Chronic ethanol consumption compromises neutrophil function in acute pulmonary Aspergillus fumigatus infection
Figures
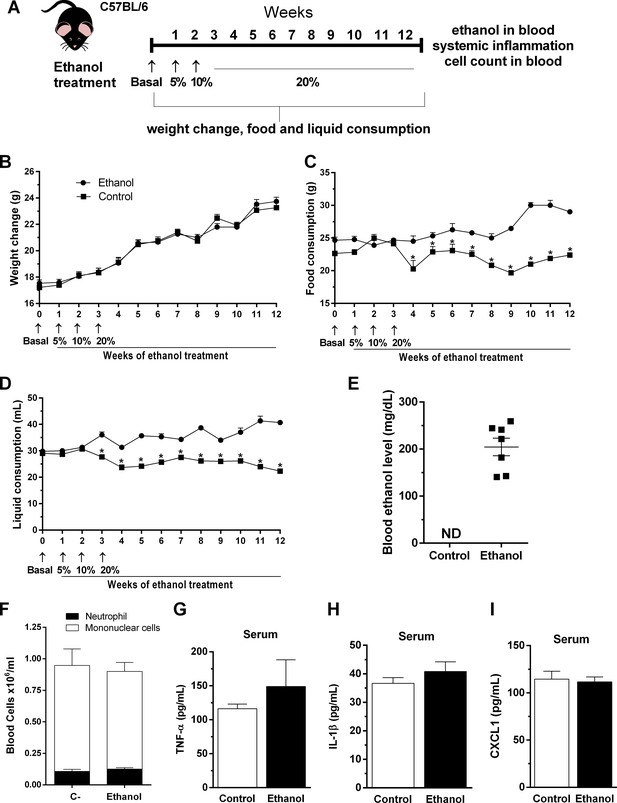
Outcome of chronic ethanol treatment in mice.
(A) Experimental design: C57BL/6 mice received ethanol 5% (v/v) in the first week, followed by 10% (v/v) in the second week (to help mice acclimate with this intervention) and were treated during 10 weeks with ethanol 20% (v/v) in their drinking water. Control group received water. (B–D) During treatment, weight change, food and liquid consumption were measured. Data are presented as Mean ± SD (15 mice per group) *Significantly different (p<0.05) in t test. After ethanol treatment, blood was collected to evaluate (E) blood ethanol levels, (F) differential cell count and (G) TNF-α, (H) IL-1β and (I) CXCL1 in serum. Data are presented as Mean ± SD (4 to 9 mice per group) and analyzed with ANOVA test. Please, also see Supplementary file 1 and Figure 1—source data 1.
-
Figure 1—source data 1
Values for the outcome of ethanol treatment in mice.
- https://cdn.elifesciences.org/articles/58855/elife-58855-fig1-data1-v2.xlsx
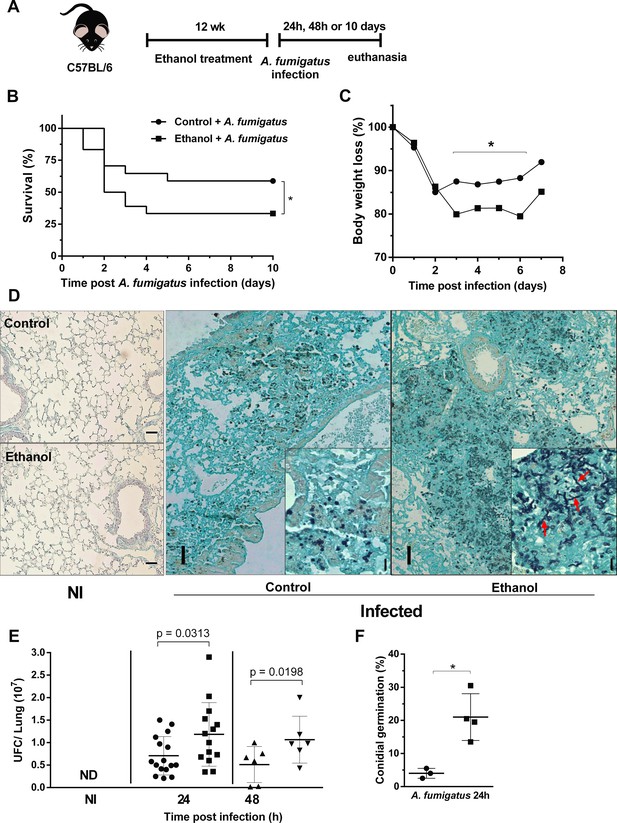
Chronic ethanol consumption leads to susceptibility associated with increased fungal load after A. fumigatus infection.
