Soluble collectin-12 mediates C3-independent docking of properdin that activates the alternative pathway of complement
Figures
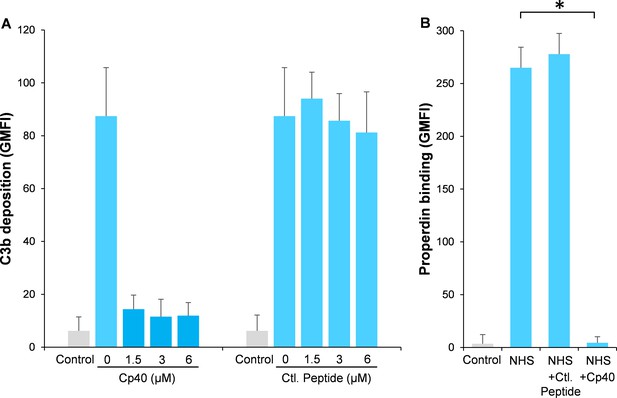
Inhibition of complement activation by compstatin analog Cp40.
NHS (20%) was preincubated with compstatin analog Cp40 or its control peptide (A: 0 ~ 6 µM; B: 6 µM) prior to inducing complement activation on A. fumigatus through incubation in the presence of Ca2+. C3b deposition (A) or properdin binding (B) was analyzed and expressed as the geometric mean fluorescence intensity (GMFI) by flow cytometry. Data are expressed as mean ± S.E.M from three independent experiments. Results are representative of at least six independent experiments. *p<0.01.
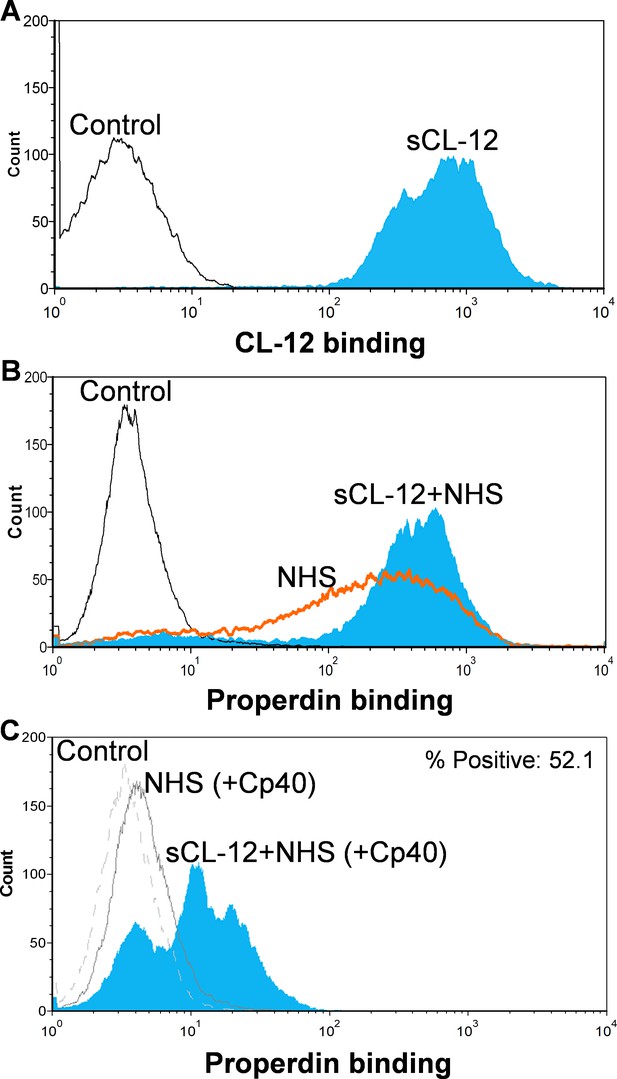
Binding of properdin to A.fumigatus in the presence of soluble CL-12 and compstatin analog Cp40.
A. fumigatus were incubated with or without soluble CL-12 (sCL-12) (5 µg/ml) prior to addition of NHS (20%) under EGTA-Mg2+ buffer. In some experiments, NHS (20%) was preincubated with compstatin analog Cp40 (6 µM) prior to induce complement activation. CL-12 (A) or properdin (B, C) binding was analyzed by flow cytometry. Results are representative of at least six independent experiments.
