Antipsychotic olanzapine-induced misfolding of proinsulin in the endoplasmic reticulum accounts for atypical development of diabetes
Figures
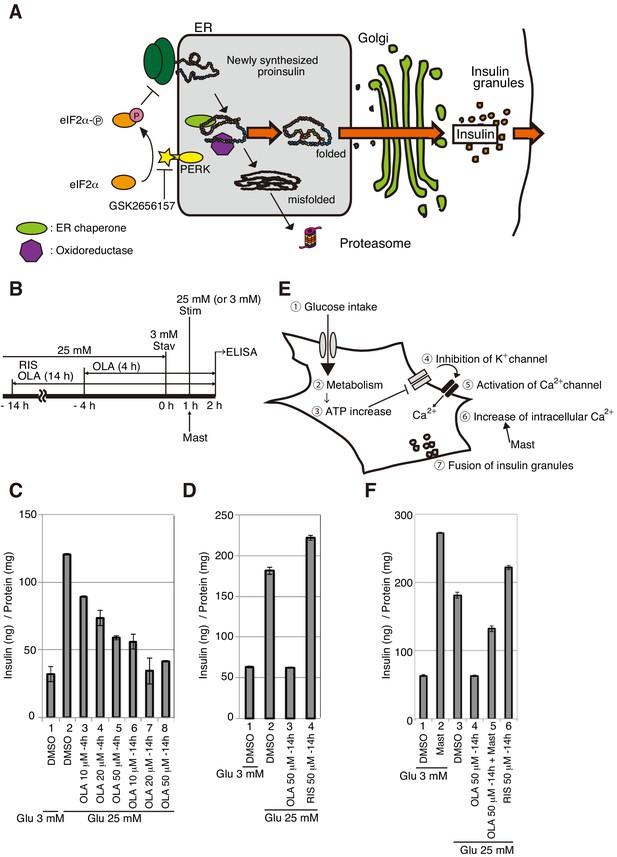
Effect of olanzapine on secretion of insulin from MIN6 cells.
(A) Schematic representation of protein quality control in the ER, PERK-mediated translational attenuation, and storage and secretion of insulin (see Introduction). GSK2656157 inhibits protein kinase activity of PERK. (B) Scheme for measuring the level of insulin secreted into medium in response to glucose stimulation. After culture in a medium containing 25 mM glucose, MIN6 cells were starved for 1 hr in medium containing 3 mM glucose (Stav), and then cultured in medium containing 25 mM glucose (Stim or 3 mM glucose as control). The amount of insulin secreted into the medium during the 1 hr incubation was determined using an ELISA. The data are normalized to the amounts of total cellular proteins and presented as the mean ± SD (n = 2). (C) MIN6 cells were pretreated with the indicated concentrations of olanzapine (OLA) for 4 hr or 14 hr in medium containing 25 mM glucose before glucose starvation as shown in (B). (D) MIN6 cells were pretreated with 50 μM olanzapine or risperidone (RIS) for 14 hr before glucose starvation as shown in (B). (E) Schematic representation of the signaling cascade for insulin secretion in response to glucose stimulation. Cells intake glucose (①). Glucose metabolism (②) triggers an increase in cellular ATP/ADP ratio (③), which inhibits K+ channels and induces depolarization (④). This leads to opening of Ca2+ channels (⑤) and increased influx of Ca2+ (⑥), inducing the fusion of insulin granules to the plasma membrane for secretion (⑦). Mastoparan (Mast) enhances insulin secretion by increasing the intracellular concentration of Ca2+ independently of glucose intake. (F) MIN6 cells were pretreated with 50 μM olanzapine or risperidone for 14 hr before glucose starvation as shown in (B). Mastoparan (Mast, 20 μM) was added at the time of glucose stimulation to aliquots of cells not pretreated or pretreated with 50 μM olanzapine for 14 hr.
