SKAP2 is required for defense against K. pneumoniae infection and neutrophil respiratory burst
Figures
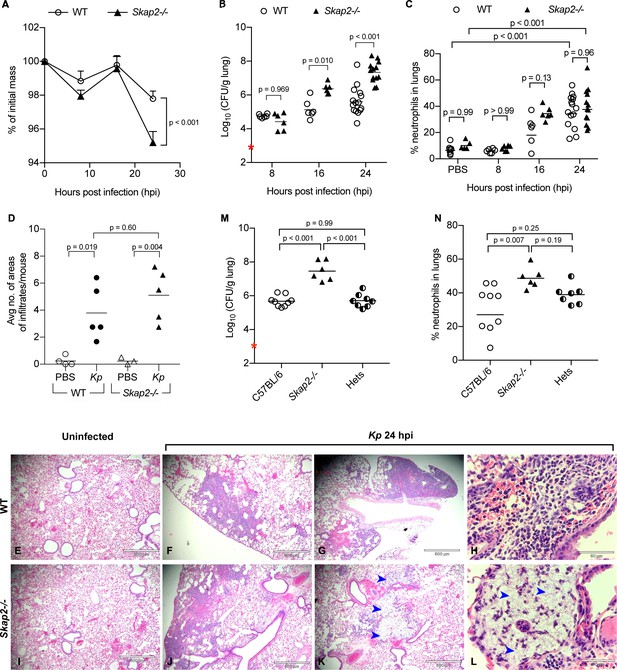
Skap2-/- mice are more susceptible to K. pneumoniae intranasal infection.
(A–L) WT (BALB/c) and Skap2-/- mice were intranasally treated with PBS or infected with 5 × 103 cfu (red asterisk) of K. pneumoniae (Kp). At the indicated time points, (A) mice were weighed, lungs were harvested and single cell suspensions were prepared for (B) CFU and (C) analysis of neutrophils (CD11b+Ly6Ghi). (D–L) At 24 hr post-infection or inoculation with PBS (mock), lungs were harvested and processed for HE-staining. (D) Lung tissue sections were scored for infiltrates of leukocytes or bacteria. Mock (E, I) or K. pneumoniae-infected (F–H, J–L) WT (E–H), and Skap2-/- (I–L), lungs were imaged at 4X (E–G, I–K) or at 40X (H, L). Blue arrows indicate bacteria. Bacterial burden (M) and live neutrophils (CD11b+Ly6Ghi) (N) from K. pneumoniae-infected C57BL/6, Skap2-/-, and Skap2+/- (Hets) littermates are shown. Data are compiled from 2 to 4 independent experiments with 2–4 mice/time point/genotype. (A) Mean ± SEM. (B–D, M–N) Each dot represents values from a mouse, and black bars represent geometric means (B, M) or means (C–D, N). Significance was assessed using (A) two-way ANOVA with Sidak’s post-test, or one-way ANOVA with Tukey’s post-test (M–N), or with Sidak’s post-test (B–C, D); log-transformed numbers were used for (B, M).
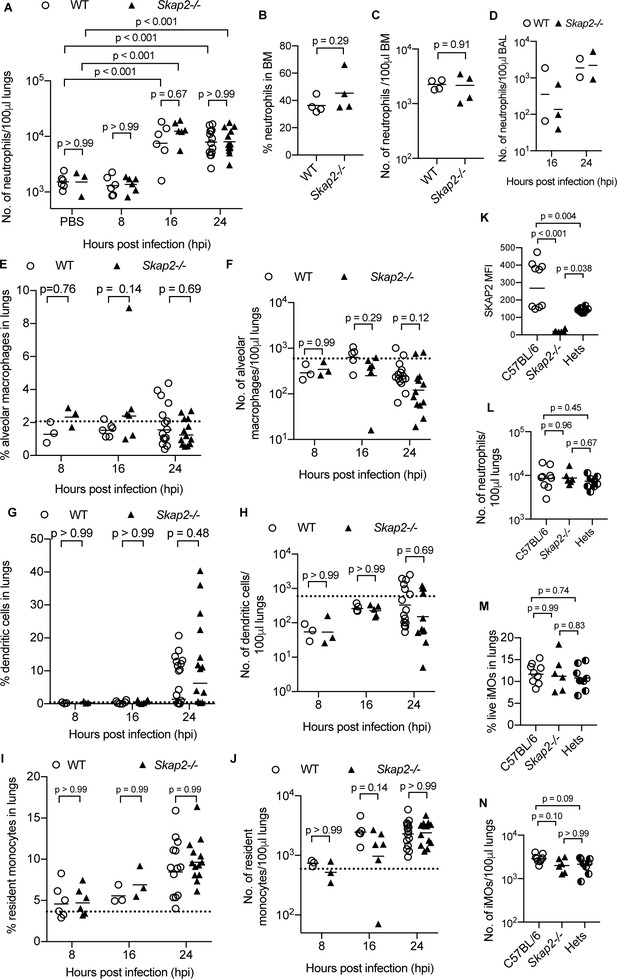
WT and Skap2-/- mice have similar numbers of immune cells in lungs after K. pneumoniae intranasal infection.
