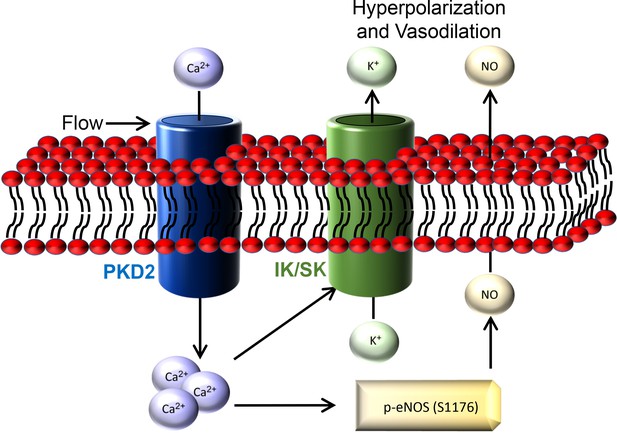
Intravascular flow stimulates PKD2 (polycystin-2) channels in endothelial cells to reduce blood pressure
Figures
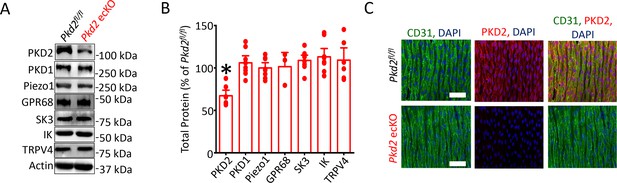
Generation and validation of Pkd2 ecKO mice.
(A) Representative Western blots illustrating the effect of tamoxifen-treatment of Pkd2fl/fl and Pkd2fl/fl: Cdh5(PAC)-creERT2 mice on PKD2, PKD1, Piezo1, GPR68, eNOS, SK3, IK and TPRV4, proteins in mesenteric arteries. (B) Mean data for proteins in mesenteric arteries of tamoxifen-treated Pkd2fl/fl: Cdh5(PAC)-creERT2 mice when compared to those in tamoxifen-treated Pkd2fl/fl mice. n = 3–8. * indicates p<0.05 versus Pkd2fl/fl. (C) En-face immunofluorescence imaging illustrating that PKD2 protein (Alexa Fluor 555) is abolished in endothelial cells of mesenteric arteries in tamoxifen-treated Pkd2fl/fl: Cdh5(PAC)-creERT2 mice (representative of 6 mesenteric arteries). CD31 (Alexa Fluor 488) and DAPI are also shown. Scale bars = 50 µm.
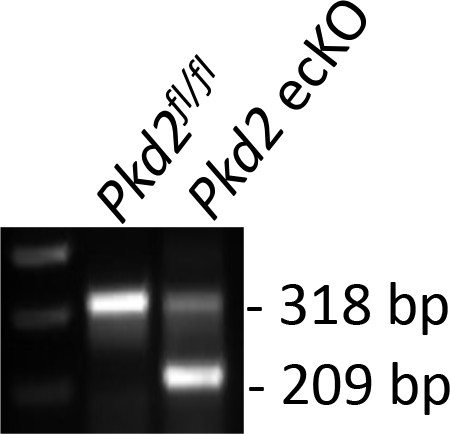
Genotyping of mouse lines.
Genomic PCR indicating that tamoxifen (1 mg/ml, i.p., 3 days) stimulated Cre-recombination in mesenteric arteries of Pkd2fl/fl: Cdh5(PAC)-creERT2 mice. Representative of n = 3.
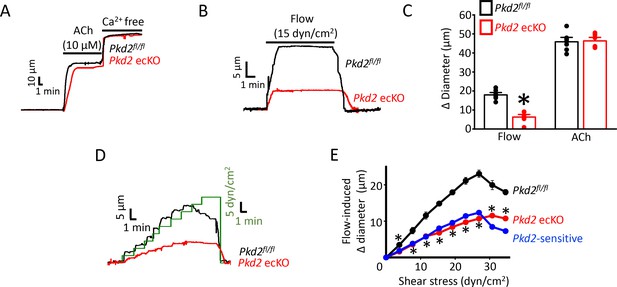
PKD2 channels contribute to intravascular flow-, but not ACh-, mediated vasodilation.