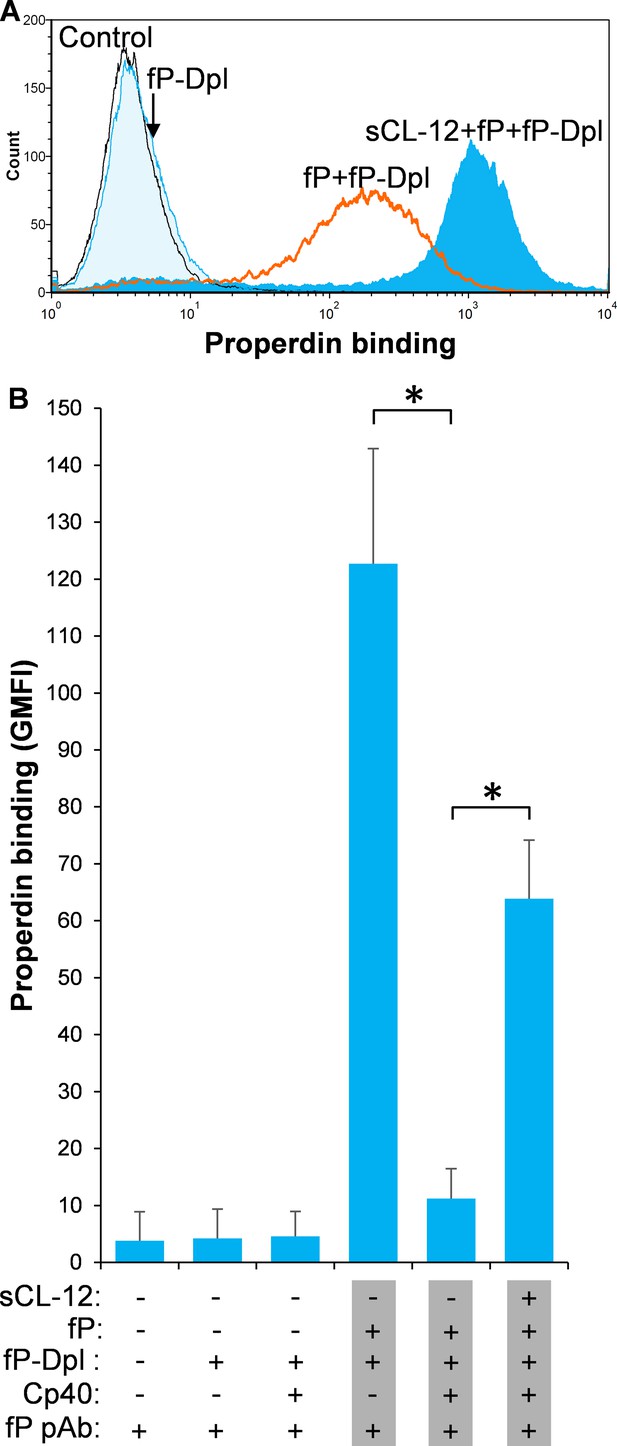
Soluble CL-12-dependent properdin binding on A.fumigatus.
A. fumigatus were incubated with or without sCL-12 (5 µg/ml) prior to addition of properdin-depleted serum (fP-Dpl) (10%) reconstituted with serum properdin (10 µg/ml) (A). In some experiments, the serum was preincubated with or without compstatin analog Cp40 (6 µM) under EGTA-Mg2+ buffer (B). Properdin binding was analyzed by flow cytometry. The GMFI was used to assess protein binding. B is expressed as mean ± S.E.M from three independent experiments. Results are representative of at least six independent experiments. *p<0.01.
