A novel acidification mechanism for greatly enhanced oxygen supply to the fish retina
Figures
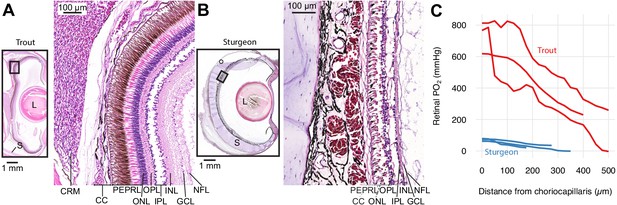
Retinal micro-anatomy and oxygen distribution in the retina of rainbow trout (Oncorhynchus mykiss) (A) and white sturgeon (Acipenser transmontanus) (B).
Histology indicates a thick retina and presence of a choroid rete mirabile in the rainbow trout and a thin retina and absence of a choroid rete mirabile in the white sturgeon. PEPRL constitutes the outer retina, and ONL to NFL constitute the inner retina. Partial pressure of O2 (PO2) distribution in the retina of rainbow trout (red) and white sturgeon (blue) (C) showing that rainbow trout has a higher PO2 (linear mixed-effects model, n = 6 [3 for each species], β ± SE = 390±75.8 mmHg, t = 5.14, p<0.001) and a steeper PO2 gradient (linear mixed-effects model, n = 6 [3 for each species], β ± SE = −1.06 ± 0.0859 mmHg µm−1, t = −12.4, p<0.001) when compared to white sturgeon. Abbreviations: CC, choriocapillaris; CRM, choroid rete mirabile; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; L, lens; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PEPRL, pigment epithelium and photoreceptor layer; S, sclera.
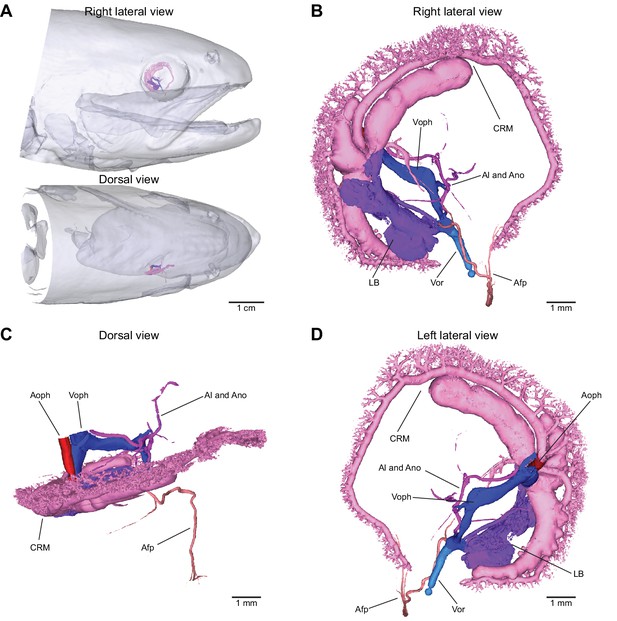
Vascular macro-anatomy of the eye of rainbow trout (Oncorhynchus mykiss).
Vascular filling with a radiopaque contrast agent and subsequent micro computed tomography allowed for three-dimensional modeling of the retinal circulation. Overview of head region (A) and right lateral (B), dorsal (C), and left lateral view (D) of the right eye. The ophthalmic artery, an extension of the efferent pseudobranchial artery, feeds into the choroid rete mirabile that supplies the choriocapillaris whose origin is seen as multiple branches off choroid rete mirabile in (B-D). The only vessel to enter the interior vitreous humor of the eye is the falciform process artery supplying the retractor lentis muscle. The falciform process artery is supplied by the lentiform body that receives its blood supply from the lentiform artery. Red to blue color gradient indicates blood flow direction from the ophthalmic artery via the retinal blood vessels to the ophthalmic vein. For an interactive three-dimensional model, see Supplementary file 1. Abbreviations: Afp, falciform process artery; Al, lentiform artery; Ano, optic nerve artery; Aoph, ophthalmic artery; CRM, choroid rete mirabile; LB, lentifrom body; Voph, ophthalmic vein; Vor, oral venous return.