(A) Original traces illustrating responses to ACh (10 µM) and Ca2+-free solution (passive diameter) in pressurized (80 mmHg) mesenteric arteries from Pkd2fl/fl and Pkd2 ecKO mice. (B) Original trace of flow-mediated dilation in pressurized (80 mmHg) mesenteric arteries from Pkd2fl/fl and Pkd2 ecKO mice. (C) Mean diameter changes in response to flow (15 dyn/cm2) or ACh (10 µM). *p<0.05 vs. Pkd2fl/fl. n = 8 for each. # p<0.05 vs. flow in the same genotype. (D) Original traces illustrating diameter responses to stepwise increases in intravascular flow in pressurized (80 mmHg) mesenteric arteries from Pkd2fl/fl and Pkd2 ecKO mice. (E) Mean data. The Pkd2-sensitive component of flow-mediated vasodilation is illustrated in blue. *p<0.05 vs. Pkd2fl/fl. n = 5 for Pkd2fl/fl, n = 4 for Pkd2 ecKO.
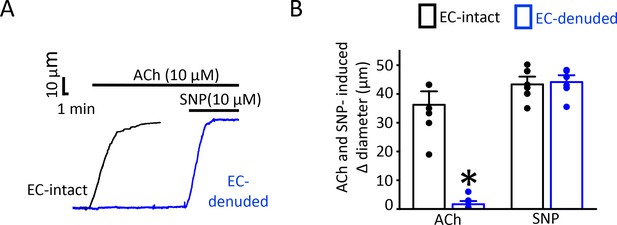
Endothelial denudation abolishes ACh-mediated vasodilation.
(A) Original traces demonstrating responses to ACh (10 µM) and SNP (10 µM) in EC-intact (black) and EC-denuded (blue) pressurized (80 mmHg) mesenteric arteries of Pkd2fl/fl. (B) Mean data. *p<0.05 vs. EC-intact. n = 8 for each.
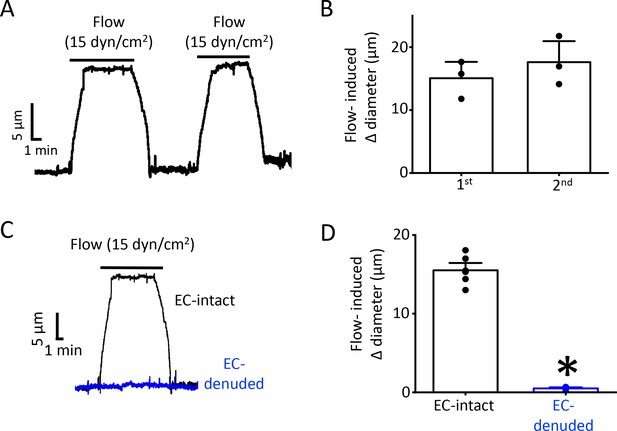
Endothelial denudation abolishes flow-mediated dilation.
(A) Original traces demonstrating reproducible flow responses (15 dyn/cm2) in pressurized Pkd2fl/fl mesenteric arteries. (B) Mean diameter changes in response to two consecutive flow (15 dyn/cm2) stimuli. n = 3. (C) Original traces demonstrating flow responses (15 dyn/cm2) in pressurized (80 mmHg) EC-intact and EC-denuded mesenteric arteries from Pkd2fl/fl mice. (D) Mean data for Pkd2fl/fl arteries. n = 7 for EC-intact. n = 6 for Pkd2fl/fl EC-intact. *p<0.05 vs.EC-intact. n = 8 for each.
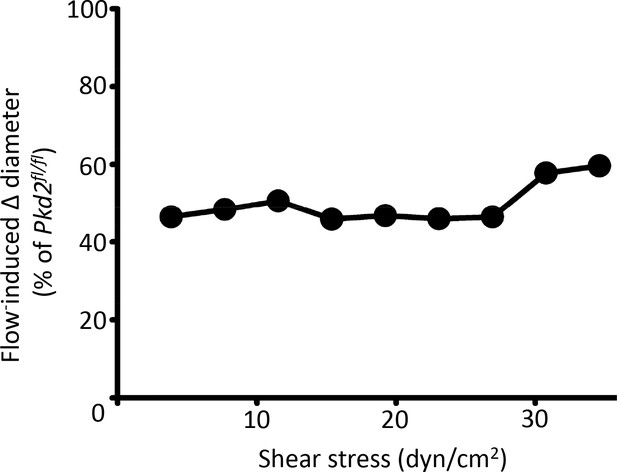
Endothelial cell PKD2 knockout attenuates flow-mediated vasodilation over a broad shear stress range.
