Structural ordering of the Plasmodium berghei circumsporozoite protein repeats by inhibitory antibody 3D11
Figures
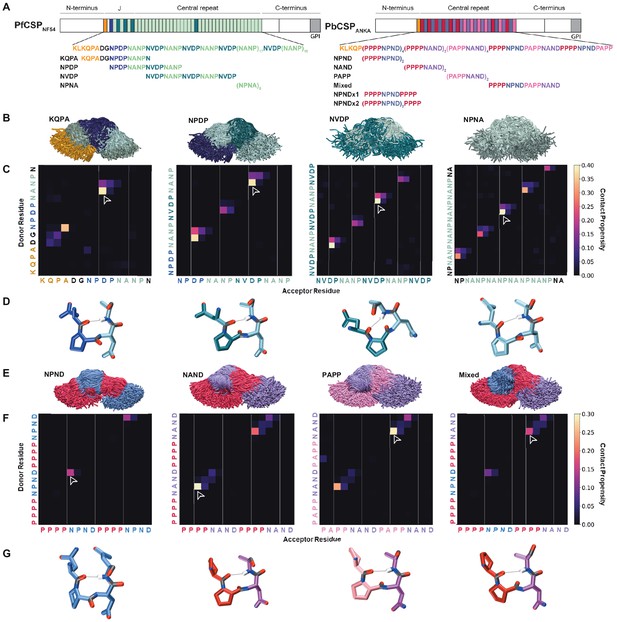
Comparison of PfCSP and PbCSP repeat sequences and structures.
(A) Schematic representations of PfCSP strain NF54 and PbCSP strain ANKA, each comprising an N-terminal domain, central repeat region, and C-terminal domain. The junctional region (J) immediately following the N-terminal domain of PfCSP is indicated. Colored bars represent each repeat motif. The sequences of each CSP central repeat region and corresponding peptides used in the study are shown below their respective schematics. (B-G) Conformational ensembles of CSP peptides in solution from molecular dynamics simulations. (B) Superposition of the conformations of the four PfCSP-derived peptides at each nanosecond. The peptides are aligned to the conformational median structure and only the backbone is shown for clarity. (C) Ensemble-averaged backbone-backbone hydrogen-bonding maps for each PfCSP peptide sequence. The propensity for hydrogen bonds between the NH groups (y-axis) and CO groups (x-axis) is indicated by the color scale on the right. (D) Sample molecular dynamics snapshots of the highest-propensity turn for each PfCSP peptide are shown as sticks with hydrogen bonds shown as gray lines. The highest-propensity turn for each peptide is indicated by the arrowhead on the corresponding hydrogen-bonding map. (E) Superposition of the conformations of the four PbCSP-derived peptides at each nanosecond. The peptides are aligned to the conformational median structure and only the backbone is shown for clarity. (F) Ensemble-averaged backbone-backbone hydrogen-bonding maps for each PbCSP peptide sequence. The propensity for hydrogen bonds between the NH groups (y-axis) and CO groups (x-axis) is indicated by the color scale on the right. (G) Sample molecular dynamics snapshots of the highest-propensity turn for each PbCSP peptide are shown as sticks with hydrogen bonds shown as gray lines. The highest-propensity turn for each peptide is indicated by the arrowhead on the corresponding hydrogen-bonding map.
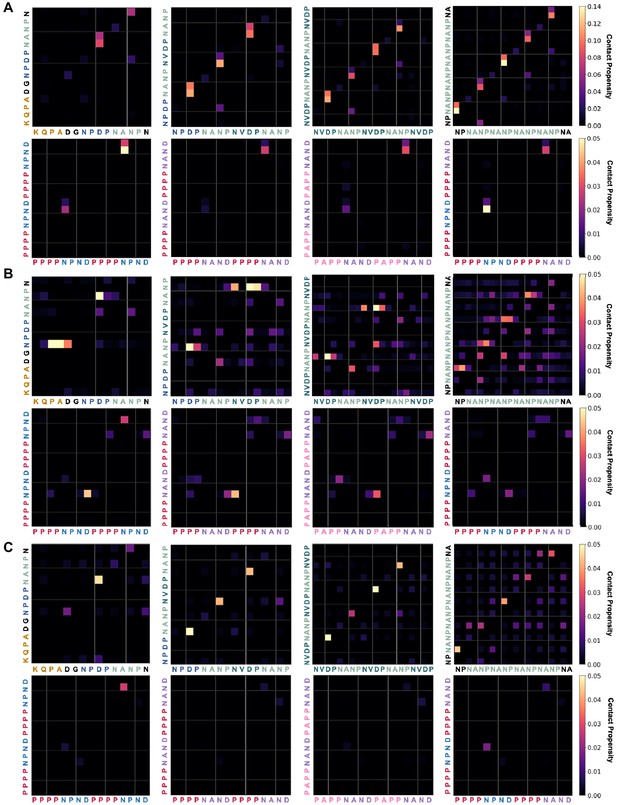
Ensemble-averaged hydrogen-bonding propensities for PfCSP- and PbCSP-derived peptides.
(A) The propensity for hydrogen bonds between the NH groups of the backbone (y-axis) and CO groups of the side chains (x-axis) is indicated by the color scale on the right. (B) The propensity for hydrogen bonds between the NH groups of the side chains (y-axis) and CO groups of the backbone (x-axis) is indicated by the color scale on the right. (C) The propensity for hydrogen bonds between the NH groups of the side chains (y-axis) and CO groups of the side chains (x-axis) is indicated by the color scale on the right.