(A–J) WT (open circles) and Skap2-/- (solid triangles) mice were intranasally inoculated with PBS or K. pneumoniae and assess for (A–D) neutrophils (CD11b+Ly6Ghi), (E–F) alveolar macrophages (CD11bint CD11chi), (F–H) dendritic cells (CD11bhi CD11chi), and (I–J) resident monocytes (CD11b+ Gr1lo). Data are compiled from 2 to 4 independent experiments (2–4 mice/time point/genotype). (K) Analysis of SKAP2 expression in C57Bl/6, Skap2-/- and Skap2+/- (Hets) by intercellular staining of SKAP2 followed by flow cytometry. (L–N) Neutrophils (CD11b+Ly6Ghi) and iMOs (CD11b+Ly6Chi from K. pneumoniae-infected lungs of C57BL/6, Skap2-/-, and Skap2+/- (Hets) littermates by intracellular staining of SKAP2 followed by flow cytometry. (K–N) Data are compiled from 3 independent experiments. (A–J, L–N) Data are shown as percentage or cell number per 100 μl of lung homogenates as indicated on the axis. (A–N) Each dot represents a mouse; bars represent means. Significance was assessed using one-way ANOVA with Sidak’s post-test (A–J), two-tailed unpaired Student’s t test (K), or with Tukey’s post-test (L–N).
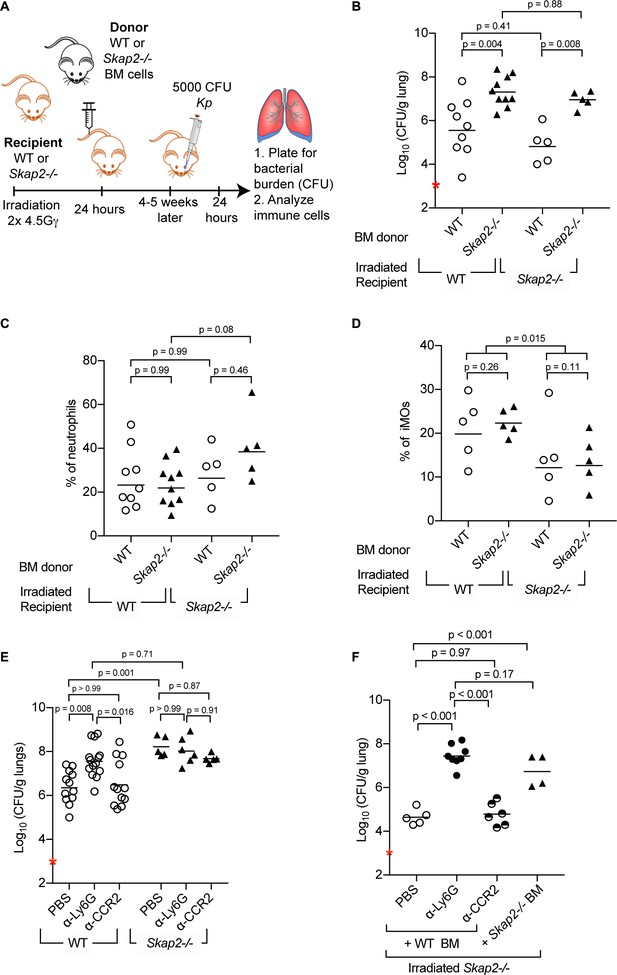
Reconstitution of Skap2-/- mice with WT bone marrow hematopoietic stem cells confers protection against K. pneumoniae.
(A) Schematic used to generate bone marrow chimeras in (B–D, F). (B–F) Mice were infected with 5 × 103 cfu K. pneumoniae (red asterisk); 24 hpi mice were sacrificed and lungs harvested. (E–F) WT and Skap2-/- mice were injected intraperitoneally with 50 μg of α-Ly6G (1A8) or 20 μg of α-CCR2 (MC-21) to deplete neutrophils and iMOs, respectively, or PBS 16 hr prior to infection. Bacterial burden (B, E, F) and percent live neutrophils (CD11b+ Ly6Ghi) (C), or inflammatory monocytes (CD11b+ Ly6Chi) (D) from K. pneumoniae-infected lungs. Data are compiled from 2 to 4 independent experiments using groups of 2–3 mice/genotype/experiment. Each dot represents a mouse, bars are geometric means (B, E–F) or means (C–D). Statistics were assessed using one-way ANOVA with (C–D) Sidak’s post-test, or (B, E–F) Tukey’s post-test. (D) Percent of iMOs were compiled, and comparison between WT and Skap2-/- irradiated recipient disregarding the donor BM were assessed by two-tailed unpaired Student’s t test.