Mean data illustrating relative dilation to shear stress in pressurized (80 mmHg) Pkd2 ecKO arteries compared with Pkd2fl/fl arteries. n = 4–5.
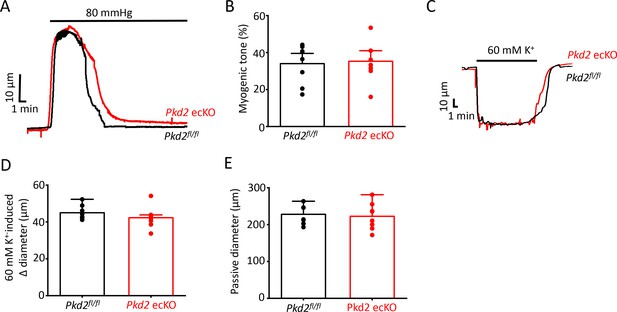
Smooth muscle-specific vasoconstriction and passive diameter are unaltered in Pkd2 ecKO arteries.
(A) Representative traces illustrating the development of myogenic tone in pressurized (80 mmHg) Pkd2fl/fl and Pkd2 ecKO arteries. (B) Mean myogenic tone in pressurized (80 mmHg) mesenteric arteries from Pkd2fl/fl and Pkd2 ecKO. n = 8 for each. (C) Representative traces illustrating 60 mm K+ constriction in pressurized (10 mmHg) arteries. (D) Mean data for 60 mM K+-induced constriction. n = 8. (E) Mean data for passive diameter (Ca2+- free PSS) in pressurized (80 mmHg) arteries. n = 8.
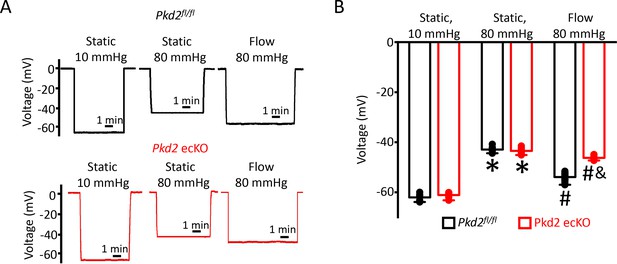
EC PKD2 channels contribute to flow-mediated arterial hyperpolarization.
(A) Original membrane potential recordings obtained from microelectrode impalements in pressurized mesenteric arteries of Pkd2fl/fl and Pkd2 ecKO mice at static (10 and 80 mmHg) and with 80 mmHg and flow (15 dyn/cm2). All three impalements in Pkd2fl/fl and Pkd2 ecKO were from the same two arteries. (B) Mean data (Pkd2fl/fl: 10 mmHg, n = 8; 80 mmHg, n = 10; 80 mmHg + flow, n = 18; Pkd2 ecKO: 10 mmHg, n = 7; 80 mmHg, n = 12; 80 mmHg + flow, n = 16). *p<0.05 for 80 mmHg static versus 10 mmHg static in same genotype. # p<0.05 for 80 mmHg + flow versus 80 mmHg static in the same genotype. and indicates p<0.05 versus Pkd2fl/fl under the same condition.
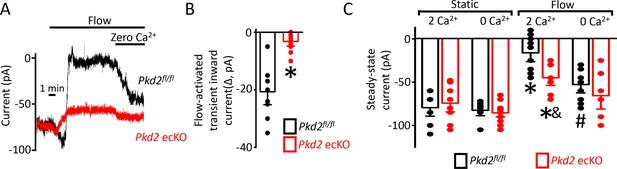
Flow reduces steady-state inward current through a PKD2-mediated, Ca2+ influx-dependent mechanism in voltage-clamped mesenteric artery endothelial cells.