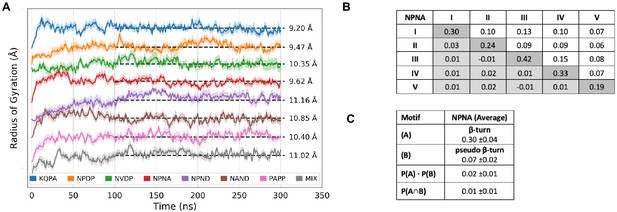
Experimental details of MD simulations.
(A) Time evolution of the radius of gyration of the different peptides from MD simulations. Shading represents the standard error of the mean computed from the different simulation repeats (see Materials and methods). The simulations are statistically converged after 100 ns. The average radius of gyration for each peptide is reported on the right. The individual peptides have been shifted on the y-axis to allow for ease of visualization. (B) The simulated propensity of having a specific hydrogen bond is shown on the diagonal, with the propensity of two specific hydrogen bonds (P(A∩B)) shown above the diagonal. Each individual NPNA motif is labelled from I-V. The difference of the simulated values from the calculated values (P(A)·P(B)) is shown below the diagonal. These differences are all well below the average standard error of mean of 0.04, confirming that the individual motifs are uncorrelated. (C) A mathematical example showing that the β-turn and pseudo β-turn propensities are independent and uncorrelated (P(A∩B)=P(A)·P(B)). This holds true for all the simulated peptides.
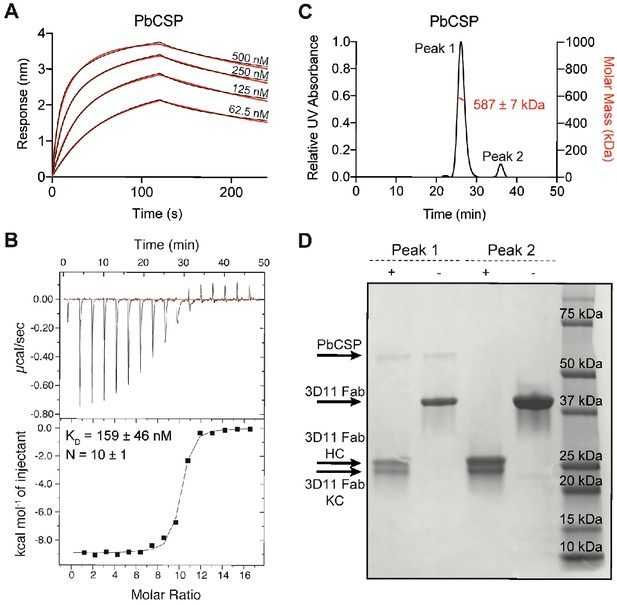
Biophysical characterization of 3D11 Fab-PbCSP binding.
(A) Binding kinetics of twofold dilutions of 3D11 Fab to PbCSP. Representative sensorgrams are shown in black and 2:1 model best fits in red. Data are representative of three independent measurements. (B) Isothermal titration calorimetry (ITC) analysis of 3D11 Fab binding to PfCSP at 37°C. Above, raw data of PbCSP (0.005 mM) in the sample cell titrated with 3D11 Fab (0.4 mM). Below, plot and trendline of heat of injectant corresponding to the raw data. KD and N values resulting from three independent experiments are indicated. Standard error values are reported as standard error of the mean (SEM). (C) Results from size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS) for the 3D11 Fab-PbCSP complex. A representative measurement of the molar mass of the 3D11 Fab-PbCSP complex is shown as the red line. Mean molar mass and standard deviation are as indicated. (D) SDS-PAGE analysis of resulting Peaks 1 and 2 from SEC-MALS. Each peak was sampled in reducing and non-reducing conditions as indicated by + and -, respectively.
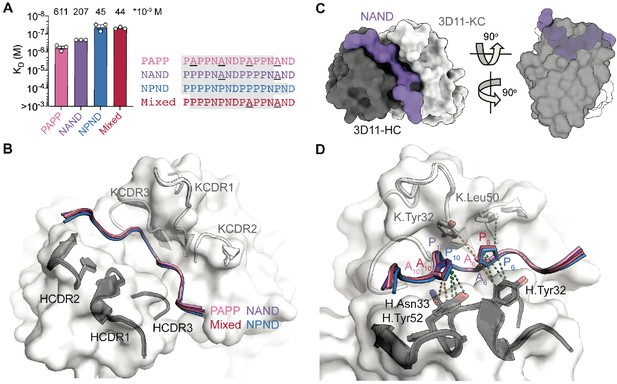
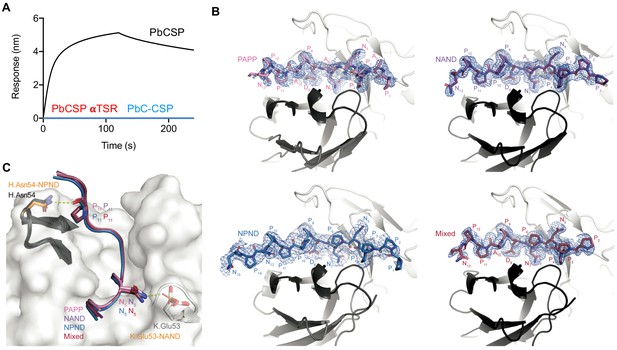
3D11 Fab binding to PbCSP repeat peptides.
(A) Affinities of 3D11 Fab for PAPP, NAND, NPND, and Mixed peptides as measured by ITC. Symbols represent independent measurements. Mean KD values are shown above the corresponding bar. Error bars represent SEM. Peptide sequences are as indicated to the right of the plot, with variable residues underlined and shaded residues indicating those resolved in the corresponding X-ray crystal structures. (B) The 3D11 Fab binds the PAPP (pink), NAND (purple), NPND (blue) and Mixed (red) peptides in nearly identical conformations. mAb 3D11 CDRs are indicated. (C) Overview and side view of the NAND peptide (purple) in the binding groove of the 3D11 Fab shown as surface representation (H-chain shown in black and K-chain shown in gray). (D) Van der Waals interactions formed by side chain atoms of both Ala and Pro residues are indicated by orange dashed lines, and those unique to Pro6 and Pro10 are indicated by green dashed lines.