(A) Experimental design: C57BL/6 mice were treated for 12 weeks with ethanol. The next day after the end of the treatment, mice were intranasally infected with 3 × 108 conidia of A. fumigatus. (B) Comparative lethality curves with P = <0.0001 in Log-rank (Mantel-Cox) test. (C) Comparative weight change curves of ethanol-fed group and control group were performed (18 mice per group). Right lungs were collected 24 and 48 hr after the infection. Homogenate from right lungs were plated and CFUs were quantified. Left lungs were fixed with formaldehyde 4% and embedded in paraffin. Sections were stained with GMS and the percentage of germlings was counted (6–16 mice per group) P value indicated in the figure in t test. (D) Representative slides of GMS staining. The insets in 24 hr images represent magnification to show germlings (red arrows) into lung tissue. (E) Fungal load and (F) fungal germination in lungs p=0.0389 in t test. Bars represent 100 µm.
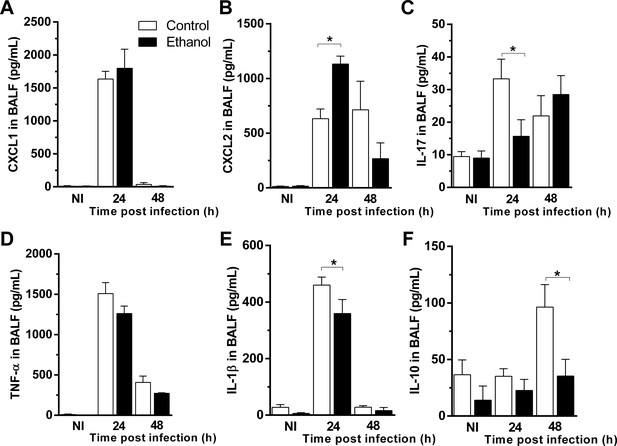
Ethanol consumption altered cytokine release in airways in mice after A. fumigatus infection.
BALF supernatants were harvested at 24 and 48 hr after infection and used for ELISA assay. (A) CXCL1, (B) CXCL2, (C) IL-17, (D) TNF, (E) IL-1β and (F) IL-10 levels in BALF. Experiments were assayed in triplicate. Data are presented as Mean ± SD (3 to 9 mice per group). *p<0.0247 in ANOVA test. Please, also see Figure 3—source data 1.
-
Figure 3—source data 1
Values for inflammatory mediators in BALF after A. fumigatus infection.
- https://cdn.elifesciences.org/articles/58855/elife-58855-fig3-data1-v2.xlsx
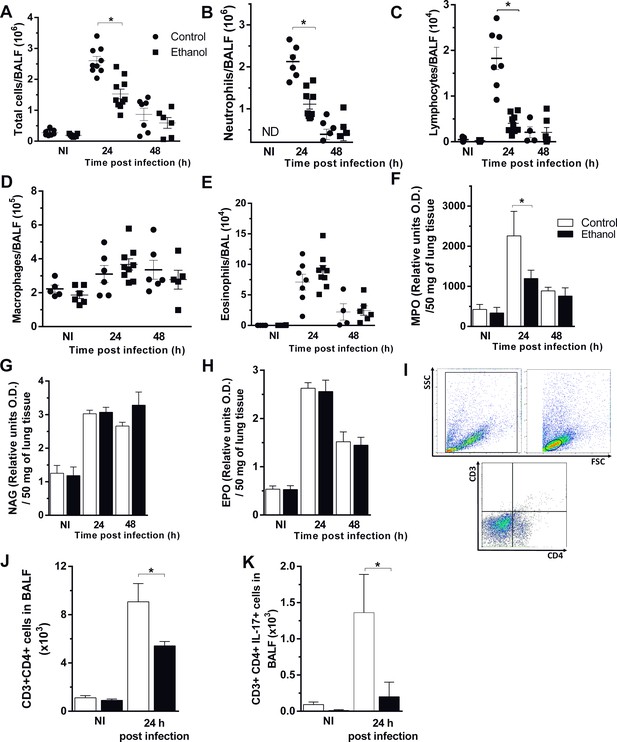
Chronic ethanol consumption affects cellular influx after A. fumigatus infection.