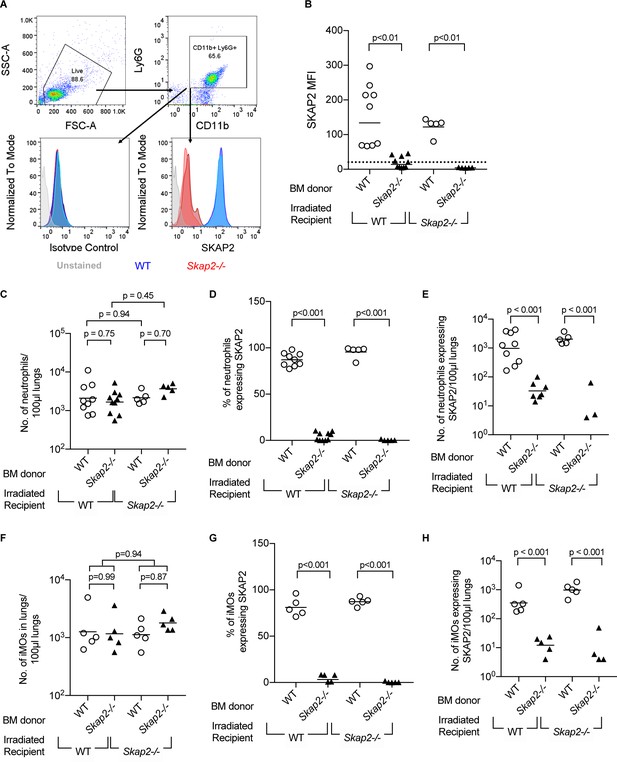
Transplantation efficiency of bone marrow reconstitution into WT and Skap2-/- recipients.
(A) Schematic for gating strategy of flow cytometry data in bone marrow chimeras. Blocks and arrows indicate the cell population used for next gating step. (B–H) Neutrophil (CD11b+ Ly6Ghi) (C–E), or inflammatory monocytes (CD11b+ Ly6Chi) (F–H) from K. pneumoniae-infected lungs were unstained or intracellularly stained with SKAP2 antibody or isotype control. (B) SKAP2 expression in CD11b+ cells shown as mean fluorescent intensity (MFI). Dotted line indicates average level from cells intracellularly stained with isotype control. Data are compiled from 2 to 4 independent experiments using 2–3 mice/genotype/experiment. Each dot represents a mouse, bar represents means. Statistical significance was assessed using one-way ANOVA with Sidak’s post-test.
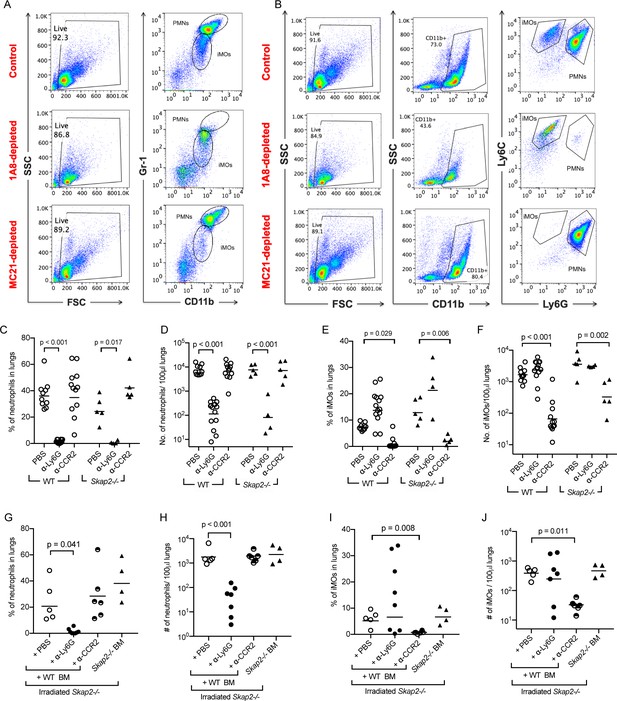
Evaluation of immune cell populations in depletion studies in WT and Skap2-/- mice.
Analysis of inflammatory monocytes (CD11b+ Gr1lo or CD11b+ Ly6Chi Ly6Glo) and neutrophils (CD11b+ Gr1hi or CD11b+ Ly6Cint Ly6Ghi) from non-depleted (PBS), α-Ly6G (clone 1A8)-depleted, or MC-21-depleted (α-CCR2) K. pneumoniae-infected lungs. (A–B) Example of flow cytometry analysis for depletion experiments. (C–F) Quantification of neutrophils (CD11b+ Gr1hi) (C–D), or inflammatory monocytes (CD11b+ Ly6Chi Ly6Glo) (E–F) from α-Ly6G, α-CCR2, or PBS-treated, and K. pneumoniae-infected WT or Skap2-/- mice based on flow cytometry analysis. (G–J) Quantification of neutrophils (G–H), or inflammatory monocytes (I–J) from α-Ly6G, α-CCR2, or PBS-treated, K. pneumoniae-infected irradiated Skap2-/- mice based on flow cytometry analysis. Data are compiled from 3 to 4 independent experiments with 2–3 mice/genotype/experiment. Each dot represents a mouse, bars represent geometric means. (D, F, H, J) were log-transformed. Significance was assessed using one-way ANOVA with Tukey’s post-test.