Experimental details of mAb 3D11 binding.
(A) 3D11 Fab binding to peptides representative of the PbCSP αTSR domain (residue 263–318; red) and the full C-terminal domain (residues 202–318; PbC-CSP; blue). PbCSP (residue 24–318) was used as a positive control (black). (B) Composite omit map electron density contoured at 1.0 sigma (blue mesh) around PbCSP peptides PAPP, NAND, NPND and Mixed in complex with the 3D11 Fab. (C) Slight differences in H-bonding at the N- and C-terminal ends of the PbCSP repeat peptides when bound to the 3D11 Fab in the crystal structures. PbCSP peptides are colored as in Figure 3. Antibody residues partaking in H-bonds are colored orange. mAb 3D11 HCDR2 is colored in black and KCDR2 in white.
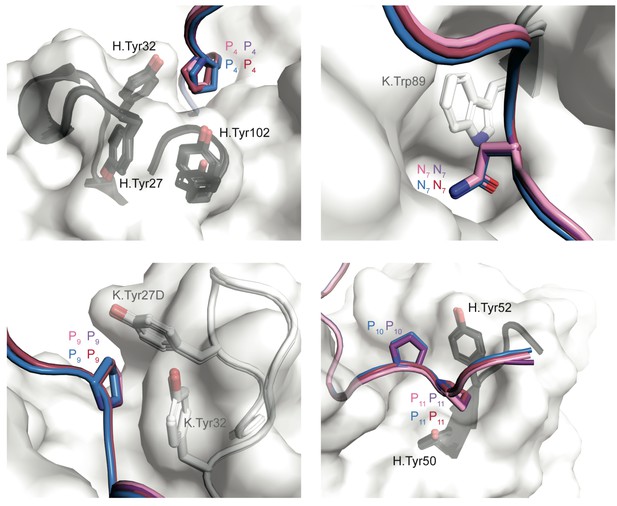
Interactions between mAb 3D11 aromatic side chains and PbCSP peptides.
Interactions formed between PbCSP peptide residues and aromatic side chains of the 3D11 Fab HCDR (black) and KCDR (white). PbCSP peptides are colored as in Figure 3.
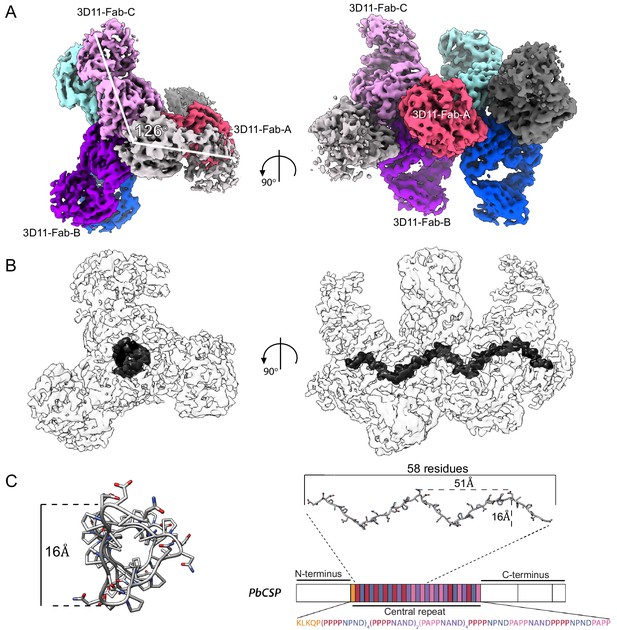
Spiral organization of the PbCSP repeat upon 3D11 Fab binding.
(A) The cryoEM map of the 3D11 Fab-PbCSP complex reveals high-resolution information for seven predominant 3D11 Fabs. Regions corresponding to Fabs are colored from pink to gray. (B) CryoEM map of the 3D11 Fab-PbCSP complex is shown as a transparent light gray surface with the PbCSP region highlighted in black. (C) The PbCSP model built into the cryoEM map is shown in dark gray as sticks and aligned to the schematic representation of the PbCSP protein sequence.
