RETRACTED: Alcohol drinking alters stress response to predator odor via BNST kappa opioid receptor signaling in male mice
Peer review process
This article was accepted for publication as part of eLife's original publishing model.
History
- Version of Record published
- Accepted Manuscript published
- Accepted
- Received
Decision letter
-
Matthew N HillReviewing Editor; University of Calgary, Canada
-
Kate M WassumSenior Editor; University of California, Los Angeles, United States
-
Nicholas GilpinReviewer
In the interests of transparency, eLife publishes the most substantive revision requests and the accompanying author responses.
[Editors’ note: the authors submitted for reconsideration following the decision after peer review. What follows is the decision letter after the first round of review.]
Thank you for submitting your work entitled "Alcohol Drinking Alters Stress Coping via Extended Amygdala Kappa Opioid Receptor Signaling in Male Mice" for consideration by eLife. Your article has been reviewed by three peer reviewers, and the evaluation has been overseen by a Reviewing Editor and a Senior Editor.
Our decision has been reached after consultation between the reviewers. Based on these discussions and the individual reviews below, we regret to inform you that your work will not be considered further for publication in eLife.
In general, the reviewers were all enthusiastic about your findings, but the consensus formed during discussion was that as is, much of the data is not mechanistically linked, which was felt to limit the ability to make causal associations and conclusions about the findings in the manuscript. The reviewers did feel that more studies could be done to formally link both the photometry and electrophysiology data with the behavior. But these were deemed to be beyond the scope of a revision at eLife, which is limited to two months. As we are enthusiastic about these findings, if you are able to address each of these concerns, we will consider your manuscript again as a new submission once these have been completed. Please see detailed comments from the reviewers below.
Reviewer #1:
This study aimed to test the role of BNST Pdyn-KOR signaling in stress coping (measured primarily via behavioral assessments during exposure to the predator odor TMT) following voluntary alcohol consumption in mice. The findings reported in this manuscript are interesting, but several issues need to be addressed. One major issue is overinterpretation of the data, as outlined in several points below, this study did not show that "altered responses to an innate stressor were associated with enhanced PFC-driven excitation of prodynorphin-containing neurons in the BNST" nor did it show that "KOR dysregulation of corticolimbic circuits underlies lasting behavioral changes to stressors that emerge after chronic drinking." This manuscript includes a set of interesting but disparate findings that provide separate pieces of evidence for stress and ethanol effects on BNST cellular activation, stress and ethanol effects on BNST KOR signaling, stress and ethanol effects on corticolimbic circuits, and stress/ethanol/KOR drug effects on stress related behavior. No experiments tested the relationship, causal or otherwise, between stress behaviors/stress response strategies and stress/ethanol effects on peptide signaling and physiology. Furthermore, the discussion of "maladaptive" and "impaired" stress coping is an overinterpretation that should either be removed or operationalized and incorporated into all analyses. The inclusion of data showing a causal relationship between stress effects on biology and behavior (or at least an association between these outcomes in the same animals) would greatly strengthen the manuscript.
1) The statement "Altered responses to an innate stressor were associated with enhanced PFC-driven excitation of prodynorphin-containing neurons in the BNST" and "KOR dysregulation of corticolimbic circuits underlies lasting behavioral changes to stressors that emerge after chronic drinking" are overinterpretations of the data. For example, it is not possible to know how the fiber photometry data in Figure 2 relates to behavior if at all. The same can be said for animals used for slice electrophysiology recordings. Why were TMT behavioral assessments not performed during fiber photometry recordings of BNST Pdyn neurons? This would provide information (at least correlative) regarding the role of BNST Pdyn signaling in these behaviors in alcohol- vs. water-exposed mice.
2) In general, terms like "maladaptive" stress coping are used too liberally. For example, "we tested whether BNST KOR/Pdyn signaling regulates maladaptive stress reactions after long-term alcohol drinking." Maladaptive how? Behavior was clearly affected by the independent variables, but there is no evidence that one response is more or less "adaptive." Also, what is meant by "negative" stress coping?
3) The use of no-injection controls instead of vehicle-injected controls is atypical and not ideal because it does not match groups for the stress of injections (Figure 1D-F). This concern is magnified in a study that examines stress coping. It is difficult to interpret drug effects in the absence of a vehicle-injected control group.
4) It is unclear when systemic or intra-BNST norBNI injections were given in relation to TMT behavioral testing. Comparing Figure 1A and 1G, it appears that systemic norBNI injections were given immediately prior to TMT behavioral testing and intra-BNST norBNI injections were given at the end of the intermittent alcohol exposure period (7-10 days prior to TMT behavioral testing). This should be clarified. If systemic and intra-BNST treatments occurred at different time points, the reason for this should be explained and its potential impact on results and interpretation should be discussed.