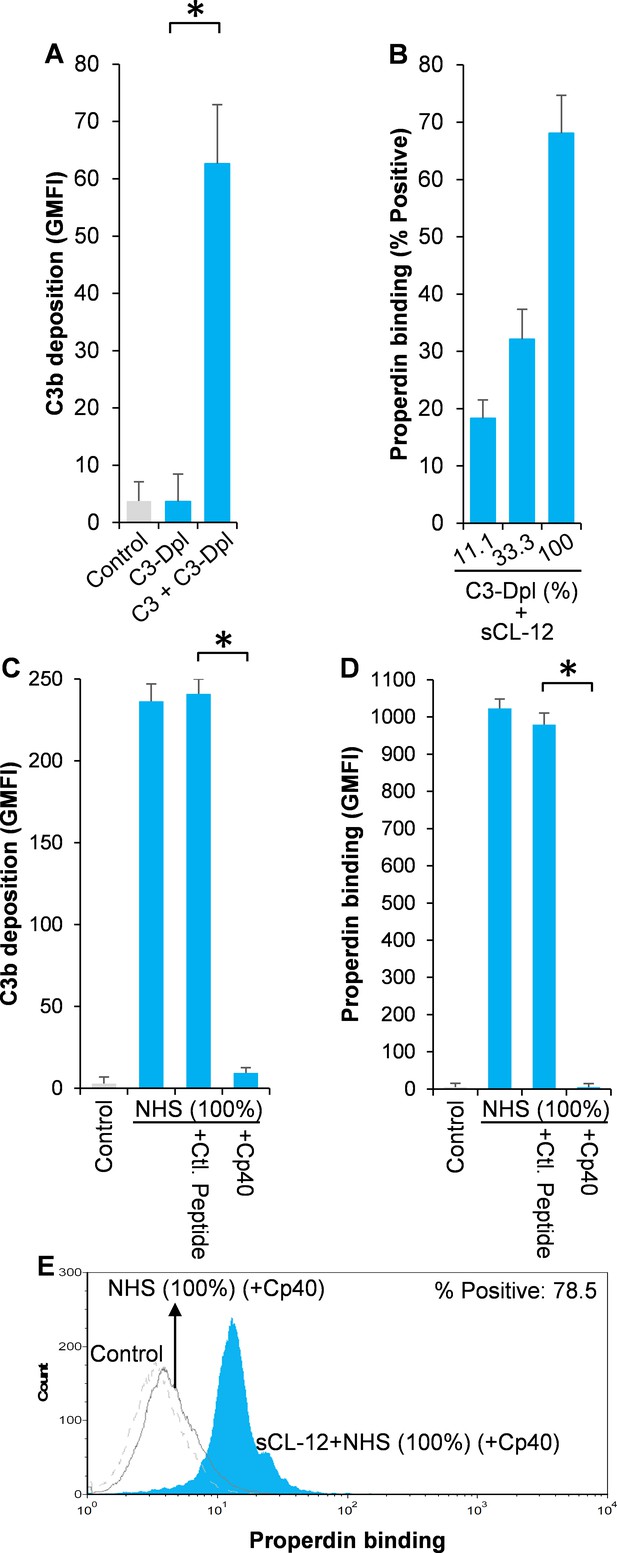
Effect of soluble CL-12 on properdin binding in C3-absent serum.
A. fumigatus was incubated with C3-depleted serum (C3-Dpl) (10%) reconstituted with exogeneous C3 (130 µg/ml) (A). In some experiments, A. fumigatus was incubated with or without sCL-12 (5 µg/ml) prior to addition of C3-Dpl (11.1 ~ 100%) (B). Inhibitory effect of compstatin analog Cp40 (20 µM) was determined on NHS (100%)-induced complement activation as described above. C3b deposition (A, C) or properdin binding (B, D, E) was analyzed and expressed as the GMFI or % positive events with respect to the C3-Dpl alone by flow cytometry. Results are representative of at least six independent experiments. *p<0.01.
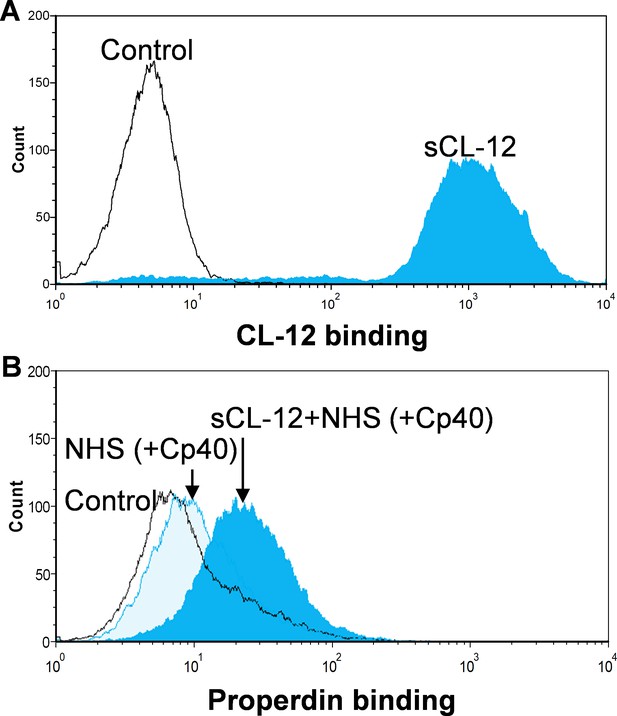
Binding of properdin to A.niger in the presence of soluble CL-12 and compstatin analog Cp40.