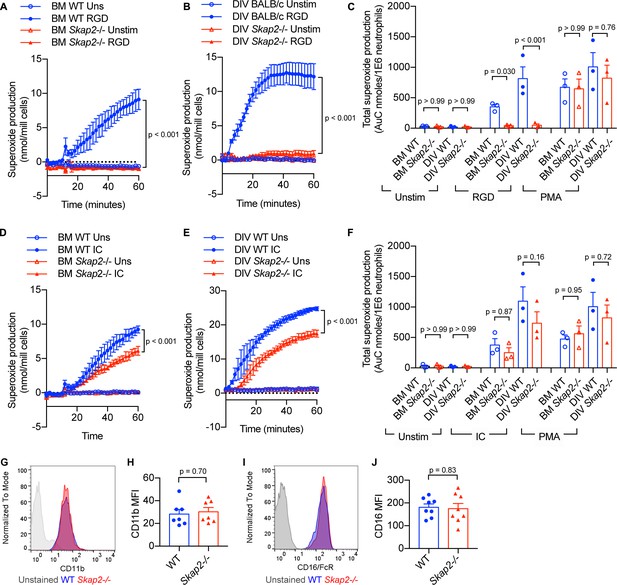
BM and DIV neutrophils require SKAP2 for integrin-activated ROS production, but not for FcγR.
(A–F) Extracellular respiratory burst of BM and DIV neutrophils. WT or Skap2-/- neutrophils were plated on a poly-RGD-coated surface (A–C), or IgG immune complex (IC)-coated surface (D–F), and superoxide production was measured by cytochrome C reduction. Unstimulated (unstim) cells were plated onto 10% FBS/PBS or stimulated with 100 nM PMA. (C, F) Total concentration of superoxide produced after 60 min. (G–J) Expression of surface receptors on DIV neutrophils of CD11b (G–H) or activating CD16 Fcγ receptor (I–J). (G–J); Blue shaded areas and bars represent WT, and red, Skap2-/-. (A–B, D–E) represent the mean ± SD of one experiment in technical triplicate assessed using two-way ANOVA with Tukey’s post-test. (C, F, H, J) represent the mean ± SEM of at least three independent experiments performed in at least technical duplicate (C, F) and were assessed using two-way ANOVA with Tukey’s post-test (C, F), or two-tailed unpaired Student’s t test (H, J).
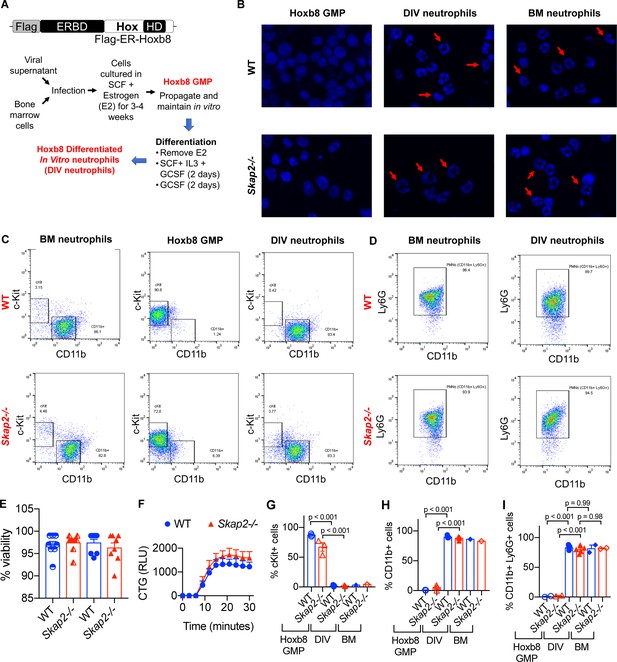
Hoxb8-transformed GMP differentiate into neutrophils with similar morphology and surface markers to that of BM-derived neutrophils.
(A) Schematic of MSCV-Hoxb8 transduction system to generate immortalized stem cell progenitors. The estrogen-binding domain (ERBD) of the estrogen receptor fused to Hoxb8 with an N-terminal Flag epitope tag allows conditional expression of Hoxb8 in the presence of estrogen. After 4 days of differentiation, cells are referred to as DIV neutrophils. (B) Nuclear morphology of BM-derived neutrophils isolated from WT or Skap2-/- mice, HoxB8 GMP, and DIV neutrophils with DAPI. Red arrows indicate polymorphonuclear nuclei. (C) Analysis of c-Kit and CD11b expression. (D) Analysis for Ly6G expression on live CD11b+ cells. (E–F) Viability of WT and Skap2-/- DIV neutrophils was assessed using (E) trypan blue exclusion test from neutrophils differentiated from Hoxb8 immortalized GMP from 2 different WT and Skap2-/- mice prior to functional studies, or (F) DIV neutrophils were plated into 10%FBS/PBS-coated wells, loaded with CellTiter Glo reagent, and chemiluminescence was detected for 30 min by plate reader. The quantification of ATP is shown as relative light units (RLU) from one experiment done in technical triplicate. (G–I) Quantification of (G) cKit+, (H) CD11b+, or (I) CD11b+ Ly6G+ cells from Hoxb8 GMP, day 4 DIV neutrophils (DIV), and BM neutrophils (BM). Results are compiled from 1 to 4 independent experiments with each dot representing one experiment and are shown as mean ± SEM. Significance was determined by one-way ANOVA with Sidak’s post-test.