(A) Original recordings of steady-state current modulation by flow (10 ml/min) and effect of removing bath Ca2+ at −60 mV in endothelial cells from Pkd2fl/fl and Pkd2 ecKO mice. (B) Mean data for flow-induced transient inward current. n = 9 for Pkd2fl/fl and n = 10 for Pkd2 ecKO. * indicates p<0.05 versus Pkd2fl/fl.(C) Mean data for steady-state currents in the presence and absence of flow and in the presence and absence of extracellular Ca2+ (Pkd2fl/fl: static + Ca2+, n = 9; static with zero Ca2+, n = 6; flow + Ca2+, n = 9; flow with zero Ca2+, n = 9 and Pkd2 ecKO: static + Ca2+, n = 9; static with zero Ca2+, n = 15; flow + Ca2+, n = 8; flow with zero Ca2+, n = 8). *p<0.05 versus static + Ca2+ conditions in the same genotype, and indicates p<0.05 vs Pkd2fl/fl under the same condition, # p<0.05 versus flow + Ca2+ in the same genotype.
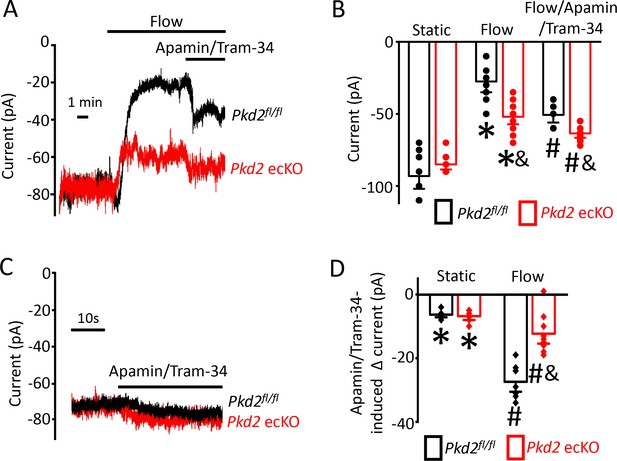
Flow-mediated PKD2 channel activation stimulates SK/IK channels in mesenteric artery endothelial cells, leading to vasodilation.
(A) Original recordings of steady-state current modulation by flow (10 ml/min) and flow plus apamin/Tram-34 (300 nM of each) at −60 mV in mesenteric artery ECs from Pkd2fl/fl and Pkd2 ecKO mice. (B) Mean data (Pkd2fl/fl: static, n = 8; flow, n = 8; flow + apamin/Tram-34, n = 7. Pkd2 ecKO: static, n = 9, flow, n = 10; flow + apamin/Tram-34, n = 9). * indicates p<0.05 versus static in the same genotype and p<0.05 vs Pkd2fl/fl in the same conditions. # p<0.05 versus flow in the same genotype. (C) Original recordings of steady-state current modulation by apamin/Tram-34 (300 nM of each) in the absence of flow at −60 mV in ECs from Pkd2fl/fl and Pkd2 ecKO mice. (D) Mean data comparing responses to apamin/Tram-34 in static and flow conditions at −60 mV (Pkd2fl/fl: static, n = 6; flow, n = 7. Pkd2 ecKO: static, n = 6; flow, n = 9). *p<0.05 versus static control. # p<0.05 versus static + apamin/Tram-34 in the same genotype. and indicates p<0.05 for Pkd2 ecKO vs Pkd2fl/fl in the same condition.
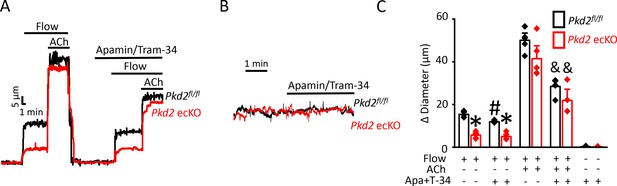
PKD2 channels contribute to intravascular flow-mediated SK/IK channel activation and vasodilation.