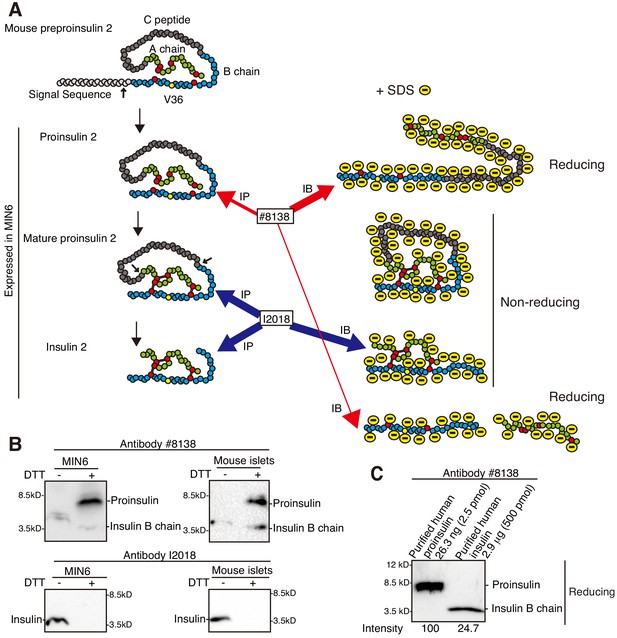
Characterization of two anti-insulin monoclonal antibodies utilized.
(A) Schematic representation of maturation of insulin 2. Each circle denotes an amino acid residue. Preproinsulin 2 comprises a signal sequence (light gray), B chain (blue), C peptide (gray), and A chain (green). Preproinsulin 2 is converted to proinsulin 2 after cleavage of the signal sequence, and proinsulin 2 becomes mature proinsulin 2 via formation of three intramolecular disulfide bonds. Mature proinsulin 2 is converted to insulin 2 after proteolysis at the residues indicated by the two arrows. The red circles represent cysteine residues that form the disulfide bonds (bars), and which are required for insulin folding and activity. The region encompassing V36, indicated by the yellow circle, is recognized by the anti-insulin monoclonal antibody #8138, which immunoprecipitates (immature) proinsulin 2 and detects proinsulin 2 and insulin 2 (B chain) after reducing SDS-PAGE. In contrast, the anti-insulin monoclonal antibody I2018 immunoprecipitates mature proinsulin 2 and insulin 2, and detects insulin 2 after non-reducing SDS-PAGE. (B) Lysates of MIN6 cells and mouse islets were analyzed by immunoblotting using #8138 and I2018 after reducing (DTT +) and non-reducing (DTT -) SDS-PAGE. (C) The indicated amounts of recombinant and purified proinsulin and insulin were subjected to reducing SDS-PAGE followed by immunoblotting using #8138.

Secretion of proinsulin 2, processed proinsulin 2 and insulin 2 from MIN6 cells.
(A) Schemes of the experiments shown in (B) and (C). (B) cDNAs encoding mouse preproinsulin 1, preproinsulin 2, proinsulin 1, and proinsulin 2 were subjected to in vitro transcription and translation in the presence of 35S-Met and 35S-Cys, and then to immunoprecipitation using #8138. A pulse (20 min)-chase (80 min) experiment was performed in MIN6 cells, and cell lysates and media were collected and subjected to immunoprecipitation using #8138. The immunoprecipitates were analyzed by reducing SDS-PAGE and autoradiography. (C) A pulse (3 or 20 min)-chase (30 min) experiment was performed in MIN6 cells, and cell lysates and media (M) were analyzed as in (B). (D) MIN6 cells untreated or treated with brefeldin A (BFA, 10 µg/ml) were subjected to pulse-chase experiment to determine changes in the levels of intracellular and extracellular proinsulin as well as processed proinsulin, as shown in the schema (top). (E) cDNAs encoding mouse proinsulin 2, B chain of mouse insulin 1, and B chain of mouse insulin 2 were treated as in (B). A pulse (20 min)-chase (40 min and 80 min) experiment was performed in MIN6 cells, and cell lysates and media were collected and subjected to immunoprecipitation using #8138 or I2018. The immunoprecipitates were analyzed by reducing and non-reducing SDS-PAGE followed by autoradiography.