Soluble CL-12 (sCL-12) (A) or properdin (B) binding was analyzed on A. niger by flow cytometry as described for A. fumigatus. Results are representative of at least six independent experiments.
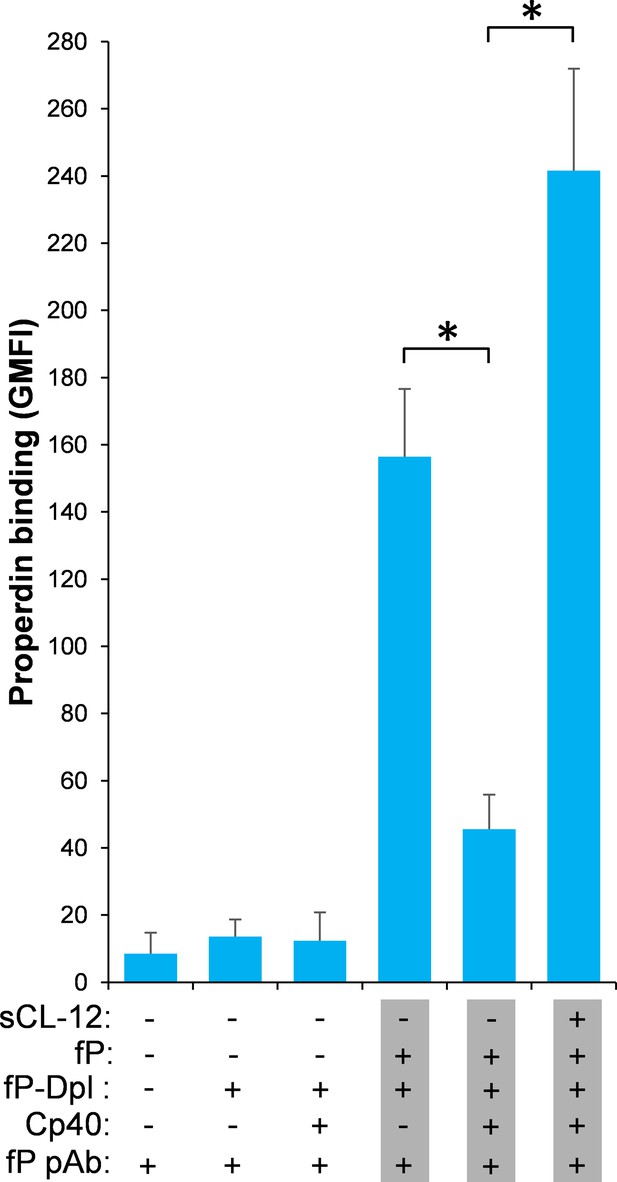
Soluble CL-12-dependent properdin binding on A.niger.
Properdin binding was analyzed with sCL-12 and properdin-depleted serum (fP-Dpl) restored with serum properdin on A. niger as described for A. fumigatus. The GMFI was used to assess protein binding. Results are expressed as mean ± S.E.M from three independent experiments. Results are representative of at least six independent experiments. *p<0.01.
