) at close-to-arrhythmic frequencies (right AP traces). The dotted line of the widest AP in the right traces shows the AP at 1.2 Hz for reference. The orange lines show the APs without BioICD current (control). (J) APD restitution curve for the original parameters of the human ventricular cardiomyocyte model, with and without BioICD channel. Orange shading is used to indicate the region of the restitution curve where the slope exceeds 1. DI, diastolic interval.
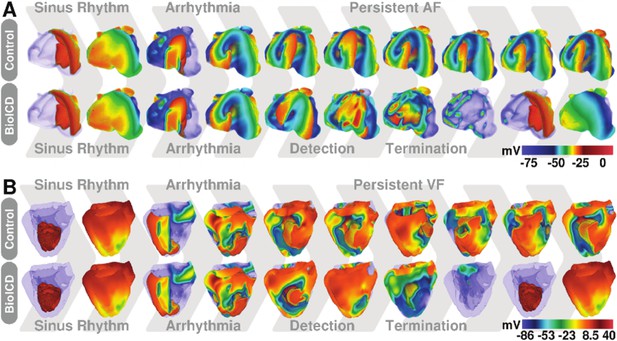
Self-restoration of excitation rhythm in anatomically realistic models of human (A) atria and (B) ventricles with reentrant arrhythmias using BioICD channel Model I (more depolarised regions are more transparent).
The upper panels show representative plots of the control situation (in the absence of a BioICD channel). Induction, during sinus rhythm, of reentrant activity by giving an extra external stimulus leads to sustained AF and VF in atria and ventricles, respectively. The lower panels show the exact same situation, but in atria and ventricles expressing the BioICD channel. Once the arrhythmia is induced, the heart itself is able to detect and terminate fibrillation in order to restore sinus rhythm.
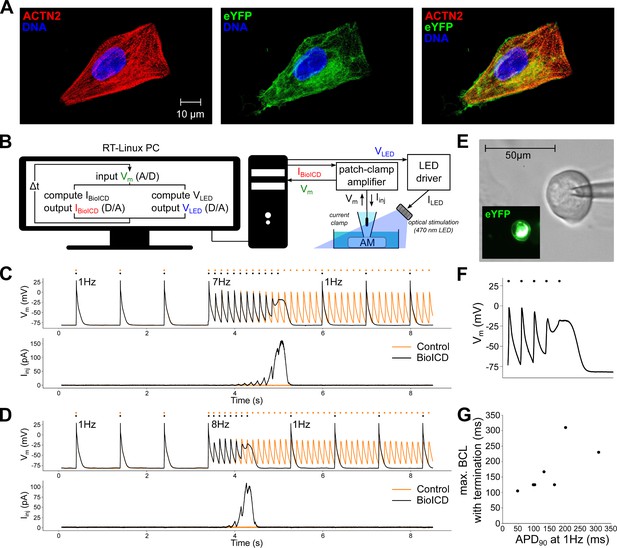
Self-restoration of excitation rhythm in cultured human atrial myocytes (AM) with the help of dynamic patch-clamping using BioICD channel Model I.
(A) Human atrial myocytes stained for (from left to right) -actinin (ACTN2), enhanced yellow fluorescent protein (eYFP) and DNA. (B) Dynamic patch-clamp set-up creating a feedback system for current injection and optical pacing. (C) Typical membrane potential () and injected current () traces with and without the BioICD current () enabled at 7 Hz. Both traces are from the same cell and were recorded 7 s apart. Dots indicate the stimulus times. (D) Typical membrane potential and injected current traces with and without the BioICD current enabled in the same cell as (C) at 8 Hz. Also here, both traces were recorded 7 s apart. (E) eYFP fluorescence produced by optogenetically modified human atrial myocyte. (F) Zoom-in of the trace in (C) showing termination of arrhythmic activity. The last optical stimulus is blocked, allowing 1 Hz activation to regain. (G) Maximal basic cycle length (BCL) for which termination still occurs as a function of baseline APD. Data obtained from 7 cells. Other abbreviations: real-time (RT), analog/digital (A/D), voltage input for the LED driver (), current output of the LED driver ().
-
Figure 5—source data 1
Dynamic clamp reading 1: CSV-file containing the raw data for the C-panel in Figure 5.
There are 22 columns, the first 11 for the voltage measurement, the next 11 for the injected current measurement. The first column indicates the time, the subsequent columns are measurements that follow each other in time.
- https://cdn.elifesciences.org/articles/55921/elife-55921-fig5-data1-v2.csv
-
Figure 5—source data 2
Dynamic clamp reading 2: CSV-file containing the raw data for the D-panel in Figure 5.
The data structure is similar to the one from the previous raw data file.
- https://cdn.elifesciences.org/articles/55921/elife-55921-fig5-data2-v2.csv
-
Figure 5—source data 3
Dynamic clamp summary table: Table containing the experimental datapoints that were plotted in Figure 5G.
- https://cdn.elifesciences.org/articles/55921/elife-55921-fig5-data3-v2.csv
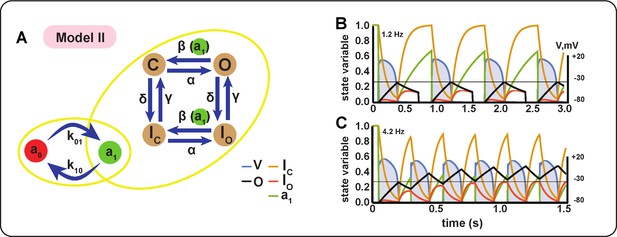
Schematic diagram and behaviour of BioICD channel Model II.