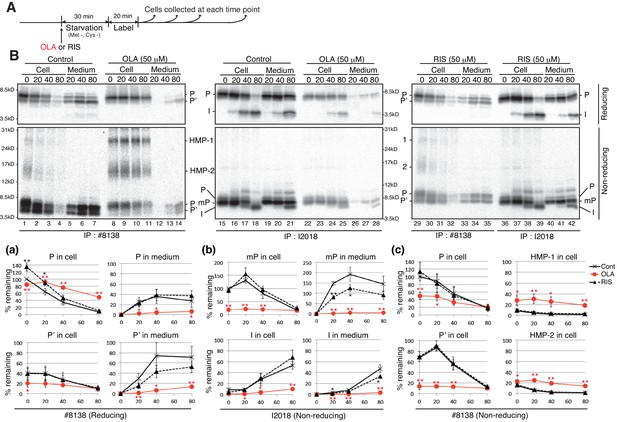
Effect of olanzapine and risperidone on maturation and secretion of proinsulin and insulin in MIN6 cells.
(A) Schemes of the experiments shown in (B). (B) MIN6 cells untreated or treated with olanzapine (50 µM) or risperidone (50 µM) from the start of starvation were subjected to pulse-chase experiment to determine changes in the levels of proinsulin (P), processed proinsulin (P’), mature prinsulin (mP), insulin (I), HMP-1 (1) and HMP-2 (2) in cells and medium. Cell lysates and media were collected and immunoprecipitated with #8138 or I2018. The immunoprecipitates were analyzed by reducing and non-reducing SDS-PAGE followed by autoradiography. The intensity of each band was determined, and the intensity of intracellular proinsulin or mature proinsulin at time 0 in control cells was defined as 100% (n = 3) [categorized in a, b and c].
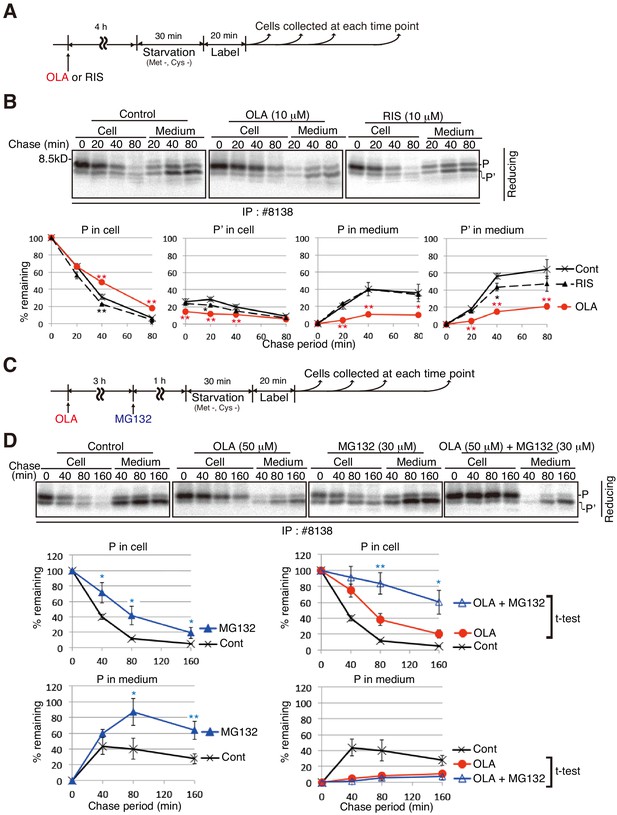
Effect of the proteasome inhibitor MG132 on the level of proinsulin accumulated intracellularly in olanzapine-treated MIN6 cells.