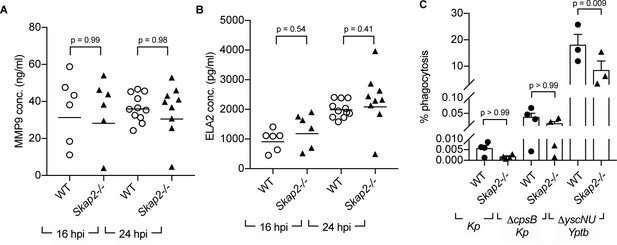
SKAP2 is not required for K. pneumoniae-stimulated degranulation nor phagocytosis.
(A–B) Levels of (A) total MMP-9, and (B) neutrophil elastase (ELA2) from cell-free supernatant from K. pneumoniae-infected WT and Skap2-/- lung homogenates were analyzed by ELISA. Data are compiled from 2 to 4 independent experiments with 2–3 mice/genotype/experiment. Each dot represents a mouse and bars represent geometric means. (C) WT and Skap2-/- DIV neutrophils were incubated with encapsulated (Kp), unencapsulated (ΔcpsB Kp) K. pneumoniae, or Yptb ΔyscNU. Percent phagocytosis was calculated as CFUbacteria with neutrophils and gentamicin/CFUbacteria without gentamicin treatment. (A–C) Data are compiled from at least 3 independent experiments performed in technical triplicate. Statistics represent mean ± SEM and were assessed using one-way ANOVA with Sidak’s post-test.