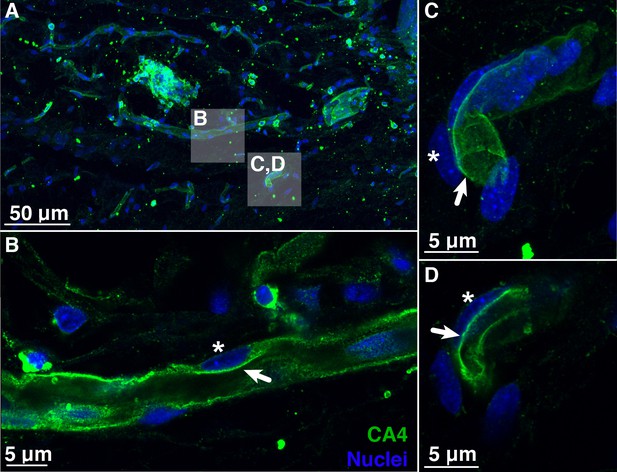
Blood vessels in the choroid rete mirabile of the rainbow trout eye, stained for the membrane-bound, carbonic anhydrase isoform 4 (CA4; green) and nuclei (blue).
(A) Overview of a choroid rete mirabile section with inserts denoting the magnified vessels shown in panels B and C. (B) Magnified blood vessel shows CA4 staining along the entire length of the vessel. The staining pattern of a single endothelial cell is consistent with an apical localization of CA4 protein (arrow), whereas the nuclei of endothelial cells (see asterisk) are located basolaterally of the CA4 staining. (C) Another magnified blood vessel shows CA4 staining (arrow) surrounding the entire vessel lumen with the cell nuclei (asterisk) found on the outside. To further illustrate the luminal orientation of CA4, panel (D) represents a cross-section of the vessel shown in C, clearly showing that CA4 staining is not found within the vessel itself, but only surrounding the lumen. Detection of CA4 was with a polyclonal antibody raised against rainbow trout CA4 that has been described previously (Georgalis et al., 2006; Gilmour et al., 2007), and nuclei were visualized with DAPI.
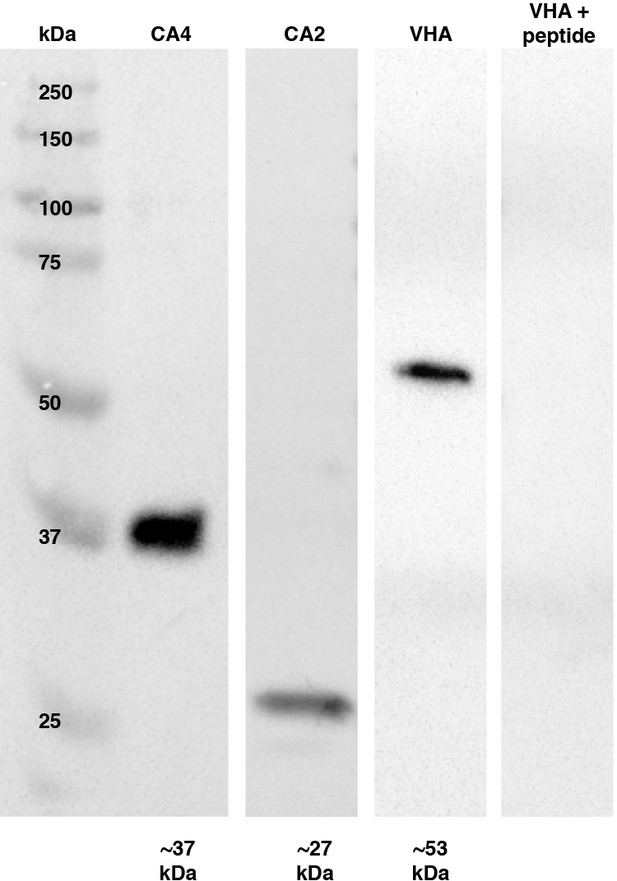
Western blot analysis of choroid rete mirabile tissue homogenates.