(A) Schemes of the experiments shown in (B). (B) MIN6 cells untreated or treated with olanzapine (10 µM) or risperidone (10 µM) for 4 hr were analyzed as in Figure 4B to determine changes in the levels of proinsulin (P) and processed proinsulin (P’) in cells and medium using #8138 and reducing SDS-PAGE. The intensity of intracellular proinsulin at time 0 was defined as 100% (n = 3). (C) Schemes of the experiments shown in (D). (D) MIN6 cells untreated or treated with olanzapine (50 µM), MG132 (30 µM) or olanzapine (50 µM) and MG132 (30 µM) were analyzed as in Figure 4B to determine changes in the level of proinsulin (P) in cells and medium using #8138 and reducing SDS-PAGE. The intensity of intracellular proinsulin at time 0 was defined as 100% (n = 3).
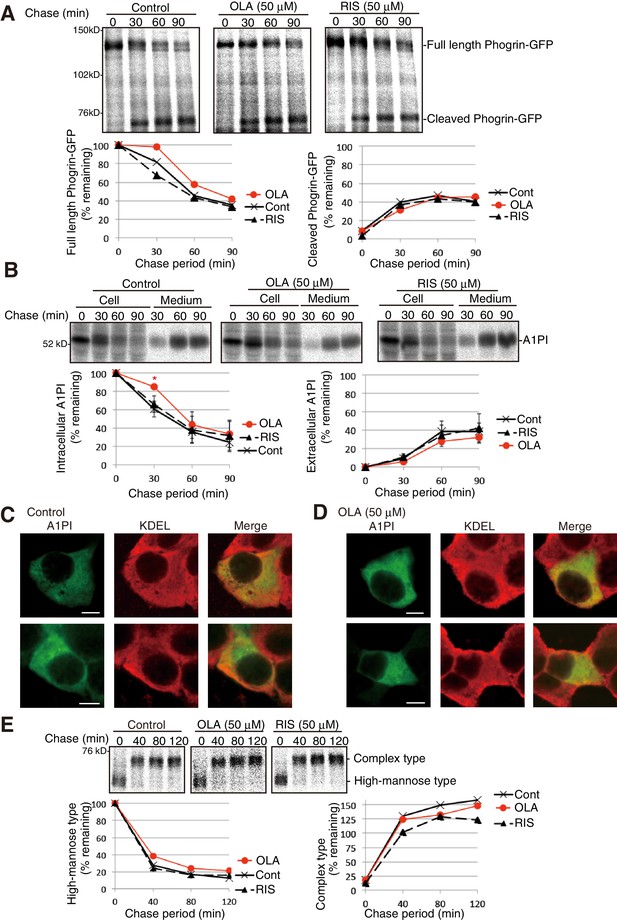
Effect of olanzapine on processing of phogrin-GFP, secretion of A1PI, and maturation of hemagglutinin in MIN6 cells.
(A) MIN6 cells transfected with a phogrin-GFP expression vector were untreated or treated with olanzapine (50 µM) or risperidone (50 µM) for 4 hr and then subjected to pulse-chase experiment in the presence of olanzapine (50 µM) or risperidone (50 µM) to determine the rate of processing of phogrin-GFP. The amounts of full-length and cleaved phogrin-GFP were determined and are shown below with the amount of full-length phogrin-GFP at time 0 defined as 100% (n = 1). (B) MIN6 cells transfected with an A1PI expression vector were untreated or treated with olanzapine (50 µM) or risperidone (50 µM) for 4 hr and then subjected to pulse-chase experiment in the presence of olanzapine (50 µM) or risperidone (50 µM) to determine the rate of secretion of A1PI. The amounts of intracellular and extracellular A1PI were determined and are shown below with the amount of intracellular A1AP at time 0, defined as 100% (n = 3). (C) (D) MIN6 cells transfected with an A1PI expression vector were untreated (C) or treated with olanzapine (50 µM) for 14 hr (D). Fixed and permeabilized cells were analyzed by immunofluorescence using anti-A1PI and anti-KDEL antibodies. Bars: 5 μm. (E) MIN6 cells transfected with a hemagglutinin expression vector were untreated or treated with olanzapine (50 µM) or risperidone (50 µM) for 4 hr and then subjected to pulse-chase experiment to determine the rate of maturation of hemagglutinin. The immunoprecipitates were digested with endoglycosidae H and then analyzed by reducing SDS-PAGE and autoradiography. The amounts of high-mannose type and complex type hemagglutinin were determined and are shown below, with the amounts of high-mannose type hemagglutinin at time 0 defined as 100% (n = 1).