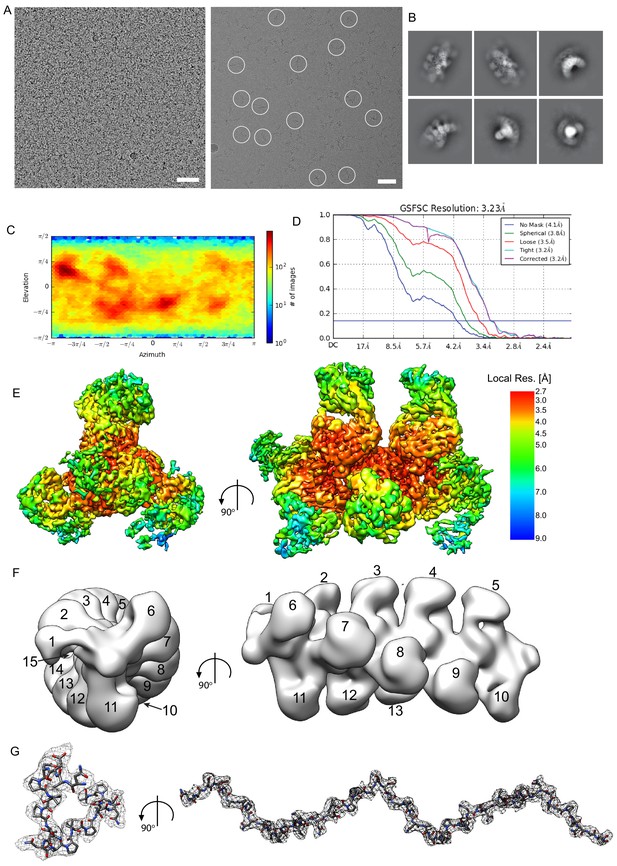
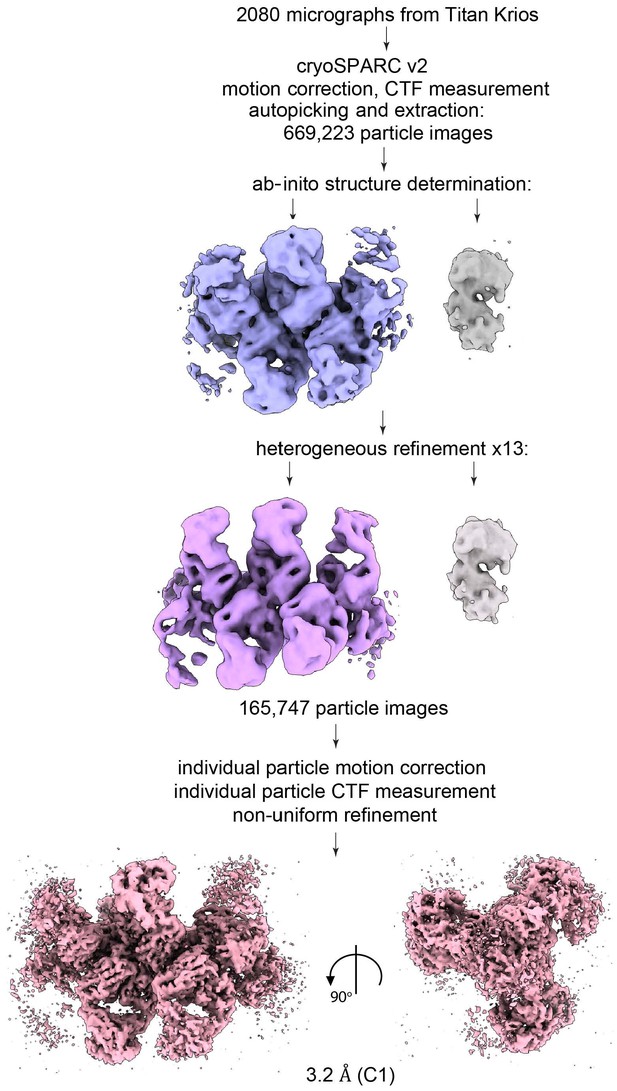
CryoEM analysis of the 3D11 Fab-PbCSP complex.
(A) Left panel – a representative cryoEM micrograph used to calculate the 3D11 Fab-PbCSP cryoEM map. Right panel – a cryoEM micrograph of the 3D11 Fab-PbCSP complex from a 200 kV screening microscope with individual particles highlighted with white circles. Scale bars: 50 nm. (B) Selected 2D class averages of the 3D11 Fab-PbCSP complex. (C) Particle orientation distribution plot. (D) Fourier shell correlation curve from the final 3D non-uniform refinement of the 3D11 Fab-PbCSP complex in cryoSPARC v2. (E) Local resolution (Å) plotted on the surface of the cryoEM map. (F) Low-pass filtered (20 Å) cryoEM map of the 3D11 Fab-PbCSP complex with observable 3D11 Fabs numbered. (G) CryoEM map of PbCSP (gray mesh) with the model shown as sticks (black carbons).
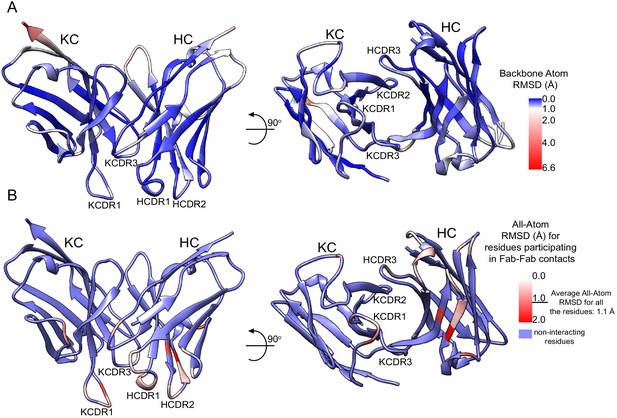
Comparison between the 3D11 Fab-PbCSP cryoEM structure and 3D11 Fab-NPND peptide crystal structure.
(A) Color representation of the backbone RMSD of the 3D11 Fab variable region and PbCSP core epitope between the 3D11 Fab-PbCSP cryoEM structure and the 3D11 Fab-NPND peptide crystal structure. (B) Color representation of the all-atom RMSD of the 3D11 Fab residues engaging in Fab-Fab contacts between the 3D11 Fab-PbCSP cryoEM structure and the 3D11 Fab-NPND peptide crystal structure. RMSD values were calculated using PyMOL (Schrodinger LLC, 2015) and plotted by color on the secondary structure of the 3D11 Fab-NPND peptide crystal structure.
3D Variability Analysis on 165,747 particle images of the 3D11 Fab-PbCSP complex.