5) Include fiber photometry data from the 2nd TMT trial (TMT2) in addition to data from TMT1 and TMT3 (Figure 2I).
Reviewer #2:
The authors have conducted a series of elegant experiments in mice to examine the hypothesis that prodynorphin/kappa opioid receptor signalling in the BNST is responsible for impaired behavioral responses to acute stress during protracted withdrawal from chronic alcohol drinking.
Overall, I think the manuscript is interesting and has high translational value for the treatment of alcohol use disorders. Their behavioral results seem robust and the experiments are well-reasoned and mostly appropriately designed. The manuscript is well written, and the figures are informative. The methodology used is appropriate and is capable to investigate many levels of the phenomenon. That said, I have some concerns with the current form of the manuscript.
1) The main behavioral effect in EtOH-drinking mice – increased time spent closed to TMT containing cotton tip – is convincing and very robust across experiments. However, I have some concerns regarding the interpretation of these behavioral changes at this point, as there may be alternative explanations that should be addressed or ruled out. For example, olfactory deficits have been repeatedly described in patients with chronic alcohol abuse, and BNST plays a critical role in olfactory function. How would the authors rule that EtOH drinking mice approached TMT simply because their olfactory impairments instead of stress-coping deficits? There are more options to investigate this question, including i) carrying out similar tests with neutral odors, ii) investigating effects of different TMT concentrations, or iii) performing different stress-coping tests during the protracted withdrawal period. At least, authors should provide a more detailed analysis of behaviour during the TMT test, including distance moved, freezing and possibly types of exploratory behaviour (i.e. flat-back approach), preferentially also during the habituation period (during which an empty cotton tip holder was placed into to the home cage), in order to strengthen the claim that increased TMT-contact reflects impaired coping with stress.
2) There is some confusion in the interpretation of results which is reflected both in the title and the impact statement of the paper, as authors did not investigate to effects of “alcohol drinking” per se but the effects of alcohol drinking during protracted alcohol withdrawal which seem to be qualitatively different. This should be clearly stated and discussed, whereas title and significance statement should be formulated accordingly.
3) According to Figure 1B, alcohol intake as well as alcohol preference levels in EtOH drinking mice remained substantially lower than in mice in the cited paper (Hwa et al., 2011); in fact, mice of the current experiments did not prefer ethanol over water. What could be an explanation for this? Was the protocol of Hwa et al., 2011 followed and increasing amounts of ethanol were induced during the first week?
4) Are there any "classical" symptoms of ethanol withdrawal (e.g. hyperactivity or convulsions) at this later time point when authors carried out their measurements?
5) Does "burying" mean defensive burying during the test? Is the movement generally directed towards TMT? The effects of EtOH drinking and of Pdyn/KOR-manipulations on burying behavior varied across experiments. This – along with a more precise description of this type of behavior – should be clearly stated and discussed.
6) Although it is mentioned in Results section that behavior in the forced swim test was only affected during early, but not during protracted abstinence, the latter data are not shown. How would the authors interpret the different time-related changes between the two behavioral tests?
7) In fiber photometry experiments, calcium activity of BNSTPDYN neurons did not differ between EtOH and H2O drinking mice during the first session (when all other behavioral, immunohistochemical and electrophysiological measurements were carried out) but only after repeated exposure to TMT. It would be nice to see correlated behavioral changes over TMT trials which may help to interpret these results. Maybe I'm mistaken but is seems that Figure 3B and 3F, 3C and 3G, as well as 3D and 3H depict the very same animals in non-injected, TMT-exposed H2O and EtOH groups, even though these were described as subsequent, separate experiments. This is not elegant and should be clarified.
8) Figure 3 and Figure 3—figure supplement 1 show that the TMT-induced increase in BNSTPdyn sEPSC frequency did not seem to depend EtOH-drinking history, while KOR antagonism also seemed to induce very similar effects in H2O and EtOH drinking mice. Rather, sEPSC/IPSC ratio of non-Pdyn cells of EtOH drinking stressed animals seemed to differ from H2O drinking mice, suggesting that EtOH-related effects here were not directly regulated by the Pdyn system. Therefore, the last sentence in Results paragraph six and third sentence of the Discussion are over-interpreting these results and should be formulated much more carefully.
9) How was injection volume and targeting of CTB controlled for across mice in tracing studies shown in Figure 4G? Are cell counts normalized in some way to expression within the BNST? It would be good to see injections sites for this experiment as well.