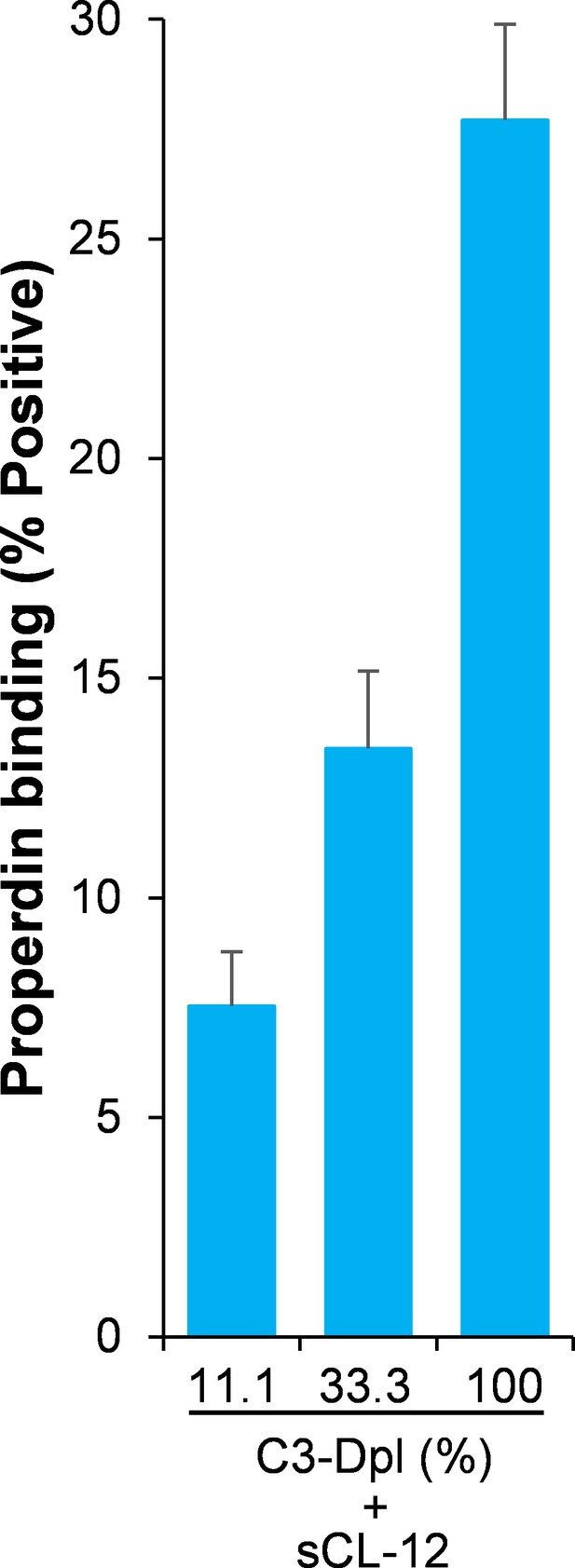
Effect of soluble CL-12 on properdin binding in C3-absent serum.
Properdin binding was analyzed with sCL-12 and C3-depleted serum (C3-Dpl) on A. niger as described for A. fumigatus. % positive events with respect to the C3-Dpl alone was used to assess protein binding. Results are representative of at least six independent experiments.
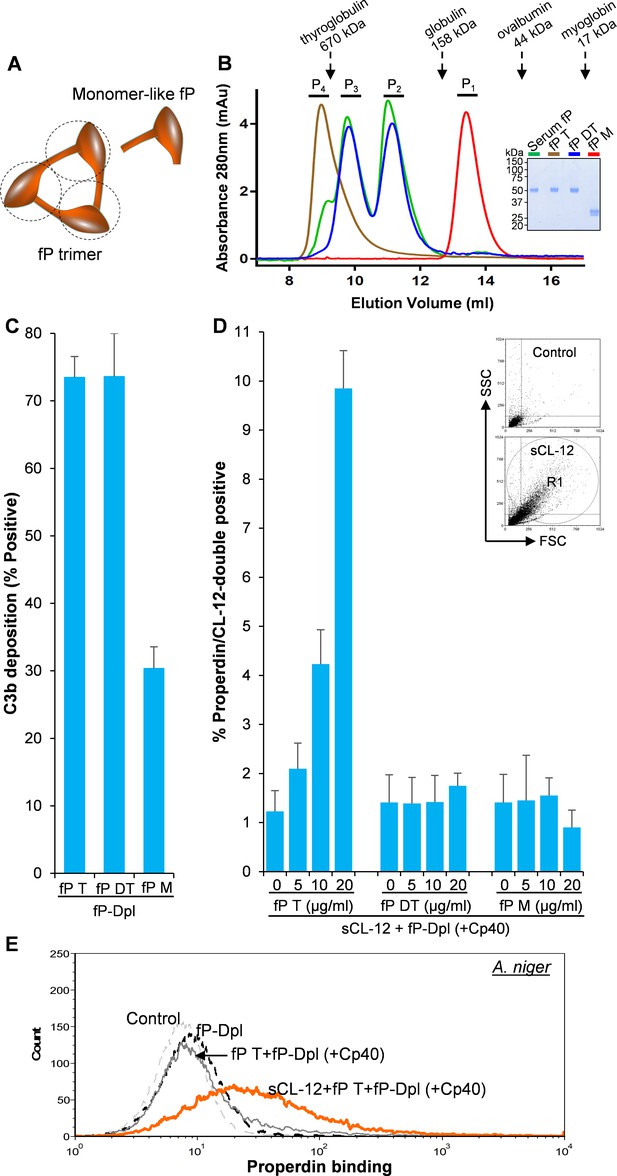
Binding specificity of soluble CL-12 towards properdin variants.