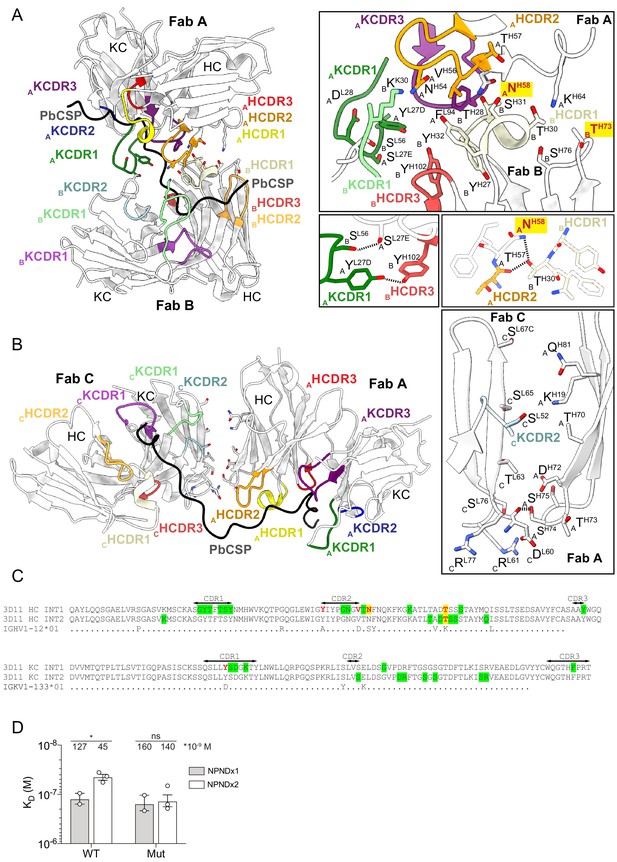
Homotypic interactions between 3D11 Fabs stabilize the 3D11 Fab-PbCSP complex.
(A and B) Close-up views of adjacent 3D11 Fabs from the cryoEM structure in complex with PbCSP (black). 3D11 Fabs bound to PbCSP form homotypic contacts with each adjacent Fab through two interfaces; one consisting of CDRs from the heavy and light chains of Fabs A and B (interface 1, A), and the second mediated by residues in FR3 of Fab A HC and FR3 of Fab C LC (interface 2, B). Variable domains of Fabs are shown in white. HCDR1, −2,–3, and KCDR1, −2 and −3 are colored yellow, orange, red, green, blue and purple, respectively. Residues forming Fab-Fab contacts are labeled with the position of the Fab in the cryoEM model (A, B or C) indicated in subscript. mAb 3D11 affinity-matured residues that engage in Fab-Fab contacts, but do not directly interact with PbCSP are highlighted in yellow with red font. Black dashed lines denote H-bonds. (C) Sequence alignment of mAb 3D11 with its inferred germline precursor. INT1 and INT2 refer to the two interfaces shown in (A) and (B). Green highlight: germline-encoded residues involved in homotypic interactions; Red: affinity-matured residues involved in homotypic interactions; Yellow highlight: affinity-matured residues involved in homotypic interactions that do not directly interact with PbCSP. (D) Binding affinity of WT 3D11 and H-58/73 germline-reverted mutant (Mut) Fabs to NPNDx1 (gray bars) and NPNDx2 (white bars) peptides as measured by ITC. Symbols represent independent measurements. Mean KD values resulting from at least two independent experiments are shown. Error bars represent standard error of the mean. An unpaired one-tailed t-test was performed using GraphPad Prism 8 to evaluate statistical significance: *p<0.05.
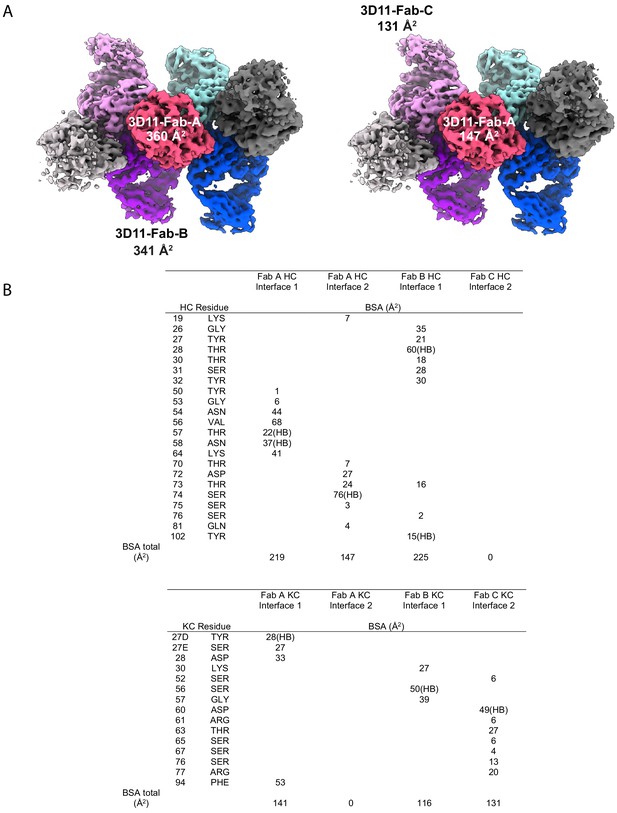
Homotypic contacts between 3D11 Fabs in the 3D11 Fab-PbCSP cryoEM structure.
(A) Main interaction interfaces between adjacent 3D11 Fabs in the 3D11 Fab-PbCSP cryoEM structure. (B) Table of contacts between 3D11 Fabs. HB: hydrogen bond (3.8 Å cut-off).