Bands matching the predicted size of respective proteins confirm the specificity of the antibodies against carbonic anhydrase 4 (CA4) and vacuolar-type H+-ATPase (VHA) in rainbow trout. The antibodies for CA2 and CA4 are well-validated, and for the custom VHA antibody, we show a complete absence of bands in the peptide preabsorption control.
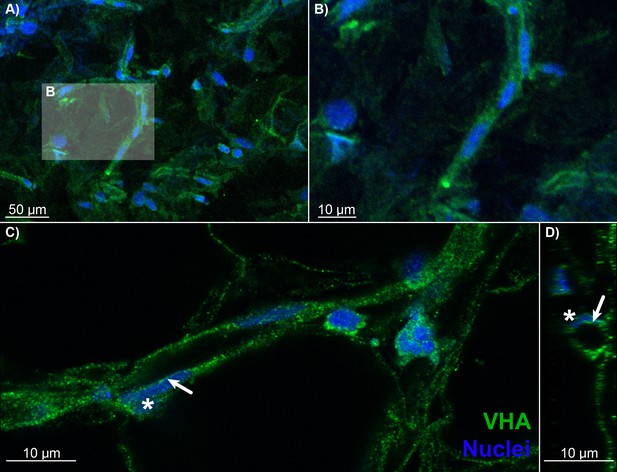
Blood vessels in the choroid rete mirabile of the rainbow trout eye, stained for the vacuolar-type proton ATPase (VHA; green) and nuclei (blue).
(A) Overview of a choroid rete mirabile section with an insert denoting the magnified vessel section in (B) that shows a single blood vessel with VHA staining. (C) Another blood vessel shows the staining pattern for VHA along the entire length of the vessel walls. In a single endothelial cell, the staining pattern for VHA indicates an apical localization of the protein, whereas the nuclei of endothelial cells (asterisk) are located basolaterally of the VHA staining. To further illustrate the luminal orientation of VHA, panel (D) represents a cross-section of the vessel shown in C, further illustrating that VHA staining is not found within the vessel itself, but only surrounding the blood lumen. Detection of VHA was with a polyclonal antibody raised against the conserved β-subunit of VHA, and nuclei were visualized with DAPI.
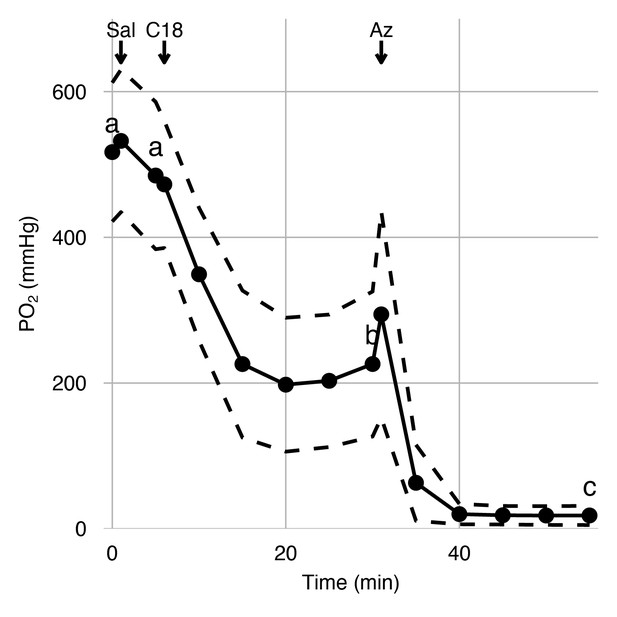
Effects of carbonic anhydrase inhibition on retinal oxygen secretion.
Choroidal PO2 was measured in anesthetized rainbow trout using an ultra-thin PO2 electrode inserted into the choroid during sequential injections of saline, C18, or acetazolamide (Az) via a dorsal aorta catheter. The saline injection did not affect choroidal PO2, but the injection of C18 and Az led to sequential reductions in choroidal PO2, as indicated by letters that differ (linear mixed-effect model, p<0.001). Black circles connected by solid lines represent mean choroidal PO2 at 15 time points, and dashed lines represent standard error of mean (n = 6).