(A) Illustrative diagram of monomer-like properdin complex. (B) SEC analysis of properdin. Inset: SDS-PAGE analysis of properdin under reducing condition. (C) A. fumigatus were incubated with fP-Dpl (10%) restored with purified serum properdin (10 µg/ml) or properdin variants (fP T: properdin tetramer; fP DT: properdin dimer/trimer; fP M: monomer-like properdin, 10 µg/ml). C3b deposition were analyzed and expressed as % Positive events with respect to the fP-Dpl alone with mean ± S.E.M from three independent experiments. (D) sCL-12 (5 µg/ml) was preincubated with the fungus prior to addition of the serum treated with Cp40 (6 µM) plus the properdin variants. Binding of properdin variants was analyzed in sCL-12-positive region (R1 in FSC vs SSC of the fungus) and expressed as % properdin/CL-12-double positive events with mean ± S.E.M from three independent experiments. (E) Binding of properdin tetramer (20 µg/ml) was determined on A. niger as above. Results are representative of at least six independent experiments.
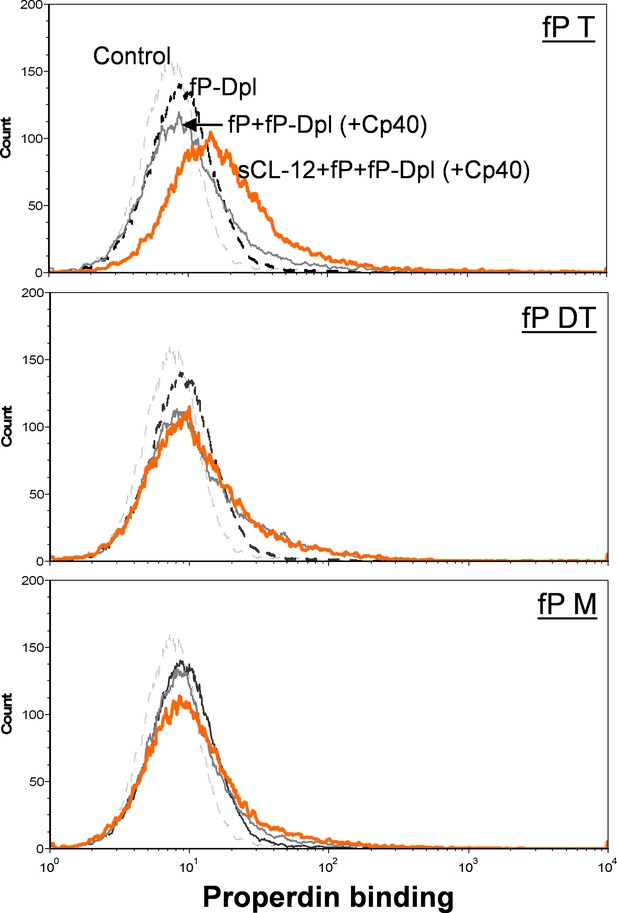
Binding of properdin variants to A.niger in the presence of soluble CL-12.
Properdin variants binding was analyzed with sCL-12 and fP-Dpl restored with properdin variants (fP T: properdin tetramer; fP DT: properdin dimer/trimer; fP M: monomer-like properdin, 10 µg/ml) as described for A. fumigatus. Results are representative of at least six independent experiments.