(A) Representative traces illustrating responses to flow (15 dyn/cm2) and flow (15 dyn/cm2) + ACh (10 µM) in the presence and absence of apamin/Tram-34 (300 nM of each) in pressurized (80 mmHg) mesenteric arteries from Pkd2fl/fl and Pkd2 ecKO mice. (B) Representative traces illustrating responses to apamin/Tram-34 (300 nM of each) in the absence of intravascular flow in pressurized (80 mmHg) mesenteric arteries. (C) Mean data (Pkd2fl/fl: flow, n = 5; flow + apamin/Tram-34, n = 5; flow + ACh (10 µM), n = 5; flow + ACh (10 µM) + apamin/Tram-34, n = 5; static + apamin/Tram-34, n = 5. Pkd2 ecKO: flow, n = 5; flow + apamin/Tram-34, n = 5; flow + ACh (10 µM), n = 5; flow + ACh (10 µM) + apamin/Tram-34, n = 4; static + apamin/Tram-34, n = 5). * indicates p<0.05 versus Pkd2fl/fl in the same condition. # indicates p<0.05 for flow + apamin/Tram-34 versus flow in the same genotype. and indicates p<0.05 for flow + ACh versus flow + ACh + apamin/Tram-34 in the same genotype.
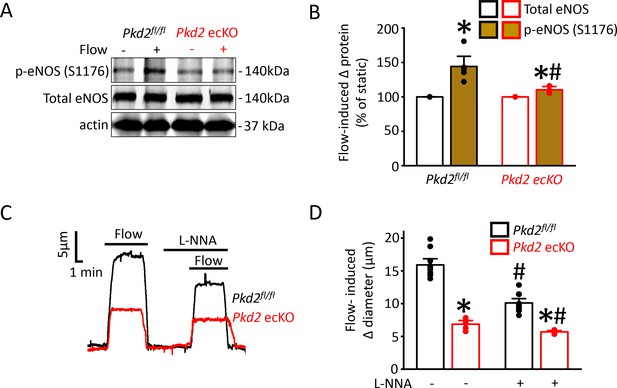
Flow-mediated PKD2 channel activation in ECs stimulates eNOS serine 1176 phosphorylation, leading to vasodilation.
(A) Original Western blots illustrating effects of flow (15 dyn/cm2) and Pkd2 ecKO on p-eNOS (S1176) and total eNOS proteins in Pkd2fl/fl and Pkd2 ecKO mesenteric arteries. (B) Mean data for flow-induced change (Δ) in proteins. n = 5 for Pkd2fl/fl. n = 4 for Pkd2 ecKO. * indicates p<0.05 versus static. # indicates p<0.05 versus same protein in Pkd2fl/fl. (C) Representative traces demonstrating flow (15 dyn/cm2)-mediated vasodilation in pressurized (80 mmHg) mesenteric arteries of Pkd2fl/fl and Pkd2 ecKO mice in the presence and absence of L-NNA (10 µM). (D) Mean data. n = 10 for Pkd2fl/fl. n = 5 for Pkd2 ecKO. * indicates p<0.05 versus Pkd2fl/fl in the same condition. # indicates p<0.05 versus flow in the absence of L-NNA (10 µM) in the same genotype.
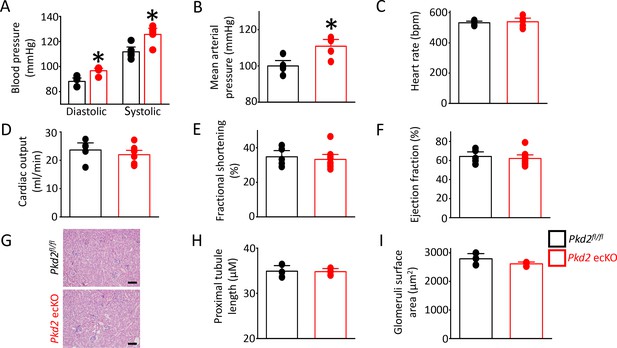
Pkd2 ecKO elevates systemic blood pressure, but does not alter cardiac function or kidney histology.
(A) Mean diastolic and systolic blood pressures in Pkd2fl/fl and Pkd2 ecKO mice (n = 5 for each). * indicates p<0.05 versus Pkd2fl/fl. (B) Mean arterial blood pressure (MAP) (n = 5 for each). * indicates p<0.05 versus Pkd2fl/fl. (C–F) Mean echocardiography data. Heart rate (HR), Cardiac output (CO), fractional shortening (FS) and ejection fraction (EF) (n = 5 Pkd2fl/fl and n = 10 for Pkd2 ecKO). (G) Representative images of H and E stained kidney cortex used for histological assessment. Scale bars = 100 µm. (H) Mean proximal tubule length (n = 15 proximal tubules measured for each group from three individual mice). (I) Mean glomeruli surface area (n = 75 glomeruli measured per group from three individual mice).