After ethanol treatment, mice were infected with A. fumigatus. BALF and lungs were harvested at 24 and 48 hr after infection for inflammatory cell infiltrates determination. (A) Total cells, (B) Neutrophil, (C) Macrophage, (D) Lymphocyte, (E) Eosinophil counts in BALF. (F) MPO, (G) NAG and (H) EPO assays in lungs. (I) Blood leukocyte count/mL. BALF cells were labeled with specific antibodies for flow cytometry. (J) Gate strategy for CD3+CD4+ T cells. (K) CD3+CD4+ cells and (L) CD3+CD4+IL-17+ cells in BALF. Experiments were assayed in triplicate. Data are presented as mean ± SD (4 to 7 mice per group). *Significantly different (p<0.01) in ANOVA test. Please, also see Figure 4—source data 1.
-
Figure 4—source data 1
Values for indirect measurement of cell infiltration in lung tissue and CD3+CD4+IL-17 cells in BALF after A. fumigatus infection.
- https://cdn.elifesciences.org/articles/58855/elife-58855-fig4-data1-v2.xlsx
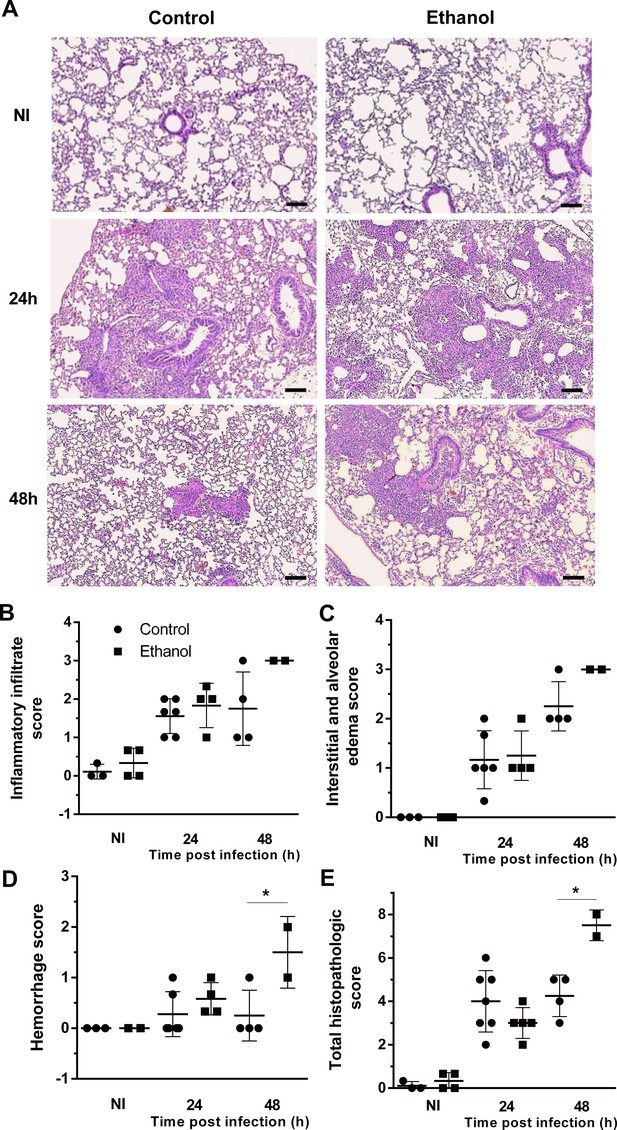
Lung histopathology is affected by ethanol treatment in mice.