10) There is no reference to Figure 4—figure supplement 1 F-H in the text. These figures suggest that – although functional connections from the mPFC to BNST may be strengthened in EtOH animals (but see concerns above about missing information on normalizing CTB counts and injection sites) – , activation of this projection upon TMT presentation does not depend on EtOH history (Figure 4—figure supplement 1H). Therefore, the first sentence in subsection “EtOH and stress interact revealing synaptic plasticity from cortical input” is misleading and should be re-formulated.
11) Out of curiosity. Ethanol consumption and preference during intermittent ethanol drinking period seemed to show a rather large variation among animals. I assume that such variation may correlate with behavioral/neuronal changes during withdrawal, i.e. heavy drinkers would show more profound stress-related impairments. Is there any effect of the amount of voluntary consumed alcohol on later outcome?
Reviewer #3:
In this study the authors characterize the effect of stress (TMT exposure) on activity of the prodynorphin/kappa opiate receptor (Pdyn/KOR) system in the BNST, in mice either following prolonged EtOH abstinence or control H2O mice. They first show that blocking BNST KORs with norBNI or genetic deletion of Pdyn in BNST normalizes the response to TMT in mice during protracted abstinence. They next showed that TMT exposure increases c-Fos expression in BNST Pdyn neurons, with greater expression in EtOH mice than H2O mice. Electrophysiological studies showed a TMT-induced non-specific increase in glutamatergic neurotransmission in the BNST, with little effect on inhibitory transmission; this effect was not sensitive to nor-BNI. Retrograde labelling indicated the mPFC likely contributed significantly to the TMT-induced increase in BNST glutamatergic transmission.
In general, this is an interesting manuscript with a novel emphasis on effects of stress after protracted abstinence. The writing is clear, but somewhat frustrating because much of the quantitative data is only found in the figure legends but not in the text in the Results section. Even then, the descriptions of the data in the figure legends appears somewhat cursory (perhaps due to relatively few figures with a tremendous number of individual panels).
1) There is one issue which remains unclear to me. It is stated "TMT increased expression of Pdyn GFP-expressing neurons in the BNST". Are the authors suggesting an increase in the number of Pdyn neurons in the BNST, an increase in Pdyn content in BNST, or both? Subsequently, it is stated that colocalization of c-Fos with Pdyn-neurons was greatest in the stressed-EtOH group. Is this due to increased c-Fos expression, increased Pdyn expression, or both?
[Editors’ note: further revisions were suggested prior to acceptance, as described below.]
Thank you for submitting your article "Alcohol Drinking Alters Stress Response to Predator Odor via BNST Kappa Opioid Receptor Signaling in Male Mice" for consideration by eLife. Your article has been reviewed by three peer reviewers, and the evaluation has been overseen by a Reviewing Editor and Kate Wassum as the Senior Editor.
The reviewers have discussed the reviews with one another and the Reviewing Editor has drafted this decision to help you prepare a revised submission.
We would like to draw your attention to changes in our revision policy that we have made in response to COVID-19 (https://elifesciences.org/articles/57162). Specifically, we are asking editors to accept without delay manuscripts, like yours, that they judge can stand as eLife papers without additional data, even if they feel that they would make the manuscript stronger. Thus the revisions requested below only address clarity and presentation.
Summary:
This manuscript reveals the prodynorphin-kappa opioid receptor system in the bed nucleus of the stria terminalis as a critical neural substrate underlying abnormal stress responses following heavy alcohol drinking. The experiments are elegant and the conclusion is supported at multiple levels (cellular, circuit, behavioral). The emphasis on the effect of stress after protracted abstinence from alcohol is novel. The data reported in this manuscript have high translational value for the treatment of alcohol use disorders.
All the reviewers were impressed by the revisions made on the manuscript and agreed that the new version had improved significantly. There are no further experiments that were viewed as necessary for this, but the reviewers did have a collection of points that require attention, please see them detailed below.
Essential revisions:
1) The rebuttal letter states that "we have added a saline-injected behavioral cohort (Figure 1G-I)." These new data strengthen the manuscript. But it is unclear if the new saline-injected controls were tested in parallel with a new norBNI-injected group, or if data from the new saline-injected group is being compared to previously collected data from the norBNI-injected group included in the first submission. If the latter, this should be stated as a caveat.
2) Elevated plus-maze tests: it is somewhat difficult to understand the presented findings, as H2O and EtOH mice with or without previous TMT exposure were not tested for anxiety in the EPM in the same experiment. If we accept that the lack of anxiogenic effect in EtOH groups in some experiments might originate from the prior exposure to TMT, how can these data be interpreted? TMT exposure is associated with maladaptive behavioral responses in EtOH drinkers, but the very same TMT exposure reduces/restores anxiety in a subsequent EPM test, a novelty-related stress situation? These discrepancies/limitations need to be clearly discussed.