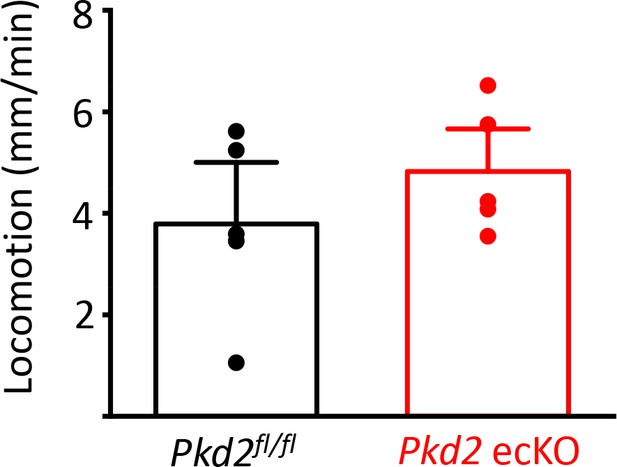
Locomotion is similar in Pkd2fl/fl and Pkd2 ecKO mice (n = 5 for each).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (M. musculus) | Pkd2fl/fl | Baltimore PKD Core Center | PMID:20862291 | Mice with Pkd2 gene flanked by loxP regions. |
| Strain, strain background (M. musculus) | Cdh5(PAC)-creERT2 | Cancer Research UK | RRID:MGI:3848984 | Mice with tamoxifen-inducible Cre recombinase that is expressed specifically in endothelial cells. |
| Strain, strain background (M. musculus) | Pkd2fl/fl: Cdh5(PAC)-creERT2 | This paper | Mouse line created in-house by mating Pkd2fl/fl with Cdh5(PAC)-creERT2. Mice with inducible endothelial cell-specific deletion of PKD2. | |
| Antibody | Anti-PKD2 (rabbit polyclonal) | Baltimore PKD Core | Rabbit mAB 3374 CT-14/4 | IF 1:200 dilution |
| Antibody | Anti-PKD2 (mouse monoclonal) | Santa Cruz | Cat. # sc-100415 RRID:AB_1127284 | WB 1:100 dilution |
| Antibody | Anti-PKD1 (mouse monoclonal) | Santa Cruz | Cat. # sc-130554 RRID:AB_2163355 | WB 1:100 dilution |
| Antibody | Anti-Piezo1 (rabbit polyclonal) | Proteintech | Cat.# 15939–1-AP. | WB 1:100 dilution |
| Antibody | Anti-SK3 antibody | Abcam | Cat. # ab28631 RRID:AB_775888 | WB 1:100 dilution |
| Antibody | Anti-IK1 Antibody (D-5) (mouse monoclonal) | Santa Cruz | Cat. # sc-365265 RRID:AB_10841432 | WB 1:100 dilution |
| Antibody | Anti-eNOS (mouse monoclonal) | Abcam | Cat. # ab76198 RRID:AB_1310183 | WB 1:100 dilution |
| Antibody | Anti-p-eNOS (rabbit monoclonal) | Cell signaling Technology | Cat. # 9571 RRID:AB_329837 | WB 1:100 |
| Antibody | Anti-GPR68 | NOVUS Biologicals | Cat. # NBP2-32747 | WB 1:100 |
| Antibody | Anti-TRPV4 (clone 1B2.6) (mouse monoclonal) | Millipore Sigma | Cat. # MABS466 | WB 1:100 |
| Antibody | Anti-Actin (mouse monoclonal) | Millipore Sigma | Cat. # MAB1501 RRID:AB_2223041 | WB 1:5000 dilution |
| Antibody | Alexa 555 secondary antibodies (anti rabbit and anti mouse) | Thermo Fisher | Cat. # A-21429 (RRID:AB_141761) and # A-31570 (RRID:AB_2536180) | IF 1:400 dilution |
| Antibody | Alexa 488 secondary antibodies (anti rat) | Thermo Fisher | Cat. # A-21470 RRID:AB_2535873 | IF 1in 400 dilution |
| Other | Nuclear staining (DAPI) | Thermo Fisher | Cat. # 3571 RRID:AB_2307445 | IF 1:1000 dilution |