Lungs were harvested at 24 and 48 hr after infection fixed with formaldehyde 4% and embedded in paraffin. (A) Sections were stained with Hematoxylin and Eosin. Samples were graded on a 0 to 5-point scale score for (B) inflammatory infiltrate, (C) interstitial and alveolar edema, (D) hemorrhage and (E) total histopathologic score. Experiments were assayed in triplicate. Data are presented as mean ± SD (with 2 to 6 mice per group). *p<0.0142 in ANOVA test. Bars represent 100 µm.
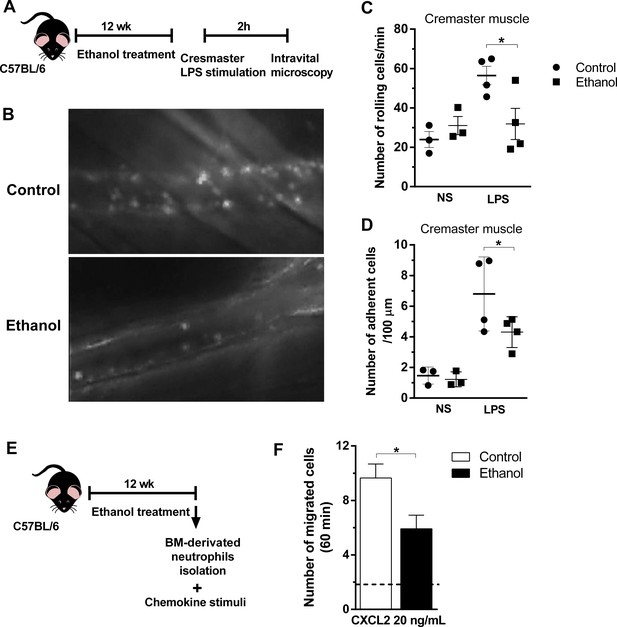
Chronic ethanol consumption reduced leukocyte rolling and adhesion in vivo and neutrophils chemotaxis ex vivo.
(A) Experimental design: after the chronic ethanol treatment, mice received an intracrostal injection of LPS. After 2 hr, mice cremaster from mouse was exposed to examine the microcirculation by intravital microscopy. Post capillaries venules were recorded. (B) Representative images from the recorded videos (please see the rich media). (C) Number of rolling cells and (D) Number of adherent cells were counted in the videos. (E) Ex vivo neutrophil chemotaxis. Experimental design: after ethanol treatment, BM-derived neutrophils were separated by density gradient and a chemotaxis assay in a Boyden chamber towards CXCL2 was performed. (F) Number of migrated neutrophils in 60 min. Data are presented as Mean ± SD (3 to 5 mice per group) *Significantly different (p<0.0028) in ANOVA test. Please, also see Figure 6—source data 1 and Figure 6-rich media videos.
-
Figure 6—source data 1
Values for bone marrow neutrophil chemotaxis.
- https://cdn.elifesciences.org/articles/58855/elife-58855-fig6-data1-v2.xlsx
Leukocyte rolling and adhesion in vivo-control mice + vehicle.
After chronic ethanol treatment, mice received an intracrostal injection of LPS or vehicle (saline). After 2 hr, mice cremaster from mouse was exposed to examine the microcirculation by intravital microscopy. Post capillaries venules were recorded. Representative recorded videos from control mice + vehicle; (Figure 6—video 2) ethanol-treated mice + vehicle; (Figure 6—video 3) LPS-stimulated control mice and (Figure 6—video 4) LPS-stimulated ethanol treated mice.
Leukocyte rolling and adhesionin vivo-ethanol-treated mice + vehicle.
Leukocyte rolling and adhesionin vivo-control mice + LPS.
Leukocyte rolling and adhesionin vivo- ethanol-treated mice + LPS.
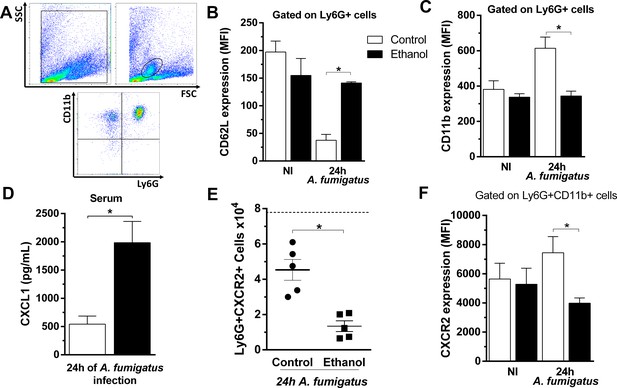
Chronic ethanol consumption impaired neutrophils activation and downregulated CXCR2 in a sepsis-like manner after A. fumigatus infection.