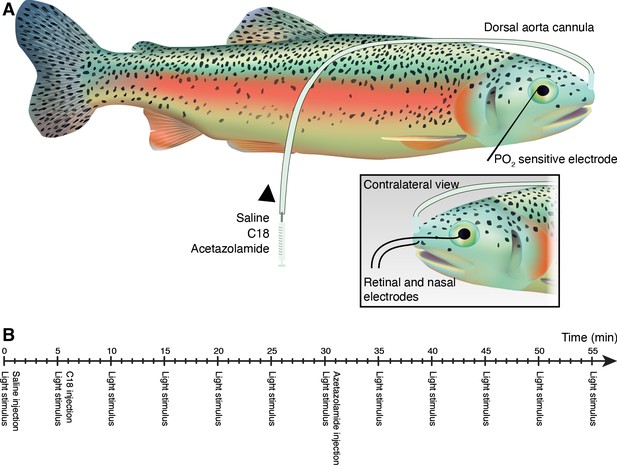
Experimental protocol for in vivo inhibition of carbonic anhydrase.
In brief, rainbow trout were anesthetized and cannulated in the dorsal aorta for injection of CA inhibitors under constant gill irrigation (A). The retina of one eye was instrumented with an ultra-thin PO2-sensitive electrode to measure choroidal PO2, and the contralateral eye was instrumented with a retinal electrode to measure the electroretinogram. After 15 min of dark acclimation, and while maintained in complete darkness, the contralateral eye was stimulated every fifth min for 55 min by an a 525 nm LED held 5 cm from the eye, while saline (control), or the CA inhibitors C18, and acetazolamide were injected via the cannula at 1, 6, and 31 min, respectively. (B) Timeline for light stimulations and injections of saline, C18, and acetazolamide. See Materials and methods for more details. Jacelyn Shu (University of British Columbia) kindly illustrated the rainbow trout.

Effects of PO2 on retinal function.
(A) Representative sets of six electroretinograms (colored traces) at high and low PO2 (right and left panels, respectively) measured after six 0.1 ms pulses of green light (black vertical arrow) and then averaged (black trace). The averaged electroretinograms were analyzed for the amplitude, V, and implicit time, IT, of the a- and b-wave (green and blue, respectively). Amplitudes (B) and implicit times (C) of the a- and b-wave in the electroretinogram of trout during progressive inhibition of retinal O2 secretion by C18 and acetazolamide (n = 4). The properties of the electroretinograms in A are marked by red symbols. Solid lines are linear mixed-effect model fits to the data (all p<0.001, see text for statistics).

Effects of the presence of a choroid rete mirabile on the morphology of the inner - and outer retina.
The transverse thickness of the inner- (A) and outer retina (B) in species with (red) and without (black) a choroid rete mirabile. Each dot represents species mean values obtained from the literature (Damsgaard et al., 2019). Effect sizes are visualized in the upper panels, showing the observed F-statistics (black vertical line) compared to a grey null-distributions of F-statistics from a phylogenetic analysis of variance simulation. This analysis showed a strong positive effect of the presence of a choroid rete mirabile on the inner retinal thickness (p<0.001, n = 31), but not on outer retinal thickness (p=0.51, n = 31).