3) What was the time interval between TMT and EPM tests across experiments? Were systemic and intra-BNST norBNI treatments repeated before EPM tests or how did the authors assume that KOR antagonism is still “working” several days later?
4) Systemic and intra-BNST administration of norBNI were administered at different timepoints i.e. 16 hrs vs. 7 days before TMT test. What was the reason for that? This is a large difference in protocols that should be discussed and evaluated more clearly.
5) The description of DREADD-experiments should be clarified at several points. It is not clear when animals received CNO treatments during the alcohol-drinking period. Was CNO administered only once (when?) to measure subsequent acute changes in EtOH consumption or was it administered repeatedly (how?) during intermittent EtOH drinking? Were the same animals tested in the TMT and EPM tests? What was the lag time between tests (and CNO treatments)?
6) The intersectional strategy for targeting Gi-DREADDs to the mPFC-BNST circuit coupled with systemic CNO injection may have off-target effects on collaterals of those mPFC that project to other brain regions – this should be acknowledged as a caveat.
7) Results and Discussion should be double checked as there are still several inaccuracies in data presentation and discussion that result in overinterpretations. E.g. in Discussion, authors claim that "Experiments with ex vivo optogenetics indicated that EtOH-drinking stressed mice had increased prefrontal cortical synaptic connectivity onto BNSTPDYN cells compared to stressed H2O drinkers and unstressed EtOH drinkers." This is not true, no parameter differed significantly between stressed EtOH and H2O drinkers; stressed EtOH drinkers only differed from non-stressed groups. Interpretation of such results should be formulated more carefully.
8) Comments on figures:
– Figure 5: Figure legends for panels E and F are missing.
– Figure 1—figure supplement 2 should be renamed to Figure 2—figure supplement 1.
– Figure 7K should denote the main effect of virus on TMT contact time, as reported in the text of the Results section.
https://doi.org/10.7554/eLife.59709.sa1Author response
[Editors’ note: the authors resubmitted a revised version of the paper for consideration. What follows is the authors’ response to the first round of review.]
Reviewer #1:
This study aimed to test the role of BNST Pdyn-KOR signaling in stress coping (measured primarily via behavioral assessments during exposure to the predator odor TMT) following voluntary alcohol consumption in mice. The findings reported in this manuscript are interesting, but several issues need to be addressed. One major issue is overinterpretation of the data, as outlined in several points below, this study did not show that "altered responses to an innate stressor were associated with enhanced PFC-driven excitation of prodynorphin-containing neurons in the BNST" nor did it show that "KOR dysregulation of corticolimbic circuits underlies lasting behavioral changes to stressors that emerge after chronic drinking." This manuscript includes a set of interesting but disparate findings that provide separate pieces of evidence for stress and ethanol effects on BNST cellular activation, stress and ethanol effects on BNST KOR signaling, stress and ethanol effects on corticolimbic circuits, and stress/ethanol/KOR drug effects on stress related behavior. No experiments tested the relationship, causal or otherwise, between stress behaviors/stress response strategies and stress/ethanol effects on peptide signaling and physiology. Furthermore, the discussion of "maladaptive" and "impaired" stress coping is an overinterpretation that should either be removed or operationalized and incorporated into all analyses. The inclusion of data showing a causal relationship between stress effects on biology and behavior (or at least an association between these outcomes in the same animals) would greatly strengthen the manuscript.
We appreciate the reviewer’s comments; however, we respectfully disagree with the characterization that there was no experiment that tested the relationship of BNST Pdyn/KOR signaling to behavior. We locally infused a KOR antagonist and used a genetic approach to knockout dynorphin in the BNST, both of which produced alterations in this behavior after alcohol exposure, supporting an interaction. However, we do agree that we should have done more work examining the physiological properties of BNST neurons and how they related to observed behaviors.
We have included the correlation between alcohol drinking history and BNST Pdyn synaptic transmission as Figure 4—figure supplement 1C-D in the revised manuscript. Regarding the overinterpretation of stress behavior, we agree, so we have toned down our discussion of coping in favor for more operationalized descriptions throughout the manuscript.
1) The statement "Altered responses to an innate stressor were associated with enhanced PFC-driven excitation of prodynorphin-containing neurons in the BNST" and "KOR dysregulation of corticolimbic circuits underlies lasting behavioral changes to stressors that emerge after chronic drinking" are overinterpretations of the data.
Again, the first point raised, we have experimentally examined and can provide correlations between the parameters measured and behavioral responses exploring this relationship in Figure 4—figure supplement 1C-D. In addition, we have newly added a pathway-specific DREADD inactivation approach of PFC to BNST to explore alcohol-induced disruption of TMT-driven behavioral responses (Figure 7). These were specific to affecting TMT behavior, but not alcohol drinking (Figure 7E-H) or anxiety-like behavior in the elevated plus maze (Figure 7N-O).