After ethanol treatment and A. fumigatus, blood was harvested at 24 hr post infection. Neutrophils were labeled with specific antibodies for flow cytometry. (A) Gate strategy to analyze neutrophils. Neutrophils were gated by size and cellular complexity and then gated again as Ly6G+CD11b+ cells. (B) CD62L (*p=0.001) and (C) CD11b expression in circulating neutrophils (*p=0.0479). (D) Serum levels of CXCL1 were measured by ELISA assay after 24 hr of infection (*p = 0.0001). (E) Ly6G+CXCR2+ cells (*p=0.0489) and (F) MFI of CXCR2 expression in blood (*p=0.007). Experiments were done at least twice. Data are presented as mean ± SD (3 to 8 mice per group). Analysis were made by ANOVA test. Dashed line represents basal levels of non-infected groups. Please, also see Figure 7—source data 1.
-
Figure 7—source data 1
Values of flow cytometry data.
- https://cdn.elifesciences.org/articles/58855/elife-58855-fig7-data1-v2.xlsx
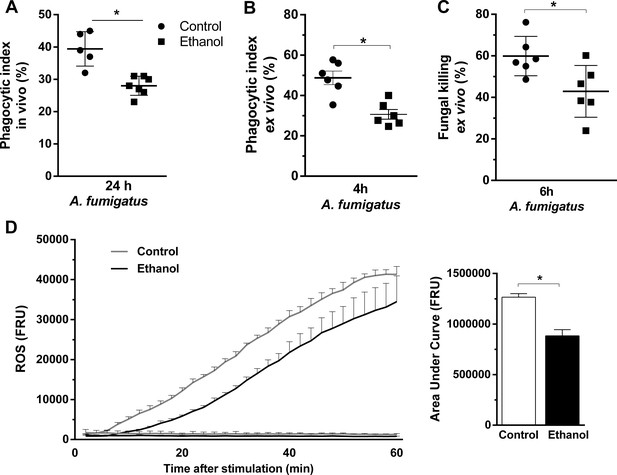
Ethanol consumption decreased neutrophils phagocytosis, killing and ROS production after A. fumigatus stimuli.
(A) Conidial phagocytosis was determined in BALF (*p=0.007). (B–D) After ethanol treatment, BM-derived neutrophils were separated by density gradient and incubated with A. fumigatus conidia. (B) Ex vivo phagocytosis was assessed by cytospin preparations from BALFs (*p=0.0013). (C) Killing assay was evaluated by cell lysis with water, the diluted samples were plated in fungal medium and colony-forming units (CFU) were determined after overnight incubation (*p=0.0238). (D) Luminometry assay was performed to evaluate neutrophil-mediated ROS production and area under curve analysis. (*p=0.057). Data are presented as mean ± SD (3 to 6 mice per group). Analysis were made by t student test.
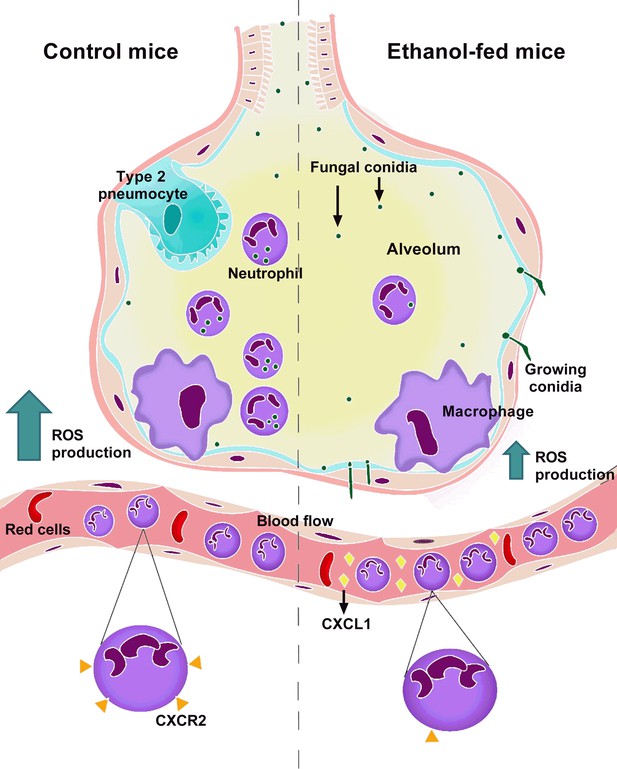
Mechanism related to chronic ethanol dysfunction in neutrophils after A. fumigatus infection.