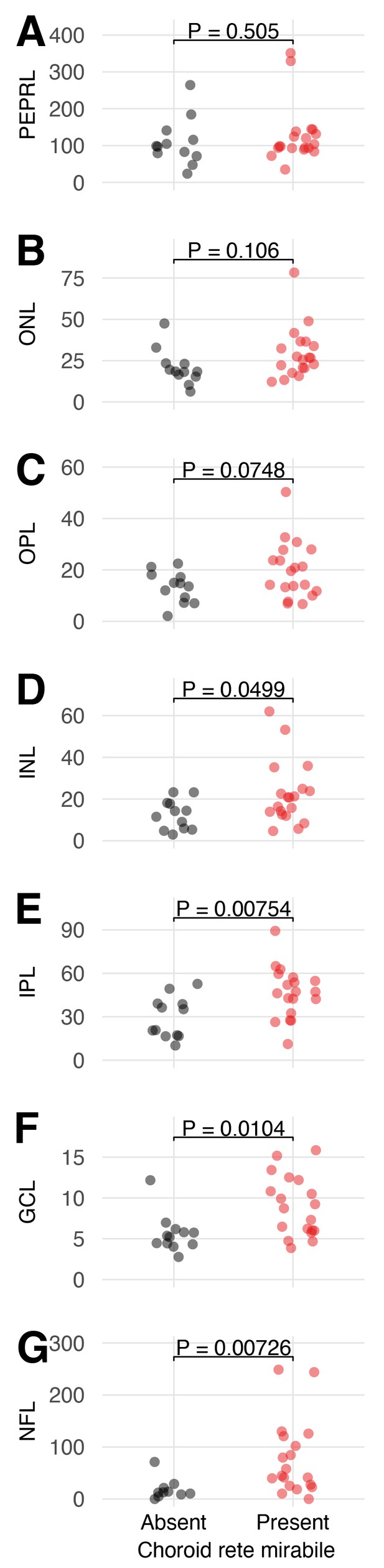
Effects of the presence of a choroid rete mirabile on the retinal thickness of the individual retinal layers.
The transverse thickness in micrometer of the seven retinal layers in species with (red) and without (black) a choroid rete mirabile: (A) pigment epithelium and photoreceptor layer (PEPRL), (B) outer nuclear layer (ONL), (C) outer plexiform layer (OPL), (D) inner nuclear layer (INL), (E) inner plexiform layer (IPL), (F) ganglion cell layer (GCL), and (G) nerve fiber layer (NFL). Each dot represents species mean values obtained from the literature (Damsgaard et al., 2019). P-values indicate the probability of the observed F-statistics lying within a null-distributions of F-statistics from a phylogenetic analysis of variance simulation.
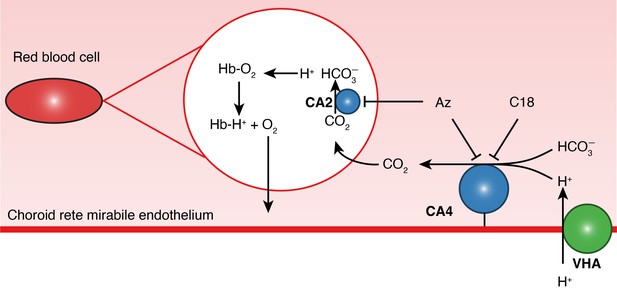
A proposed pathway for oxygen secretion in the choroid rete mirabile of teleost fishes.
Endothelial H+-secretion by vacuolar-type H+-ATPase (VHA, green) drives CO2 production facilitated by a plasma-accessible carbonic anhydrase (CA4, blue). This CO2 diffuses across the red blood cell membrane and is then dehydrated by carbonic anhydrase 2 (CA2, blue), producing H+s which bind to hemoglobin (Hb) and release O2. C18 is a membrane-impermeable CA inhibitor that inhibits only the paCA (Rummer et al., 2013). Acetazolamide (Az) is a membrane-permeable CA inhibitor that rapidly inhibits both CA2 and CA4.
Additional files
-
Supplementary file 1
Three-dimensional overview of the vasculature of the trout eye.
Micro-CT-generated interactive overview of the ocular vasculature in a rainbow trout injected with a radiopaque contrast agent in the ventral aorta. Open the file in Adobe Acrobat Reader nine or higher and activate the 3D feature by clicking on the model. Then use the cursor to interact with the model or select pre-defined views similar to Figure 1—figure supplement 1.
- https://cdn.elifesciences.org/articles/58995/elife-58995-supp1-v1.pdf
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/58995/elife-58995-transrepform-v1.pdf