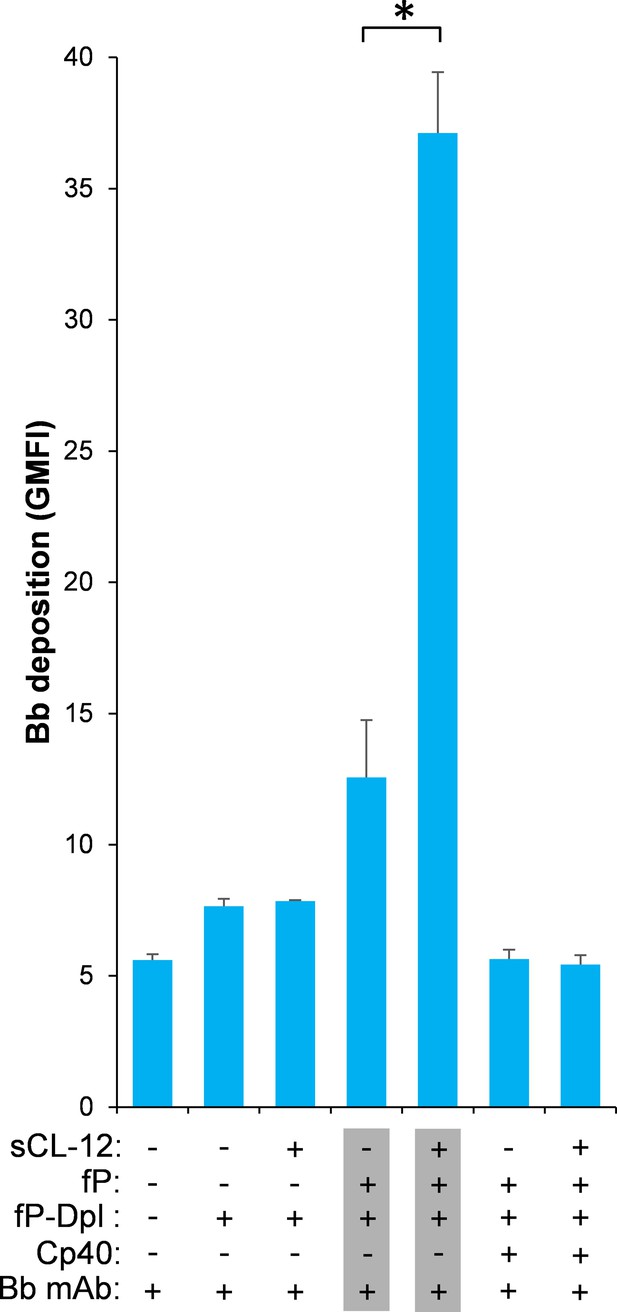
Effect of soluble CL-12 on properdin-directed AP C3 convertase assembly on A.fumigatus.
A. fumigatus were incubated with fP-Dpl (10%) in the presence or absence of sCL-12 (5 µg/ml) as described above. In some experiments, the serum was restored with serum properdin (10 µg/ml) in the presence of Cp40 (6 µM). Bb deposition was analyzed by flow cytometry. The GMFI was used to assess protein binding and expressed as mean ± S.E.M from three independent experiments. Results are representative of at least six independent experiments. *p<0.01.