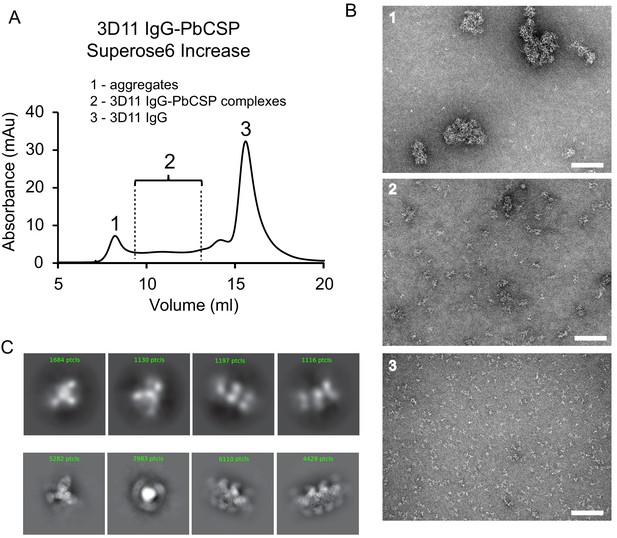
Negative-stain EM analysis of 3D11 IgG-PbCSP complexes.
(A) SEC chromatogram of 3D11 IgG-PbCSP complexes. (B) Representative negative-stain (NS) micrographs of particles present in selected SEC fractions: 1 – soluble aggregates, 2 - 3D11 IgG-PbCSP complexes, 3 – unbound 3D11 IgGs. Scale bars: 100 nm. (C) Comparison of NS 2D class averages of the 3D11 IgG-PbCSP complex (upper panels) to 2D class averages of the 3D11 Fab-PbCSP complex (lower panels).
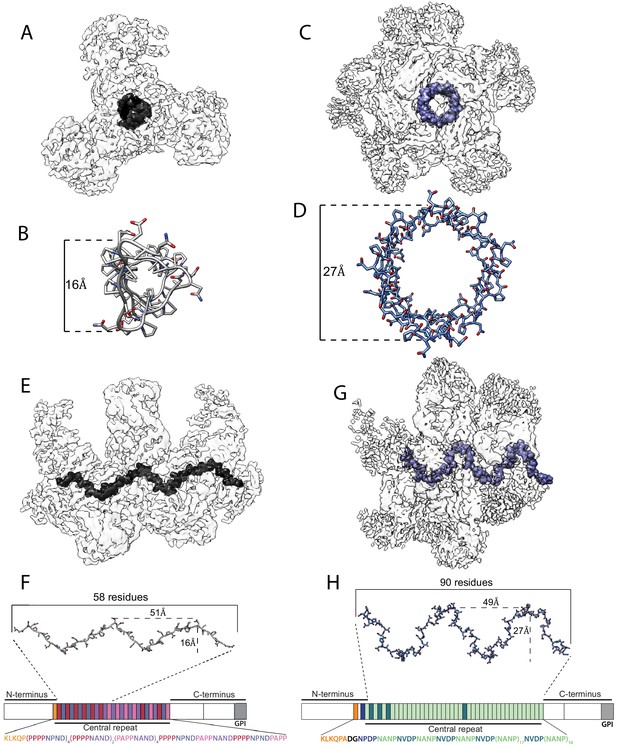
Comparison between cryoEM structures of 3D11 Fab-PbCSP and 311 Fab-PfCSP (PDB ID: 6MB3) (Oyen et al., 2018).
CryoEM maps of the 3D11 Fab-PbCSP (A and E) and 311 Fab-PfCSP (C and G) complexes are shown as a transparent light gray surface with the CSP density highlighted in black for PbCSP and in blue for PfCSP. The CSP models built into the cryoEM maps are shown as gray for PbCSP; (B and F) or blue for PfCSP; (D) and H) sticks and aligned to the schematic representations of their respective protein sequences.
Tables
X-ray crystallography data collection and refinement statistics.
Despite binding in nearly identical conformations, differences exist in the molecular details of 3D11 Fab binding to each peptide that provide key insights into mAb 3D11 recognition of PbCSP. Our crystal structures revealed that more van der Waals contacts were formed by a Pro residue in the PPPP and NPND motifs compared to an Ala at the same position in the PAPP and NAND motifs (Figure 3D). Consequently, the epitopes of the NAND, NPND and Mixed peptides had a slightly greater buried surface area (BSA; 753, 762, and 765 Å2, respectively) than the PAPP peptide (743 Å2), which only consists of Ala-containing motifs (Supplementary file 2). In particular, Pro10 of the PPPP motif found in the NAND and NPND peptides forms more van der Waals interactions with antibody residues H.Asn33 and H.Tyr52 compared to Ala10 of the PAPP motif present in PAPP and Mixed peptides. Similarly, Pro6 of the NPND motif in the NPND and Mixed peptides makes additional interactions with antibody residue K.Leu50 that are not present for Ala6 of the NAND motif within the PAPP and NAND peptides (Supplementary file 2). These differences in interactions observed at the atomic level directly relate to the binding affinities measured by ITC, where the PbCSP peptides that bury more surface area in the 3D11 paratope have the highest binding affinities (Figure 3A).