In normal conditions, infection with A. fumigatus in mice causes a huge inflammatory response, characterized by neutrophil chemokines release. The recruited neutrophils clear the infection by phagocytosis and ROS-mediated killing. In contrast, in a condition of chronic ethanol consumption, despite the correct induction of inflammatory response, there is an increase of CXCR2 ligands in blood flow, causing CXCR2 downregulation. This leads to lower neutrophils recruitment, culminating in substantial fungal burden into the lungs from ethanol-fed mice.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6J | Multidisciplinary center for Biological Investigation on Laboratory Animal Science (CEMIB) -Unicamp | C57BL/6JUnib | |
| Genetic reagent (Aspergillus fumigatus) | Strain A1163 | A. fumigatus CEA17 isolate (CEA10 derivative) and converted to pyrG+ via A. niger pyrG gene ectopic insertion Fedorova et al., 2008; Malacco et al., 2019. | ||
| Cell line (M. musculus) | Primary bone marrow neutrophils | This paper | C57BL/6JUnib | Freshly isolated from C57BL/6J (M. musculus) |
| Peptide, recombinant protein | Recombinant murine MIP-2 (CXCL2) | PeproTech | Cat# 250–15 | Chemotaxis (20 ng/ml) |
| Antibody | Purified NA/LE CD16/CD32 Clone 2.4G2 - FC Block (Rat monoclonal) | BD Biosciences | Cat# 553140 | FACS (1:100) |
| Antibody | anti-CD3-FITC (Rat monoclonal) | BD Biosciences | Cat# 555274 | FACS (1:100) |
| Antibody | anti-CD4-APC (Rat monoclonal) | BD Biosciences | Cat# 553051 | FACS (1:200) |
| Antibody | anti-IL-17a-PE (Rat monoclonal) | BD Biosciences | Cat# 559502 | FACS (1:100) |
| Antibody | anti-Ly6G-BV421 (Rat monoclonal) | BD Biosciences | Cat# 562737 | FACS (1:50) |
| Antibody | anti-CXCR2-PE (Rat monoclonal) | R and D Systems | Cat# FAB2164P | FACS (1:10) |
| Antibody | anti-CD62L-APC (Rat monoclonal) | BD Biosciences | Cat# 553152 | FACS (1:100) |
| Antibody | anti-CD11b-FITC (Rat monoclonal) | BD Biosciences | Cat# 553310 | FACS (1:100) |
| Commercial assay or kit | Mouse TNF-a ELISA kit | R and D Systems | Cat# DY410 | |
| Commercial assay or kit | Mouse IL-1b ELISA kit | R and D Systems | Cat# DY401 | |
| Commercial assay or kit | Mouse CXCL1 ELISA kit | R and D Systems | Cat# DY453 | |
| Commercial assay or kit | Mouse CXCL2 ELISA kit | R and D Systems | Cat# DY452 | |
| Commercial assay or kit | Mouse IL-17 ELISA kit | R and D Systems | Cat# DY421 | |
| Commercial assay or kit | Mouse IL-10 ELISA kit | R and D Systems | Cat# DY417 | |
| Software, algorithm | Prism | GraphPad | ||
| Software, algorithm | FlowJo | BD |
Additional files
-
Supplementary file 1
Percentage of neutrophil precursors in bone marrow after ethanol treatment.
- https://cdn.elifesciences.org/articles/58855/elife-58855-supp1-v2.docx