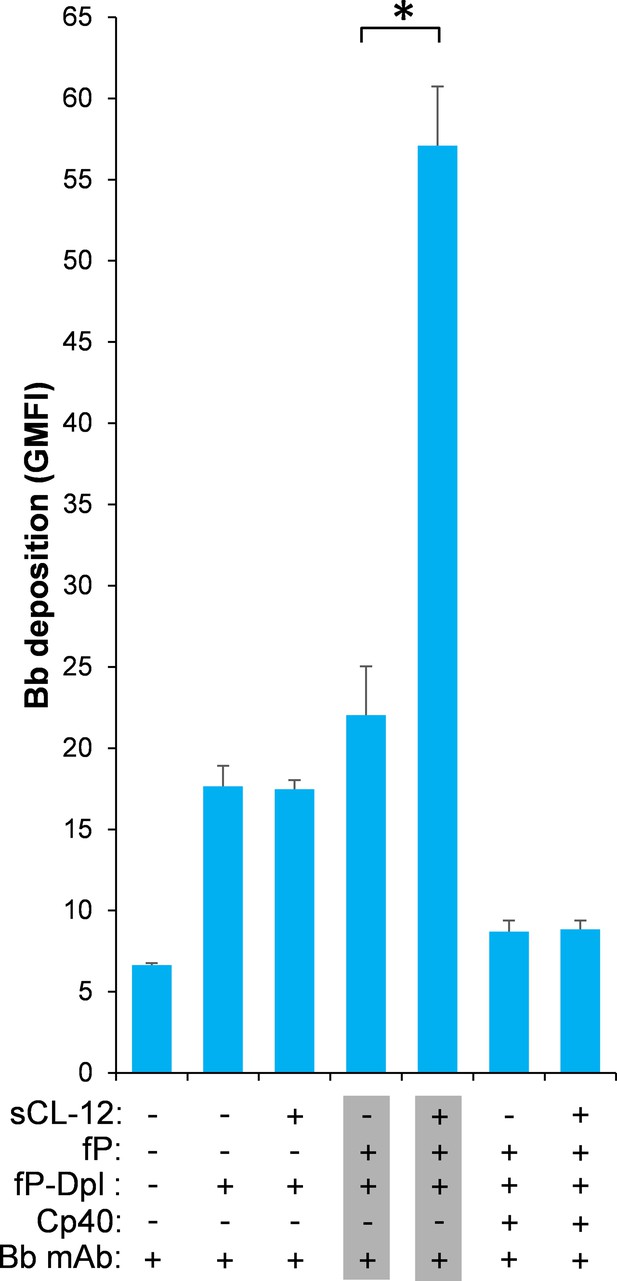
Effect of soluble CL-12 on properdin-directed AP C3 convertase assembly on A.niger.
Bb deposition on A. niger was determined with sCL-12 and fP-Dpl restored with serum properdin as described for A. fumigatus. The GMFI was used to assess protein binding and expressed as mean ± S.E.M from three independent experiments. Results are representative of at least six independent experiments. *p<0.01.
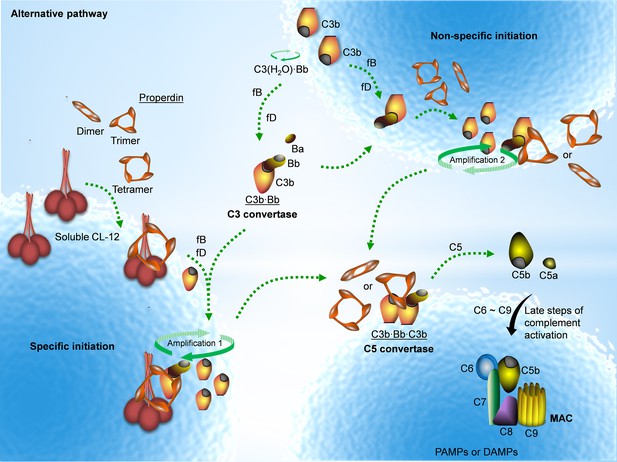
Proposed avenues of alternative pathway activation.
Specific initiation: In addition to the non-specific AP activation, the AP is also specifically triggered by pattern- recognition molecules (PRMs), as soluble CL-12. Upon opsonization of target surfaces, soluble CL-12 specifically recruits properdin organized as higher oligomer (ex. tetramer) to initiate in situ assembly of C3bBb or promotes surface recruitment of preformed fluid-phase C3bBb. When the constant turnover of the in situ assembly drives around, C3b production is highly amplified (amplification 1). Non-specific initiation: AP activation often occurs when C3 thioester is spontaneously activated in the fluid phase and forms initiation C3 convertase C3(H2O)Bb on nearby AP activator surfaces. The initiation is independent of the trigger, and starts to create AP C3 convertase (C3bBb) based on the low level of non-specifically anchored nascent C3b in collaboration with factor B (fB) and factor D (fD). When the AP C3 convertase is stabilized through the association of the complement positive regulator properdin, the productivity of C3b is highly amplified (amplification 2). Both avenues of the specific and non-specific responses contribute to the feedback C3 amplification loop, which substantially potentiates formation of AP C3 convertase, C5 convertase (C3bBbC3b) and C5b-9 membrane attack complex (MAC) for complement activation.