For example, it is not possible to know how the fiber photometry data in Figure 2 relates to behavior if at all.
The reviewer raises a good point. In light of the comments on photometry, we have removed this data set from the manuscript, as it is simply too preliminary.
The same can be said for animals used for slice electrophysiology recordings.
We agree, and have provided a correlation between drinking behavior and electrophysiology in Figure 4—figure supplement 1C-D.
Why were TMT behavioral assessments not performed during fiber photometry recordings of BNST Pdyn neurons? This would provide information (at least correlative) regarding the role of BNST Pdyn signaling in these behaviors in alcohol- vs. water-exposed mice.
We agree that this was not explored sufficiently. In light of other concerns about this experiment, we have removed the photometry from the manuscript.
2) In general, terms like "maladaptive" stress coping are used too liberally. For example, "we tested whether BNST KOR/Pdyn signaling regulates maladaptive stress reactions after long-term alcohol drinking." Maladaptive how? Behavior was clearly affected by the independent variables, but there is no evidence that one response is more or less "adaptive." Also, what is meant by "negative" stress coping?
In our interpretation, a lack of avoidance of predator odor is maladaptive in the sense it is more likely to result in the wild to lead to an interaction with a predator, which could lead to death. However, in light of the reviewer’s concerns, we can speak to the measured variables and limit our interpretation to a small section of discussion.
3) The use of no-injection controls instead of vehicle-injected controls is atypical and not ideal because it does not match groups for the stress of injections (Figure 1D-F). This concern is magnified in a study that examines stress coping. It is difficult to interpret drug effects in the absence of a vehicle-injected control group.
Initially we thought that the period of time between injection and behavior, 16 hours, would limit this potential confound. In response to this concern, we have added a saline-injected behavioral cohort (Figure 1G-I) and saline-injected slice physiology cohort (Figure 4E-H), and our interpretations of the data have not changed.
4) It is unclear when systemic or intra-BNST norBNI injections were given in relation to TMT behavioral testing. Comparing Figure 1A and 1G, it appears that systemic norBNI injections were given immediately prior to TMT behavioral testing and intra-BNST norBNI injections were given at the end of the intermittent alcohol exposure period (7-10 days prior to TMT behavioral testing). This should be clarified. If systemic and intra-BNST treatments occurred at different time points, the reason for this should be explained and its potential impact on results and interpretation should be discussed.
We apologize and have clarified these details in the Materials and methods section.
5) Include fiber photometry data from the 2nd TMT trial (TMT2) in addition to data from TMT1 and TMT3 (Figure 2I).
As suggested above, we have removed the photometry data.
Reviewer #2:
[…]
1) The main behavioral effect in EtOH-drinking mice – increased time spent closed to TMT containing cotton tip – is convincing and very robust across experiments. However, I have some concerns regarding the interpretation of these behavioral changes at this point, as there may be alternative explanations that should be addressed or ruled out. For example, olfactory deficits have been repeatedly described in patients with chronic alcohol abuse, and BNST plays a critical role in olfactory function. How would the authors rule that EtOH drinking mice approached TMT simply because their olfactory impairments instead of stress-coping deficits? There are more options to investigate this question, including i) carrying out similar tests with neutral odors, ii) investigating effects of different TMT concentrations, or iii) performing different stress-coping tests during the protracted withdrawal period. At least, authors should provide a more detailed analysis of behaviour during the TMT test, including distance moved, freezing and possibly types of exploratory behaviour (i.e. flat-back approach), preferentially also during the habituation period (during which an empty cotton tip holder was placed into to the home cage), in order to strengthen the claim that increased TMT-contact reflects impaired coping with stress.
This is an interesting point, and we thank the reviewer for the suggestion. In part, we felt that the reversal of the phenotypes by BNST-specific manipulations ruled out olfactory deficits, but we now examined this using two additional approaches. First, we examined the TMT-induced corticosterone response, and found that TMT elicited increases in both alcohol and control mice, suggesting the alcohol mice can still smell the odor (Figure 1E). Second, we then tested how animals interacted with peanut oil, an odorant that elicits approach behavior (Figure 1—figure supplement 2A-C). We found no difference in approach to peanut oil between the alcohol and water groups.