| 3D11-PAPP | 3D11-NAND | 3D11-NPND | 3D11-Mixed | |
|---|---|---|---|---|
| Beamline | APS-23-ID-D | APS-23-ID-D | NSLS-II-17-ID-1 | APS-23-ID-B |
| Wavelength (Å) | 1.033170 | 1.033200 | 0.979329 | 1.033167 |
| Space group | P3221 | P3221 | P3221 | P3221 |
| Cell dimensions | ||||
| a,b,c (Å) | 59.3, 59.3, 233.5 | 59.7, 59.7, 234.9 | 59.9, 59.9, 235.0 | 60.3, 60.3, 233.7 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 |
| Resolution (Å)* | 40.0–1.60 (1.70–1.60) | 40.0–1.55 (1.65–1.55) | 40.0–2.27 (2.37–2.27) | 40.0–1.55 (1.65–1.55) |
| No. molecules in ASU | 1 | 1 | 1 | 1 |
| No. observations | 1,210,903 (196,555) | 684,564 (117,091) | 450,057 (47,142) | 1,423,235 (247,601) |
| No. unique observations | 64,371 (10,497) | 70,664 (11,753) | 23,398 (2,556) | 72,981 (12,222) |
| Multiplicity | 18.8 (18.7) | 9.5 (9.7) | 19.1 (17.4) | 19.5 (20.3) |
| Rmerge (%)† | 10.3 (84.7) | 8.4 (80.1) | 13.8 (57.1) | 8.3 (78.0) |
| Rpim (%)‡ | 2.4 (20.1) | 2.9 (26.5) | 3.2 (13.5) | 1.9 (17.6) |
| <I/σ I> | 16.3 (1.5) | 13.8 (1.5) | 19.0 (4.1) | 19.6 (1.7) |
| CC½ | 99.9 (68.0) | 99.9 (56.7) | 99.9 (93.5) | 99.9 (84.3) |
| Completeness (%) | 99.9 (100.0) | 98.3 (97.2) | 99.3 (94.4) | 100.0 (100.0) |
| Refinement Statistics | ||||
| Reflections used in refinement | 64,275 | 70,660 | 23,327 | 72,843 |
| Reflections used for R-free | 1999 | 1986 | 1173 | 2000 |
| Non-hydrogen atoms | 3823 | 3915 | 3665 | 3858 |
| Macromolecule | 3411 | 3423 | 3382 | 3439 |
| Water | 384 | 380 | 259 | 359 |
| Heteroatom | 28 | 112 | 24 | 60 |
| Rwork§/Rfree¶ | 15.9/18.8 | 16.4/18.4 | 16.6/22.2 | 16.6/18.1 |
| Rms deviations from ideality | ||||
| Bond lengths (Å) | 0.016 | 0.010 | 0.006 | 0.011 |
| Bond angle (°) | 1.43 | 1.15 | 0.87 | 1.22 |
| Ramachandran plot | ||||
| Favored regions (%) | 98.9 | 98.0 | 97.7 | 98.2 |
| Allowed regions (%) | 1.1 | 2.0 | 2.3 | 1.8 |
| B-factors (Å2) | ||||
| Wilson B-value | 27.1 | 24.0 | 32.0 | 26.3 |
| Average B-factors | 35.0 | 31.4 | 35.2 | 31.2 |
| Average macromolecule | 33.6 | 29.4 | 34.8 | 29.7 |
| Average heteroatom | 54.4 | 54.8 | 54.4 | 57.6 |
| Average water molecule | 46.3 | 41.9 | 38.3 | 41.2 |
-
* Values in parentheses refer to the highest resolution bin.
† Rmerge = Σhkl Σi | Ihkl, i - < Ihkl > | / Σhkl < Ihkl > .
-
‡ Rpim = Σhkl [1/(N – 1)]1/2 Σi | Ihkl, i - < Ihkl > | / Σhkl < Ihkl > .
§ Rwork = (Σ | |Fo | − |Fc | |) / (Σ | |Fo |) - for all data except as indicated in footnote ¶.
-
¶ 5% of data were used for the Rfree calculation.
CryoEM data collection and refinement statistics.
| Data collection | |
|---|---|
| Electron microscope | Titan Krios G3 |
| Camera | Falcon 3EC |
| Voltage (kV) | 300 |
| Nominal magnification | 75,000 |
| Calibrated physical pixel size (Å) | 1.06 |
| Total exposure (e- /Å2) | 42.7 |
| Number of frames | 30 |
| Image processing | |
| Motion correction software | cryoSPARCv2 |
| CTF estimation software | cryoSPARCv2 |
| Particle selection software | cryoSPARCv2 |
| 3D map classification and refinement software | cryoSPARCv2 |
| Micrographs used | 2080 |
| Particles selected | 669,223 |
| Global resolution (Å) | 3.2 |
| Particles contributing to final map | 165,747 |
| Model building | |
| Modeling software | Coot, phenix.real_space_refine |
| Number of residues built | 3085 |
| RMS (bonds) | 0.002 |
| RMS (angles) | 0.56 |
| Ramachandran favored (%) | 95.8 |
| Rotamer outliers (%) | 0.5 |
| Clashscore | 6.27 |
| MolProbity score | 1.63 |
| EMRinger score | 2.54 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Recombinant DNA reagent | pcDNA3.4-3D11 Fab HC (plasmid) | This paper | N/A | 3D11 Fab heavy chain gene in pcDNA3.4 TOPO vector |
| Recombinant DNA reagent | pcDNA3.4-3D11 Fab 58/73 HC (plasmid) | This paper | N/A | 3D11 Fab germline-reverted mutant heavy chain gene in pcDNA3.4 TOPO vector |
| Recombinant DNA reagent | pcDNA3.4-3D11 Fab KC (plasmid) | This paper | N/A | 3D11 Fab light chain gene in pcDNA3.4 TOPO vector |
| Recombinant DNA reagent | pcDNA3.4-PbCSP-6xHis (plasmid) | This paper | N/A | PbCSP gene with His tag in pcDNA3.4 TOPO vector |
| Recombinant DNA reagent | pcDNA3.4-PbC-CSP-6xHis (plasmid) | This paper | N/A | PbC-CSP gene with His tag in pcDNA3.4 TOPO vector |
| Recombinant DNA reagent | pcDNA3.4-PbCSP-αTSR-6xHis (plasmid) | This paper | N/A | PbCSP αTSR gene with His tag in pcDNA3.4 TOPO vector |
| Cell line (Homo sapiens) | FreeStyle 293 F cells | Thermo Fisher Scientific | Cat# R79007 | |
| Cell line (Mus musculus) | 3D11 hybridoma cell line | Yoshida et al., 1980 | BEI Resources #MRA-100; RRID:AB_2650479 | |
| Chemical compound | GIBCO FreeStyle 293 Expression Medium | Thermo Fisher Scientific | Cat# 12338026 | |