(A) Markov diagram for BioICD Model II. (B) and (C) Traces of voltage () and state variables at 1.2 Hz and 4.2 Hz, respectively.
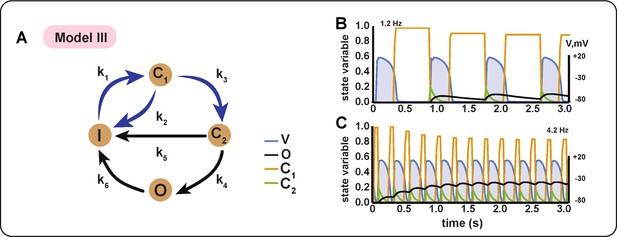
Schematic diagram and behaviour of BioICD channel Model III.
(A) Markov diagram for BioICD Model III. (B) and (C) Traces of voltage () and state variables at 1.2 Hz and 4.2 Hz, respectively.
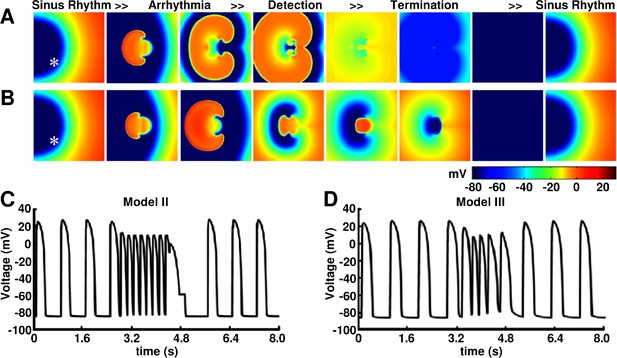
Anti-arrhythmic action of BioICD Model II and III in monolayers of human ventricular tissue (TNNP model).
(A and B) Pseudocolor plots of subsequent time frames of the process of biological auto-detection and termination of reentrant tachyarrhythmias, followed by restoration of sinus rhythm, as demonstrated by using BioICD Model II and Model III, respectively. (C and D) Voltage traces from representative cardiomyocytes (white asterisks) in the simulation domains for Model II and Model III, respectively.
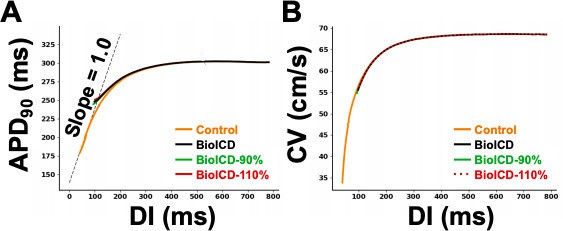
1D cable action potential duration (APD) restitution.
(A) Dynamic APD restitution curve for the original parameters of the human ventricular cardiomyocyte model, with and without BioICD channel. (B) Conduction velocity restitution curve for the TNNP model (tenTusscher and Panfilov, 2006) with and without BioICD channel for different channel densities. For all three channel densities (90, 100 and 110% of the model described in the main manuscript), the curves lie on top of each other.
Videos
Self-restoration of cardiac excitation rhythm after VT in monolayers of human ventricular tissue.
The activation of the BioICD current is shown in grey, as an overlay on the voltage map in the BioICD case.
Self-restoration of cardiac excitation rhythm after VF in monolayers of human ventricular tissue.
The activation of the BioICD current is shown in grey, as an overlay on the voltage map in the BioICD case.
Self-restoration of cardiac excitation rhythm after VT in monolayers of human ventricular tissue, in the presence of 20% fibroblasts.
The activation of the BioICD current is shown in grey, as an overlay on the voltage map in the BioICD case.
Self-restoration of cardiac excitation rhythm after VT in monolayers of human ventricular tissue, in the presence of an anatomically realistic scar (3.4 cm long), surrounded by a grey zone, where was reduced by 80%, by 70%, by 69% and by 62%.
The activation of the BioICD current is shown in purple, as an overlay on the voltage map in the BioICD case.
Failure of self-restoration of cardiac excitation rhythm after VT in monolayers of human ventricular tissue, in the presence of a large anatomically realistic scar (5.8 cm long), surrounded by a grey zone, where was reduced by 80%, by 70%, by 69% and by 62%.
The activation of the BioICD current is shown in grey, as an overlay on the voltage map in the BioICD case.
Self-restoration of cardiac excitation rhythm after VF in anatomically realistic human ventricles.
Self-restoration of cardiac excitation rhythm after AF in anatomically realistic human atria.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo-sapiens) | Conditionally immortalised human atrial cell line | doi:10.1093/eurheartj | ||
| Gene (Chlamydomonas reinhardtii) | ChR2(H134R) | Addgene | plasmid #26973 | source of the H134R variant of channelrhodopsin 2 |
| Genetic reagent | LVV-ChR2(H134R) | This paper | Lentiviral vector particles to transduce and express ChR2(H134R) | |
| Software, algorithm | graphics processing unit-usable code | doi:10.1152/ajp-heart.00109.2006, doi:10.1038/srep20835 | ||
| Software, algorithm | RTXI dynamic clamp software | doi:10.1371/journal. pcbi.1005430 |
Additional files
-
Source code 1
Key component of the graphics processing unit-usable code.
- https://cdn.elifesciences.org/articles/55921/elife-55921-code1-v2.cu
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/55921/elife-55921-transrepform-v2.docx