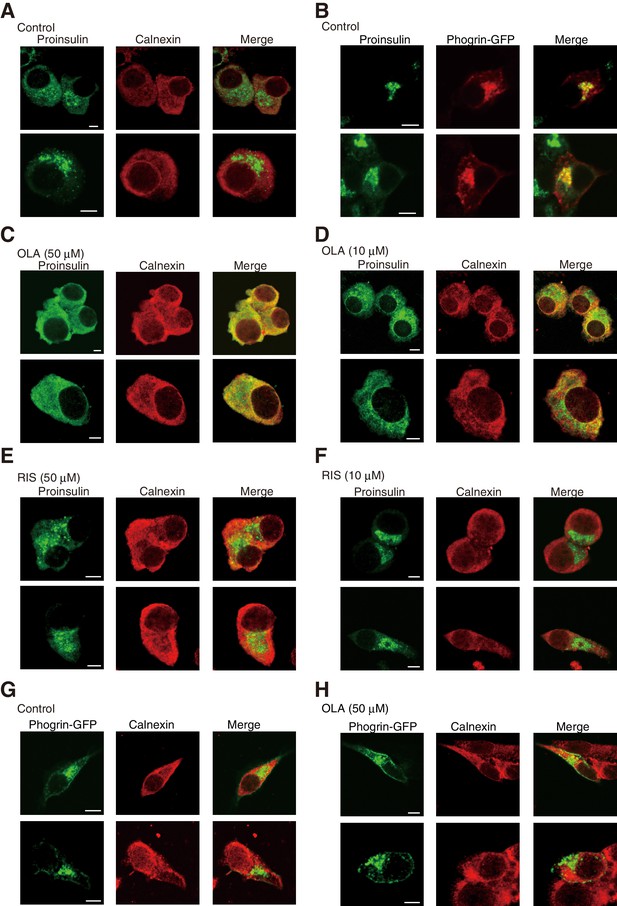
Effect of olanzapine on localization of proinsulin and phogrin-GFP in MIN6 cells (Bars: 5 μm).
(A) MIN6 cells were analyzed by immunofluorescence using anti-insulin #8138 and anti-calnexin antibodies. (B) MIN6 cells transfected with a phogrin-GFP expression vector were analyzed by immunofluorescence using anti-insulin #8138 and anti-GFP antibodies. (C) – (F) MIN6 cells treated with (C) olanzapine (50 µM), (D) olanzapine (10 µM), (E) risperidone (50 µM), or (F) risperidone (10 µM) for 14 hr were analyzed by immunofluorescence using anti-insulin #8138 and anti-calnexin antibodies. (G) (H) MIN6 cells transfected with a phogrin-GFP expression vector were (G) untreated or (H) treated with olanzapine (50 µM) for 14 hr, and then analyzed by immunofluorescence using anti-GFP and anti-calnexin antibodies.
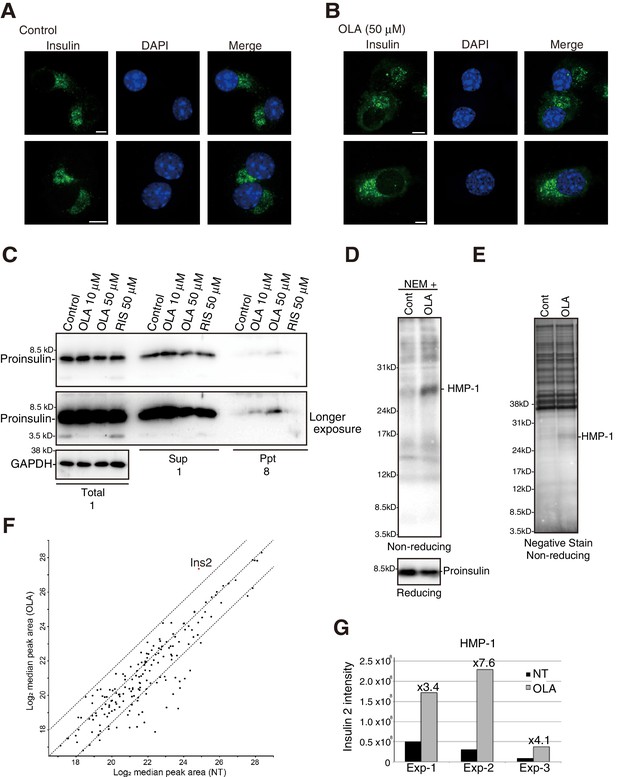
Effect of olanzapine on localization of insulin as well as solubility and oligomerization of proinsulin in MIN6 cells.