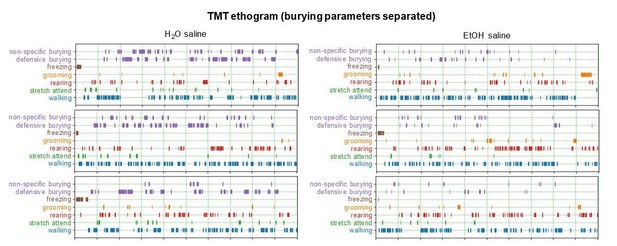
Regarding a more rigorous examination of behavior during the TMT test, we now provide ethograms/Gantt plots of mouse TMT responses after alcohol, water, alcohol + norBNI, and water + norBNI (Figure 1—figure supplement 1). On the reviewer’s request, we included observations of burying, freezing, grooming, rearing, stretch-attend (similar to the suggested flat-back approach), and walking. As the mice generally spend most time burying compared to the other behaviors, we decided to forego formally quantifying the minimal time spent freezing, grooming, etc. We now include distance traveled in our measures related to drug/viral manipulations, as well.
2) There is some confusion in the interpretation of results which is reflected both in the title and the impact statement of the paper, as authors did not investigate to effects of “alcohol drinking” per se but the effects of alcohol drinking during protracted alcohol withdrawal which seem to be qualitatively different. This should be clearly stated and discussed, whereas title and significance statement should be formulated accordingly.
Thank you for the comment, we have clarified this in the revised manuscript.
3) According to Figure 1B, alcohol intake as well as alcohol preference levels in EtOH drinking mice remained substantially lower than in mice in the cited paper (Hwa et al., 2011); in fact, mice of the current experiments did not prefer ethanol over water. What could be an explanation for this? Was the protocol of Hwa et al., 2011 followed and increasing amounts of ethanol were induced during the first week?
There are multiple environmental differences from the original paper including diet, cage types, sipper tubes, bedding, enrichment, etc. We did not ramp up the mice, just started at 20% ethanol. While it is plausible that this drives differences, others have published the escalation over time without the increasing of ethanol concentrations. We have now added blood ethanol concentrations in Figure 1D to show these mice drink to >80 mg/dl intoxication. As reviewer 1 also commented, we have included a brief discussion on this in the revised manuscript, subsection “BNST KOR/Pdyn gates stress reactions after EtOH”.
4) Are there any "classical" symptoms of ethanol withdrawal (e.g. hyperactivity or convulsions) at this later time point when authors carried out their measurements?
We did not overtly measure any classic symptoms of withdrawal. We have added a subtle difference in anxiety-like behavior at this protracted time point; however, after TMT exposure, the group difference dissipates. We have included distance traveled and elevated plus maze behavior and as additional measures in the revision.
5) Does "burying" mean defensive burying during the test? Is the movement generally directed towards TMT? The effects of EtOH drinking and of Pdyn/KOR-manipulations on burying behavior varied across experiments. This – along with a more precise description of this type of behavior – should be clearly stated and discussed.
We have purposefully kept “burying” as including both defensive burying, movement directed towards the TMT, and non-specific burying around the home cage. In Author response image 1, we show a detailed analysis of the burying behavior, split into the two directional categories.
6) Although it is mentioned in Results section that behavior in the forced swim test was only affected during early, but not during protracted abstinence, the latter data are not shown. How would the authors interpret the different time-related changes between the two behavioral tests?
This is a good question. However, we have decided to remove the early withdrawal data, as it was removed from the central question we asked.
7) In fiber photometry experiments, calcium activity of BNSTPDYN neurons did not differ between EtOH and H2O drinking mice during the first session (when all other behavioral, immunohistochemical and electrophysiological measurements were carried out) but only after repeated exposure to TMT. It would be nice to see correlated behavioral changes over TMT trials which may help to interpret these results. Maybe I'm mistaken but is seems that Figure 3B and 3F, 3C and 3G, as well as 3D and 3H depict the very same animals in non-injected, TMT-exposed H2O and EtOH groups, even though these were described as subsequent, separate experiments. This is not elegant and should be clarified.
In light of the photometry data being somewhat preliminary, we have removed it from the manuscript.
8) Figure 3 and Figure 3—figure supplement 1 show that the TMT-induced increase in BNSTPdyn sEPSC frequency did not seem to depend EtOH-drinking history, while KOR antagonism also seemed to induce very similar effects in H2O and EtOH drinking mice. Rather, sEPSC/IPSC ratio of non-Pdyn cells of EtOH drinking stressed animals seemed to differ from H2O drinking mice, suggesting that EtOH-related effects here were not directly regulated by the Pdyn system. Therefore, the last sentence in Results paragraph six and third sentence of the Discussion are over-interpreting these results and should be formulated much more carefully.
We appreciate the reviewers comment and will adjust our verbiage. We have also eliminated the non-Pdyn cells, as these data are not helpful.