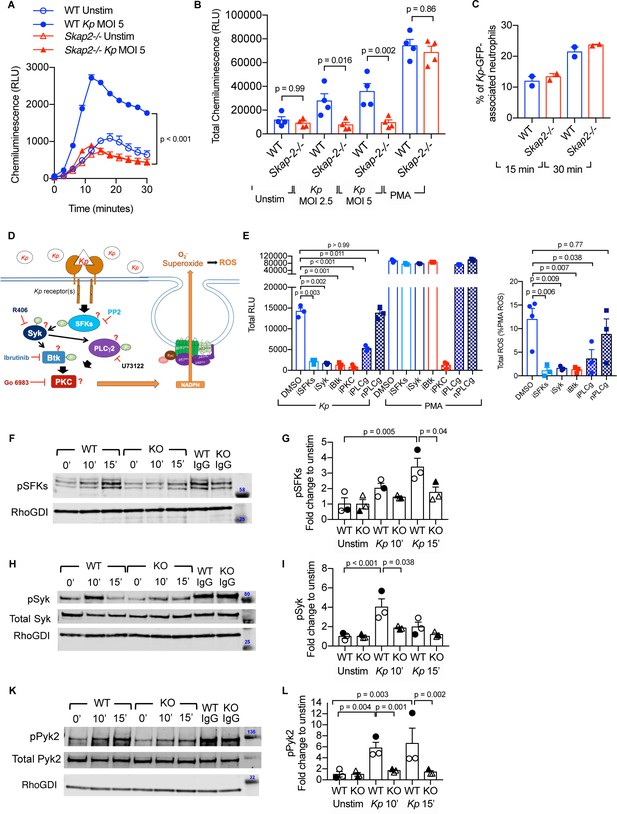
K. pneumoniae-stimulated ROS production requires SKAP2-dependent activation of tyrosine kinases.
(A–C) Respiratory burst of WT and Skap2-/- DIV neutrophils infected with Kp using isoluminol-chemiluminsence. Unstimulated (unstim), Kp-infected, and 100 nM PMA-treated cells were seeded on FBS-coated wells. (A) Representative experiment performed in triplicate of ROS (RLU) production following K. pneumoniae stimulation. (B) Total ROS (total RLU) produced after 30 min of stimulation was calculated as the total area under the curve shown in (A) of 4 independent experiments performed in technical triplicate. (C) WT and Skap2-/- neutrophils incubated with GFP-expressing K. pneumoniae (Kp-GFP) at MOI 40 for 15 or 30 min, stained with DAPI, and analyzed by flow cytometry for GFP-associated neutrophils. (D) Schematic of potential K. pneumoniae-activated signaling pathways tested by inhibitors. (E) Respiratory burst of WT DIV neutrophils untreated or treated with inhibitors using isoluminol-chemiluminescence assay. DIV neutrophils were pre-treated with DMSO, PP2 (iSFKs), R406 (iSyk), Ibrutinib (iBtk), Go 6083 (iPKC), U73122 (iPLCγ), or U73134 (nPLCγ/non-inhibitory analog of PLCγ inhibitor) for 10 min at 37°C and then infected with MOI 2.5 of K. pneumoniae or treated with 100 nM PMA and measured for 30 min. Total RLU was calculated as area under the curve. Data are a representative figure from 3 independent experiments showing mean ± SD performed in technical triplicate. Significance was assessed using one-way ANOVA with Sidak’s post-test. (F–L) WT and Skap2-/- neutrophils were infected with Kp for 10 or 15 min or stimulated with IC for 10 min at 37°C. Lysates were analyzed by western blot for pSFKs (Y416), pSyk (Y352), pPyk2 (Y402), and RhoGDI. Blots were then stripped and re-probed for total Syk or Pyk2. Data are compiled from 3 independent experiments. (B–C) Data are compiled from 2 to 4 independent experiments performed in technical triplicate. Statistics represent mean ± SEM. (F, H, K) Representative blot shown. (G, I, L) Solid symbols indicate values of blot shown. Bars indicate mean. Significance was assessed using one-way ANOVA (B) with Sidak’s post-test between WT and Skap-2-/-, or (G, I, L) between time points within each genotype, or two-way ANOVA with Sidak’s post-test between WT and Skap2-/- within the same point.
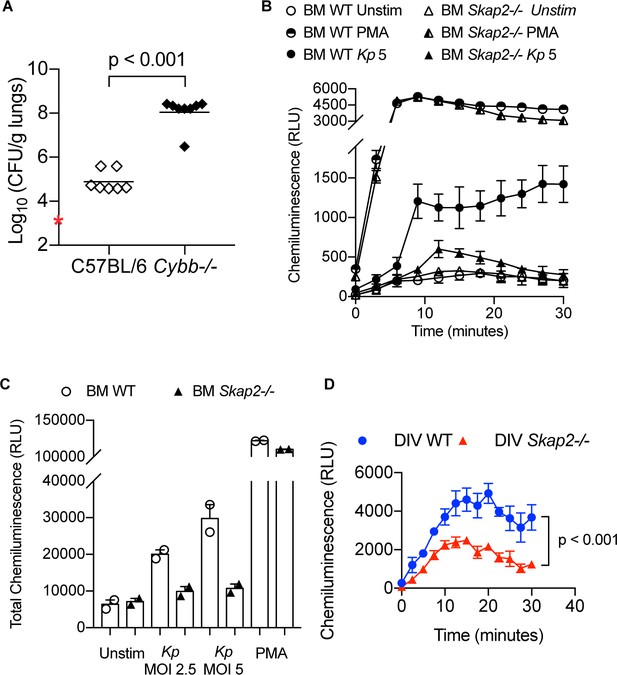
ROS restricts K. pneumoniae infection in lungs and is induced by K. pneumoniae after infection of BM neutrophils.
(A) Bacterial burden of wild-type C57BL/6 and Cybb-/- mice retropharyngeally infected with 5 × 103 cfu K. pneumoniae for 24 hr. Data are compiled from 2 independent experiments with 3–4 mice/genotype/experiment. Each dot represents a mouse and bars represent geometric means. Significance was assessed using two-tailed unpaired Student’s t test. (B–C) Respiratory burst of WT and Skap2-/- BM neutrophils with K. pneumoniae at the indicated MOI using an isoluminol-chemiluminesence assay. (B) Representative experiment of ROS (RLU) production following stimulation with K. pneumoniae, or PMA done in technical duplicate. (C) Total ROS (total RLU) produced after 30 min of stimulation from one experiment shown as mean ± SD. (D) Respiratory burst of WT and Skap2-/- BM neutrophils with Y. pseudotuberculosis ΔyscF at MOI 10 using an isoluminol-chemiluminesence assay.
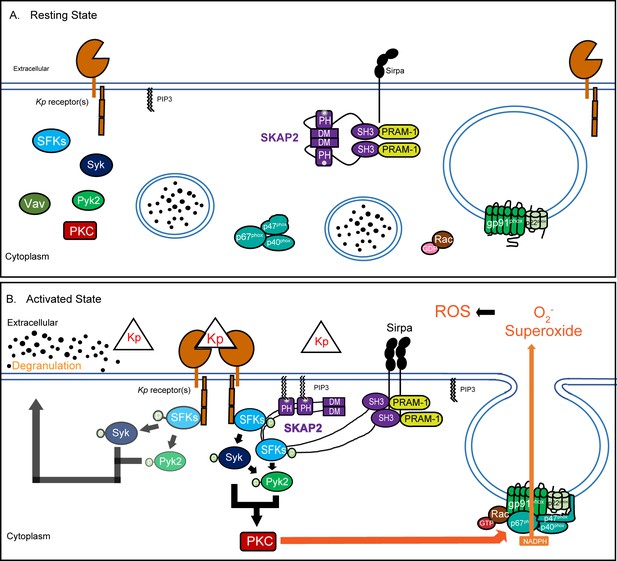
Proposed model of K. pneumoniae-stimulated signaling pathway.