MIN6 cells untreated (A) or treated with olanzapine (50 µM) (B) for 14 hr were analyzed by immunofluorescence using anti-insulin I2018 antibody. Bars: 5 μm. (C) MIN6 cells treated with DMSO (control), olanzapine (10 or 50 μM) or risperidone (50 μM) for 14 hr were lysed in 1% NP40. After centrifugation at 14,000 rpm for 10 min, supernatant and precipitate were analyzed by immunoblotting using anti-insulin #8138 and anti-GAPDH antibodies. Eight times greater amounts were used to analyze precipitate than total and supernatant. (D)-(G) MIN6 cells untreated or treated with olanzapine (50 µM) for 4 hr were lysed with 1% NP40 buffer containing 10 mM NEM. (D) Cell lysates were analyzed by reducing and non-reducing SDS-PAGE followed by immunoblotting using #8138. (E) Cell lysates were subjected to immunoprecipitation using #8138, and then to negative staining after non-reducing SDS-PAGE. (F) Gels at the position of HMP-1 were excised and analyzed by mass spectrometry. The results are shown by the scatter plot of log2 of the median peak area from three independent experiments between untreated cells (X axis) and olanzapine-treated cells (Y axis). A 5.7-fold increase by olanzapine treatment was observed for Ins2 as shown in the red circle. (G) Intensities of Ins2-derived fragments in untreated and olanzapine-treated cells in each experiment are shown along with the fold-induction.
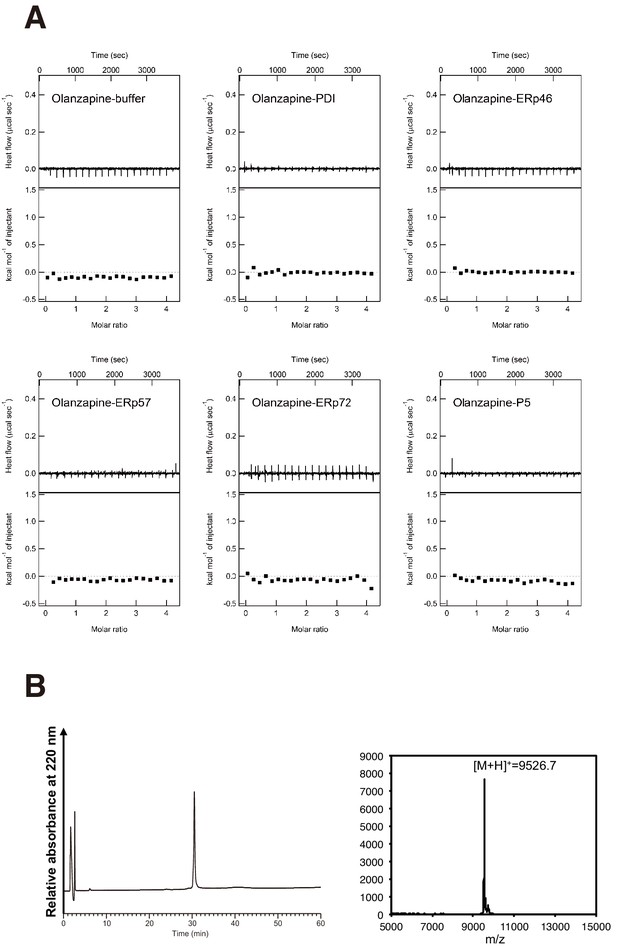
Interaction of olanzapine with various oxidoreductases.