9) How was injection volume and targeting of CTB controlled for across mice in tracing studies shown in Figure 4G? Are cell counts normalized in some way to expression within the BNST? It would be good to see injections sites for this experiment as well.
While injection volume was consistent across mice in tracing studies, we did not normalize to expression within the BNST. This remains a limitation, so we have removed this data set from the revision.
10) There is no reference to Figure 4—figure supplement 1 F-H in the text. These figures suggest that – although functional connections from the mPFC to BNST may be strengthened in EtOH animals (but see concerns above about missing information on normalizing CTB counts and injection sites) – , activation of this projection upon TMT presentation does not depend on EtOH history (Figure 4—figure supplement 1H). Therefore, the first sentence in subsection “EtOH and stress interact revealing synaptic plasticity from cortical input” is misleading and should be re-formulated.
We have adjusted our verbiage here, thank you.
11) Out of curiosity. Ethanol consumption and preference during intermittent ethanol drinking period seemed to show a rather large variation among animals. I assume that such variation may correlate with behavioral/neuronal changes during withdrawal, i.e. heavy drinkers would show more profound stress-related impairments. Is there any effect of the amount of voluntary consumed alcohol on later outcome?
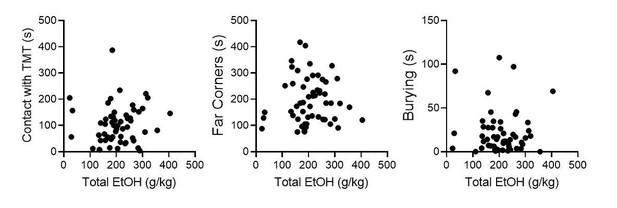
We agree, this is an interesting question. We have conducted a series of correlations including all mice used for TMT behavioral testing, including Pdyn-GFP mice used in slice physiology, saline-injected controls, GFP-injected controls, PBS-injected controls. There does not appear to be a robust correlation between total alcohol consumption and TMT behavior.
Reviewer #3:
[…]
In general, this is an interesting manuscript with a novel emphasis on effects of stress after protracted abstinence. The writing is clear, but somewhat frustrating because much of the quantitative data is only found in the figure legends but not in the text in the Results section. Even then, the descriptions of the data in the figure legends appears somewhat cursory (perhaps due to relatively few figures with a tremendous number of individual panels).
We thank the reviewer for his/her positive comments. In the revised manuscript, we now include descriptions of the data in the body of the text instead of the figure legends.
1) There is one issue which remains unclear to me. It is stated "TMT increased expression of Pdyn GFP-expressing neurons in the BNST". Are the authors suggesting an increase in the number of Pdyn neurons in the BNST, an increase in Pdyn content in BNST, or both? Subsequently, it is stated that colocalization of c-Fos with Pdyn-neurons was greatest in the stressed-EtOH group. Is this due to increased c-Fos expression, increased Pdyn expression, or both?
This is an interesting question, so we quantified Pdyn and Oprk1 expression after alcohol and TMT exposure using fluorescent in situ hybridization, shown in Figure 2F-J. There was greater Pdyn expression in the EtOH + TMT group compared to H2O+TMT group, so it appears there is an interaction of greater c-Fos activity in a greater number of Pdyn-expressing cells in the dBNST.
[Editors’ note: what follows is the authors’ response to the second round of review.]
Essential revisions:
1) The rebuttal letter states that "we have added a saline-injected behavioral cohort (Figure 1G-I)." These new data strengthen the manuscript. But it is unclear if the new saline-injected controls were tested in parallel with a new norBNI-injected group, or if data from the new saline-injected group is being compared to previously collected data from the norBNI-injected group included in the first submission. If the latter, this should be stated as a caveat.
We thank the reviewers for bringing up this point regarding whether additional norBNI mice were tested at the same time as the newer saline-injected controls. Yes, saline-injected controls were added along with additional nor-BNI injected experimental mice for consistency.
2) Elevated plus-maze tests: it is somewhat difficult to understand the presented findings, as H2O and EtOH mice with or without previous TMT exposure were not tested for anxiety in the EPM in the same experiment. If we accept that the lack of anxiogenic effect in EtOH groups in some experiments might originate from the prior exposure to TMT, how can these data be interpreted? TMT exposure is associated with maladaptive behavioral responses in EtOH drinkers, but the very same TMT exposure reduces/restores anxiety in a subsequent EPM test, a novelty-related stress situation? These discrepancies/limitations need to be clearly discussed.