| Chemical compound | GIBCO Hybridoma-SFM | Thermo Fisher Scientific | Cat# 12045076 | |
| Chemical compound | FectoPRO DNA Transfection Reagent | VWR | Cat# 10118–444 | |
| Chemical compound | Fetal bovine serum | Thermo Fisher Scientific | Cat# 12483–020 | |
| Antibody | 3D11 IgG (mouse monoclonal) | Yoshida et al., 1980 | N/A | Purified from 3D11 hybridoma cell line; See Materials and methods |
| Recombinant protein | 3D11 Fab | This paper | N/A | See Materials and methods for concentrations and masses used, and buffer conditions |
| Recombinant protein | 3D11 Fab H-58/73 | This paper | N/A | See Materials and methods for concentrations and masses used, and buffer conditions |
| Recombinant protein | PbCSP | This paper | N/A | See Materials and methods for concentrations and masses used, and buffer conditions |
| Peptide | PAPP (PAPPNANDPAPPNAND) | This paper | N/A | Derived from PbCSP repeat region |
| Peptide | NAND (PPPPNANDPPPPNAND) | This paper | N/A | Derived from PbCSP repeat region |
| Peptide | NPND (PPPPNPNDPPPPNPND) | This paper | N/A | Derived from PbCSP repeat region |
| Peptide | Mixed (PPPPNPNDPAPPNAND) | This paper | N/A | Derived from PbCSP repeat region |
| Peptide | NPNDx1 (PPPPNPNDPPPP) | This paper | N/A | Derived from PbCSP repeat region |
| Peptide | NPNDx2 (PPPPNPNDPPPPNPNDPPPPNPND) | This paper | N/A | Derived from PbCSP repeat region |
| Software, algorithm | GROMACS 5.1.4 | Abraham et al., 2015; Berendsen et al., 1995 | http://manual.gromacs.org/documentation/5.1.4/; RRID:SCR_014565 | |
| Software, algorithm | LINCS | Hess et al., 1997; Hess, 2008 | N/A | |
| Software, algorithm | Particle-Mesh Ewald algorithm | Darden et al., 1993; Essmann et al., 1995 | N/A | |
| Software, algorithm | Nosé-Hoover thermostat | Nosé, 1984; Hoover, 1985 | N/A | |
| Software, algorithm | Parrinello-Rahman algorithm | Parrinello and Rahman, 1981 | N/A | |
| Software, algorithm | VMD | Humphrey et al., 1996 | https://www.ks.uiuc.edu/Research/vmd/; RRID:SCR_001820 | |
| Software, algorithm | Matplotlib | Hunter, 2007 | https://matplotlib.org/; RRID:SCR_008624 | |
| Software, algorithm | Octet Data Analysis Software 9.0.0.6 | ForteBio | https://www.fortebio.com/products/octet-systems-software | |
| Software, algorithm | MicroCal ITC Origin 7.0 Analysis Software | Malvern | https://www.malvernpanalytical.com/ | |
| Software, algorithm | ASTRA | Wyatt | https://www.wyatt.com/products/software/astra.html; RRID:SCR_016255 | |
| Software, algorithm | GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/; RRID:SCR_002798 | |
| Software, algorithm | EPU | ThermoFisher Scientific | https://www.fei.com/software/ | |
| Software, algorithm | SBGrid | SBGrid Consortium | https://sbgrid.org/; RRID:SCR_003511 | |
| Software, algorithm | cryoSPARC v2 | Punjani et al., 2017 | https://cryosparc.com/ | |
| Software, algorithm | Phenix (phenix.refine; phenix.real_space_refine) | Adams et al., 2010 | https://www.phenix-online.org/; RRID:SCR_014224 | |
| Software, algorithm | UCSF Chimera | Pettersen et al., 2004 | https://www.cgl.ucsf.edu/chimera/; RRID:SCR_004097 | |
| Software, algorithm | UCSF ChimeraX | Goddard et al., 2018 | https://www.cgl.ucsf.edu/chimerax/; RRID:SCR_015872 | |
| Software, algorithm | Coot | Emsley et al., 2010 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/; RRID:SCR_014222 | |
| Software, algorithm | PyMOL | The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC. | https://pymol.org/2/#products; RRID:SCR_000305 | |
| Software, algorithm | PDBePISA | Krissinel and Henrick, 2007 | https://www.ebi.ac.uk/pdbe/pisa/; RRID:SCR_015749 | |
| Other | Homemade holey gold grids | Marr et al., 2014 | N/A | |
| Other | Homemade carbon grids | Booth et al., 2011 | N/A |
Additional files
-
Supplementary file 1
Hydrogen-bonding propensities from simulations of peptides in solution.
(A) Hydrogen-bonding propensity for each simulated motif and lifetime of each β-turn for the four PfCSP-derived peptides. (B) Hydrogen-bonding propensity for each simulated motif and lifetime of each β-turn for the four PbCSP-derived peptides.
- https://cdn.elifesciences.org/articles/59018/elife-59018-supp1-v1.docx
-
Supplementary file 2
Table of contacts between 3D11 Fab and PbCSP peptides.
Rows are shaded according to the number of times interactions are observed between all four crystal structures, summed in the final column.
- https://cdn.elifesciences.org/articles/59018/elife-59018-supp2-v1.docx
-
Supplementary file 3
Table of contacts between one of the 3D11 Fabs and PbCSP in the cryoEM.
- https://cdn.elifesciences.org/articles/59018/elife-59018-supp3-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/59018/elife-59018-transrepform-v1.pdf