(A) ITC measurements of the interaction between olanzapine and PDI, ERp46, ERp57, ERp72, or P5. (B) (left) HPLC profile of reduced and denatured purified proinsulin. Proteins were eluted using a 20–60% linear gradient of CH3CN in 0.05% trifluoroacetic acid at an increasing rate of 1 %/min and at a flow rate of 1.0 ml/min, and elution was monitored at 220 nm. (right) MALDI-TOF/MS spectra derived from the peak around 30 min. The observed [M+H]+ is 9526.7, corresponding to the calculated [M+H]+ (9526.9).
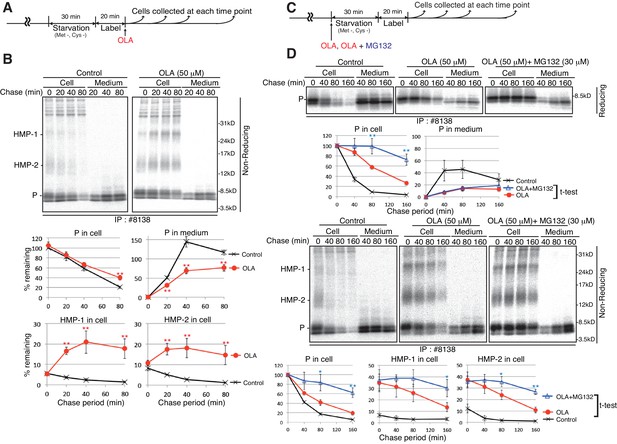
Effect of the proteasome inhibitor MG132 on the levels of proinsulin, HMP-1, and HMP-2 in olanzapine-treated MIN6 cells.
(A) Schemes of the experiments shown in (B). (B) MIN6 cells pulse-labeled for 20 min were chased for the indicated periods with or without 50 µM olanzapine to determine changes in the levels of proinsulin (P), HMP-1 (1), and HMP-2 (2) in cells and medium as in Figure 4B using #8138 and non-reducing SDS-PAGE. The intensity of intracellular proinsulin at time 0 was defined as 100% (n = 3). (C) Schemes of the experiments shown in (D). (D) MIN6 cells untreated, treated with olanzapine (50 µM), or olanzapine (50 µM) and MG132 (30 µM) were analyzed as in Figure 4B to determine changes in the levels of proinsulin (P), HMP-1 (1), and HMP-2 (2) in cells and medium using #8138 and reducing and non-reducing SDS-PAGE. The intensity of intracellular proinsulin at time 0 was defined as 100% (n = 3).
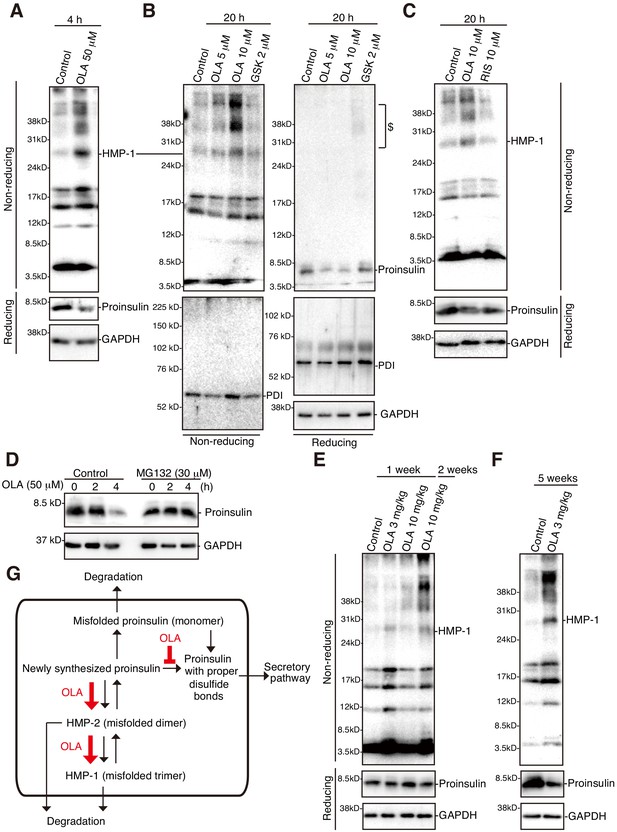
Effect of olanzapine on proinsulin oligomerization in mouse islets.