(A) At resting state, the homodimer SKAP2 is in an autoinhibited conformation because of binding of the DM domains; SKAP2 is constitutively associated with PRAM-1 but is apart from other components of the signaling pathways. (B) Activation of neutrophils through the binding of K. pneumoniae leads to production of PIP3 which binds SKAP2, relieving the autoinhibited conformation and revealing sites for docking and centralization of other signaling molecules. SKAP2 docking sites may centralize and retain signaling molecules thereby increasing their local concentration to facilitate increased phosphorylation and amplification of their signals. Alternatively, SKAP2 may directly activate one or more tyrosine kinases, including SFKs, Syk, and Pyk2, leading to ROS production.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | C57BL/6J | Jackson Laboratory | RRID:IMSR_JAX:000664 | |
| Genetic reagent (M. musculus) | B6.129S5-Skap2Gt(VICTR20)21Lex/Mmjax | Jackson Laboratory | RRID:MGI:4353994 | |
| Genetic reagent (M. musculus) | B6.129S-Cybbtm1Din/J | Jackson Laboratory | RRID:IMSR_JAX:002365 | |
| Genetic reagent (M. musculus) | BALB/c BALB/cAnNTac | Taconic Laboratory | RRID:IMSR_TAC:balb | |
| Genetic reagent (Klebsiella pneumoniae) | ATCC 43816 KPPR1 | GenBank ATCC | GenBank: CP009208.1 | |
| Antibody | Rat Anti-Ly6G monoclonal antibody, unconjugated, Clone 1A8 | Fisher Scientific | BD Biosciences Cat# 551459, RRID:AB_394206 | (100 ul of 50 ug/ml) |
| Antibody | Anti-CCR2 (MC21) | Dr. Matthias Mack Mack et al., 2001 | Cat# CCR2 (MC21), RRID:AB_2314128 | (100 ul of 200 ug/ml) |
| Antibody | Rat anti-mouse CD16/CD32 Mouse BD Fc Block | BD Biosciences | BD Biosciences Cat# 553142, RRID:AB_394657 | (1:200 dilution) |
| Antibody | Rat monoclonal anti-mouse/human α-CD11b-PE or α-CD11b-PacBlue (clone M1/70) | Biolegend | BioLegend Cat# 101207, RRID:AB_312790 BioLegend Cat# 101223, RRID:AB_755985 | (1:300 dilution) |
| Antibody | Rat monoclonal anti-mouse α-Ly6G PE-Cy7 (clone 1A8) | Biolegend | BioLegend Cat# 127617, RRID:AB_1877262 | (1:300 dilution) |
| Antibody | Hamster monoclonal anti-mouse α-CD11c-PerCP-Cy5.5 (clone HL3) | BD Biosciences | BD Biosciences Cat# 560584, RRID:AB_1727422 | (1:300 dilution) |
| Antibody | Rat monoclonal anti-mouse α-Gr1-FITC or α-Gr1-APC (clone RB6-8C5) | BioLegend | BioLegend Cat# 108411, RRID:AB_313376 BioLegend Cat# 108405, RRID:AB_313370 | (1:300 dilution) |
| Antibody | Rat monoclonal anti-mouse α-Ly6C-AlexaFluor647 (clone HK1.4) | BioLegend | BioLegend Cat# 128010, RRID:AB_1236550 | (1:300 dilution) |
| Antibody | Rabbit polyclonal anti-mouse/human α-SKAP2 | Proteintech | Proteintech Cat# 12926–1-AP, RRID:AB_2189317 | (1:200 dilution or 1.57 ug/ml) |
| Antibody | rabbit IgG polyclonal isotype antibody | Proteintech | Proteintech Cat# 30000–0-AP, RRID:AB_2819035 | (1.57 ug/ml) |
| Antibody | Alexa Fluor 488 goat anti-rabbit secondary antibody | Thermo Fisher Scientific | Thermo Fisher Scientific Cat# A-11034, RRID:AB_2576217 | (1:250 dilution) |
| Antibody | Rabbit polyclonal anti-human serum albumin | Sigma-Aldrich | Sigma-Aldrich Cat# A0433, RRID:AB_257887 | (1:400 dilution) |
| Antibody | Rabbit anti-human/mouse Phospho-Src Family (Y416) | Cell Signaling Technology | Cell Signaling Technology Cat# 2101, RRID:AB_331697 | (1:500) |
| Antibody | Rabbit anti-human/mouse monoclonal Phospho-Zap-70 (Y319)/Syk (Y352) | Cell Signaling Technology | Cell Signaling Technology Cat# 2717, RRID:AB_2218658 | (1:500) |
| Antibody | Rabbit anti-human/mouse polyclonal Phospho-Pyk2 (Y402) | Cell Signaling Technology | Cell Signaling Technology Cat# 3291, RRID:AB_2300530 | (1:500) |