We agree with the reviewers that the results using the elevated plus maze (EPM) are difficult to interpret. While we have not tested it statistically, it appears that TMT changed baseline values for control group, occluding a difference following alcohol. We have added the following text to more directly address this difference: “While EtOH mice also showed increased anxiety-like behavior in the elevated plus maze during protracted withdrawal, this group difference was eliminated following TMT exposure, as seen in our control drug/virus experiments after BNST norBNI, Pdyn deletion, and mPFC-BNST inhibition, suggesting long-lasting impact of TMT on performance in the elevated plus maze. […] This points to the elevated plus maze as a distinct, novelty-related probe, which may not be robust to differentiate EtOH-related phenotypes after a confounding TMT exposure.”
3) What was the time interval between TMT and EPM tests across experiments? Were systemic and intra-BNST norBNI treatments repeated before EPM tests or how did the authors assume that KOR antagonism is still “working” several days later?
We apologize for the confusion. The time interval between TMT and EPM tests was three days, which is now clarified in the Materials and methods. Systemic and intra-BNST norBNI administration were a single injection and were not repeated before EPM tests for fear of compounding dose concentrations. We have added the following text, “norBNI is known for its ultra-long duration of action (Munro et al., 2012). […] Therefore, it is likely that norBNI was on board at the time of TMT testing.”
4) Systemic and intra-BNST administration of norBNI were administered at different timepoints i.e. 16 hrs vs. 7 days before TMT test. What was the reason for that? This is a large difference in protocols that should be discussed and evaluated more clearly.
We apologize for the lack of information, so we have added these points to the drug methods, “Intra-BNST administration of norBNI was a single, bilateral infusion of drug. Since animals underwent stereotaxic surgery for drug delivery, 7 days was the minimum time for post-operative recovery before TMT behavioral testing. […] Therefore, it is likely that norBNI was on board at the time of TMT testing.”
5) The description of DREADD-experiments should be clarified at several points. It is not clear when animals received CNO treatments during the alcohol-drinking period. Was CNO administered only once (when?) to measure subsequent acute changes in EtOH consumption or was it administered repeatedly (how?) during intermittent EtOH drinking? Were the same animals tested in the TMT and EPM tests? What was the lag time between tests (and CNO treatments)?
We thank the reviewers for allowing us to further describe the DREADD experimental methods. “To test DREADD-mediated inhibition on EtOH consumption, saline and CNO were administered 20 min before EtOH drinking on two final test days. EtOH and H2O fluid consumption were measured after 1, 4, and 24 hr. During protracted withdrawal 7 days later, CNO was administered 20 min before the TMT test, and again 20 min before testing in the EPM, which occurred after 3 days.”
6) The intersectional strategy for targeting Gi-DREADDs to the mPFC-BNST circuit coupled with systemic CNO injection may have off-target effects on collaterals of those mPFC that project to other brain regions – this should be acknowledged as a caveat.
We thank the reviewers for this important suggestion. Text has been added acknowledging this caveat in the Discussion: “It is also possible that the systemic CNO injection may impact behavior via effects on collaterals of those PFC-BNST that project to other brain regions. This is a caveat, and intra-BNST delivery of CNO would be more direct.”
7) Results and Discussion should be double checked as there are still several inaccuracies in data presentation and discussion that result in overinterpretations. e.g. in Discussion, authors claim that "Experiments with ex vivo optogenetics indicated that EtOH-drinking stressed mice had increased prefrontal cortical synaptic connectivity onto BNSTPDYN cells compared to stressed H2O drinkers and unstressed EtOH drinkers." This is not true, no parameter differed significantly between stressed EtOH and H2O drinkers; stressed EtOH drinkers only differed from non-stressed groups. Interpretation of such results should be formulated more carefully.
We apologize for the overinterpretations of the results. We have edited this sentence in the Discussion to state, “experiments with ex vivo optogenetics indicated that EtOH-drinking stressed mice had increased prefrontal cortical synaptic connectivity onto BNST PDYN cells compared to unstressed EtOH drinkers.”
8) Comments on figures:
– Figure 5: Figure legends for panels E and F are missing.
We apologize for this oversight, the figure legend for 5E and 5F are: E. Average EtOH Preference ratio / 24 hours per group across time. F. Average EtOH Preference per mouse across the 6 weeks.
– Figure 1—figure supplement 2 should be renamed to Figure 2—figure supplement 1.
While we acknowledge that Figure 2 does not have a supplementary figure, we believe that Figure 1—figure supplement 2 conceptually aligns closer to the behavioral characterization of Figure 1 instead of the BNST c-Fos and in situ data shown in Figure 2.
– Figure 7K should denote the main effect of virus on TMT contact time, as reported in the text of the Results section.
We now have denoted the main effect of virus on TMT contact with a bracket between the groups in the figure key.
https://doi.org/10.7554/eLife.59709.sa2