(A)-(C) Isolated mouse islets were untreated or treated with the indicated concentration of olanzapine, GSK2656157 (GSK, 2 μM) or risperidone (10 μM) for the indicated period and analyzed by immunoblotting using anti-insulin #8138, anti-PDI and anti-GAPDH antibodies after reducing and non-reducing SDS-PAGE. $ denotes aggregated proinsulin. (D) Isolated mouse islets were treated or untreated with MG132 (30 μM) for 1 hr, then treated with olanzapine (50 μM) for the indicated period, and analyzed by immunoblotting using anti-insulin #8138 and anti-GAPDH antibodies after reducing SDS-PAGE. (E) (F) Islets were isolated from mice the indicated week after daily oral administration of the indicated dose of olanzapine, and analyzed by immunoblotting using anti-insulin #8138 and anti-GAPDH antibodies after reducing and non-reducing SDS-PAGE. (G) Model for olanzapine-induced β-cell dysfunction (see text).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Mus musculus) | Insulinoma | Miyazaki et al., 1990 | MIN6 | The cell line has been authenticated and tested negative for mycoplasma. |
| Recombinant DNA reagent | p3xFlag-CMV-14 | Sigma-Aldrich | ||
| Recombinant DNA reagent | pcDNA3.1(+) | ThermoFisher | ||
| Recombinant DNA reagent | Phogrin-GFP | Saito et al., 2011 | ||
| Recombinant DNA reagent | A1PI | Ninagawa et al., 2015 | ||
| Recombinant DNA reagent | HA | Gething and Sambrook, 1982 | ||
| Antibody | Anti-insulin (Mouse monoclonal) | Cell signaling | Cat#: #8138 | WB (1:1000), IP (1:400), IF (1:100) |
| Antibody | Anti-insulin (Mouse monoclonal) | Sigma-Aldrich | Cat#: I2018 | WB (1:1000), IP (1:400) |
| Antibody | Anti-GFP (Mouse monoclonal) | Roche | Cat#: 11814460001 | IP (1:400), IF (1:100) |
| Antibody | Anti-calnexin (Rabbit polyclonal) | Enzo Life Sciences | Cat#: ADI-SPA-865 | WB (1:1000), IF (1:100) |
| Antibody | Anti-PDI (Rabbit polyclonal) | Enzo Life Sciences | Cat#: ADI-SPA-890 | WB (1:1000) |
| Antibody | Anti-A1AT (Rabbit polyclonal) | Dako | Cat#: A0012 | IP (1:400), IF (1:100) |
| Antibody | Anti-GAPDH (Rabbit polyclonal) | Trevigen | Cat#: 2275-PC-100 | WB (1:1000) |
| Antibody | Anti-HA (Rabbit polyclonal) | Recenttec | Cat#: R4-TP1411100 | IP (1:400) |
| Antibody | Anti-KDEL (Mouse monoclonal) | MBL | Cat#: M181-3 | IF (1:1000) |
Additional files
-
Supplementary file 1
Data of mass spectrometric analysis Proteins quantified in all samples (see Figure 8F) are summarized.
- https://cdn.elifesciences.org/articles/60970/elife-60970-supp1-v1.xlsx
-
Supplementary file 2
Information of resources Nucleotide sequences of primers used are shown.
- https://cdn.elifesciences.org/articles/60970/elife-60970-supp2-v1.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/60970/elife-60970-transrepform-v1.al.docx