| Antibody | Rabbit anti-human/mouse polyclonal RhoGDI | Cell Signaling Technology | Cell Signaling Technology Cat# 2564, RRID:AB_2274313 | (1:1000) |
| Antibody | Rabbit anti-human/mouse polyclonal Syk | Cell Signaling Technology | Cell Signaling Technology Cat# 2712, RRID:AB_2197223 | (1:500) |
| Antibody | Rabbit anti-human/mouse polyclonal Pyk2 | Cell Signaling Technology | Cell Signaling Technology Cat# 3292, RRID:AB_2174097 | (1:500) |
| Antibody | Goat Anti-rabbit IgG (H+L) (DyLight 800 4X PEG Conjugate) | Cell Signaling Technology | Cell Signaling Technology Cat# 5151, RRID:AB_10697505 | (1:20,000) |
| Recombinant DNA Reagent | MSCVneo-HA-ER-Hoxb8 | Wang et al., 2006 | In-house (Sykes lab @ MGH) | |
| Peptide, recombinant protein | Stem cell factors | Cho-SCF | In-house | |
| Peptide, recombinant protein | Recombinant murine Stem cell factors | Peprotech | Cat. #: AF-250 | (50 ng/ml) |
| Peptide, recombinant protein | Recombinant murine Interleukin-3 | Peprotech | Cat. #: 213–13 | (10–50 ng/ml) |
| Peptide, recombinant protein | Recombinant murine Interleukin-6 | Peprotech | Cat. #: 216–16 | (10 ng/ml) |
| Peptide, recombinant protein | Recombinant human G-CSF | Peprotech | Cat. #: 300–23 | (50 ng/ml) |
| Peptide, recombinant protein | Fibronectin human plasma | Sigma | Cat. #: F0895 | (10 ug/ml) |
| Peptide, recombinant protein | β-estradiol | Sigma | Cat. #: E2758 | (0.5 uM) |
| Peptide, recombinant protein | Firbonectin-like protein polymer genetically engineered | Sigma Aldrich | Cat. #: F5022 | (15 ug/ml) |
| Peptide, recombinant protein | Albumin from human serum | Sigma | Cat. #: A9511 | (20 ug/ml) |
| Chemical compound | Hexadimethrine bromide (polybrene) | Sigma Aldrich | Cat. #: 107689 | (8 ug/ml) |
| Commercial assay or kit | Mouse total MMP9 DuoSet ELISA | R and D Systems | Cat. #: DY6718 | |
| Commercial assay or kit | Mouse neutrophil elastase/ELA2 DuoSet ELISA | R and D Systems | Cat. #: DY4517 | |
| Chemical compound | 4-Aminophthalhydrazide (isoluminol) | Sigma | Cat. #: A8264 | (50 uM) |
| Chemical compound | Peroxidase from horseradish | Sigma | Cat. #: P6782 | (15 U/ml) |
| Chemical compound, drug | PP2 | Selleck | Cat. #: S7008 | (10 nM) |
| Chemical compound, drug | R406 | Selleck | Cat. #: S2194 | (2 uM) |
| Chemical compound, drug | Ibrutinib (PCI-32765) | Selleck | Cat. #: S2680 | (1 uM) |
| Chemical compound, drug | Gouml 6983, PKC inhibitor | Abcam | Cat. #: ab144414 | (10 uM) |
| Chemical compound, drug | U-73122 | Sigma | Cat. #: U6756 | (1 uM) |
| Chemical compound, drug | U-73433 | Sigma | Cat. #: U6756 | (1 uM) |
| Chemical compound, drug | G418/Geneticin | Thermo Scientific | Cat. #: 10131035 | (1 mg/ml) |
| Other | Immunolon 4HBX 96-well plates | Fisher Scientific | Cat. #: 3855 | |
| Other | Cytochrome c from equine heart | Sigma | Cat. #: C7752 | (100 uM) |
| Other | Ficoll-Paque-Plus | Pharmacia/GE Healthcare | Cat. #: GE17-1440-02 | (1:1 dilution) |
| Other | Percoll | Sigma-Aldrich | Cat. #: P1644 | |
| Other | e-Myco Mycoplasma PCR Detection Kit | Bulldog Bio | Cat. #: 25233 |
Additional files
-
Source data 1
Figure data files.
- https://cdn.elifesciences.org/articles/56656/elife-56656-data1-v2.zip
-
Supplementary file 1
Raw values of western blot analysis for phopho-Src Family Kinases, phospho-Syk, phospho-Pyk2.
WT and Skap2-/- (KO) DIV neutrophils were infected with wild-type Kp, or stimulated with IgG IC (as positive control) for 10 min at 37°C. Lysates were prepared and analyzed by western blot for phosphoproteins, total proteins, and RhoGDI as indicated. Quantification of protein level was assessed using Licor.
- https://cdn.elifesciences.org/articles/56656/elife-56656-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/56656/elife-56656-transrepform-v2.pdf





