Burst mitofusin activation reverses neuromuscular dysfunction in murine CMT2A
Figures
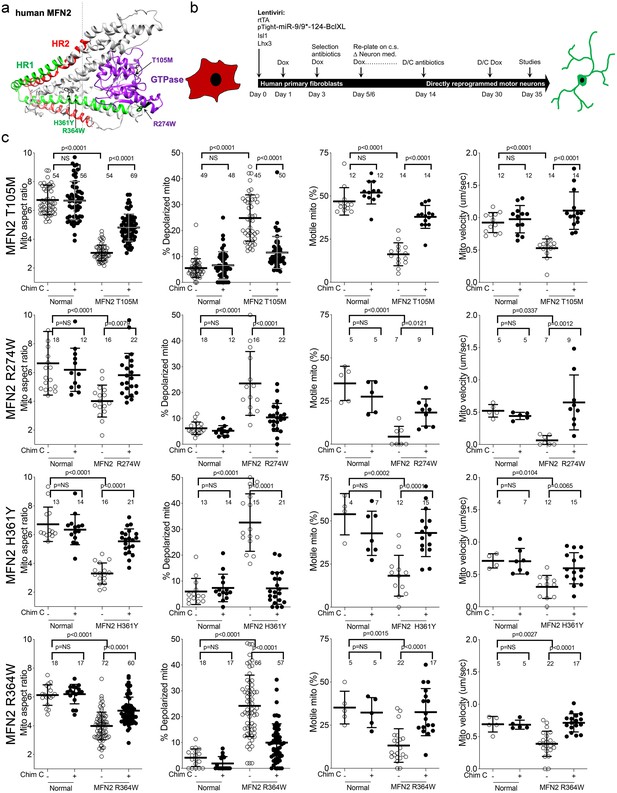
Mitochondrial abnormalities in reprogrammed CMT2A patient motor neurons and their improvement after mitofusin activation.
(a) Model structure of MFN2 showing location of CMT2A patient mutations. (b) Schematic depiction of fibroblast reprogramming procedure to produce motor neurons. (c) Mitochondrial testing in reprogrammed motor neurons from four CMT2A patients with different MFN2 mutations and representative of three normal control subjects. Open circles are baseline; closed circles are 48 hr after addition of mitofusin activator Chimera C (100 nM). Each circle is one neuron from two or three independent reprogrammings. P values from ANOVA.
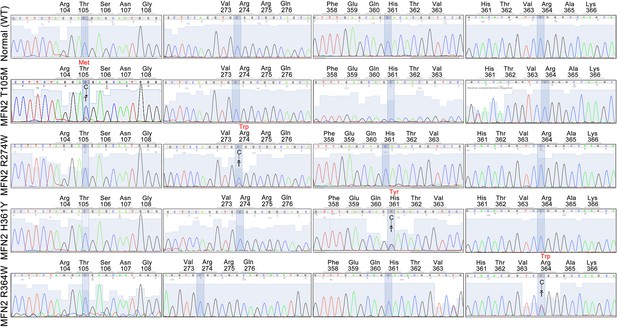
Genotyping of CMT2A patient cells.
Each cell line underwent Sanger sequencing for all 4 MFN2 mutation loci. A representative (of 3) normal control lines is shown. Shaded areas show each mutation locus. Encoded amino acids are on top; red amino acid is mutant form.
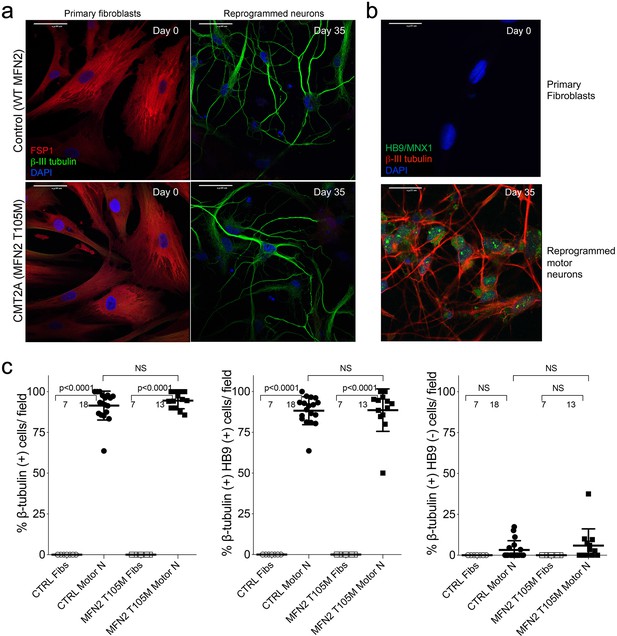
Direct reprogramming of human skin fibroblasts to neurons.
(a) Dual immunolabeling of parental fibroblasts (left) and reprogrammed neurons (right) from normal (top) and CMT2A MFN2 T105M patient (bottom). Red fibroblast-specific protein 1(FSP1) labels fibroblasts; green β-III-tubulin labels neurons. (b) Dual immunolabeling of parental fibroblasts (top) and reprogrammed neurons (bottom) with β-III-tubulin (red) and motor neuron-specific HB9/MNX1 (green). (c) Quantitative data for reprogramming efficiency of control (CTRL) and CMT2A fibroblasts from panels a and b. β-III-tubulin positive cells (left) are neurons; β-III-tubulin positive, HB9/MNX1 positive cells (middle) are motor neurons; β-III-tubulin positive, HB9/MNX1 negative cells are neurons that do not express the motor neuron marker (right). Motor neuron reprogramming efficiency was >90%, and did not differ in CMT2A. P values by ANOVA.
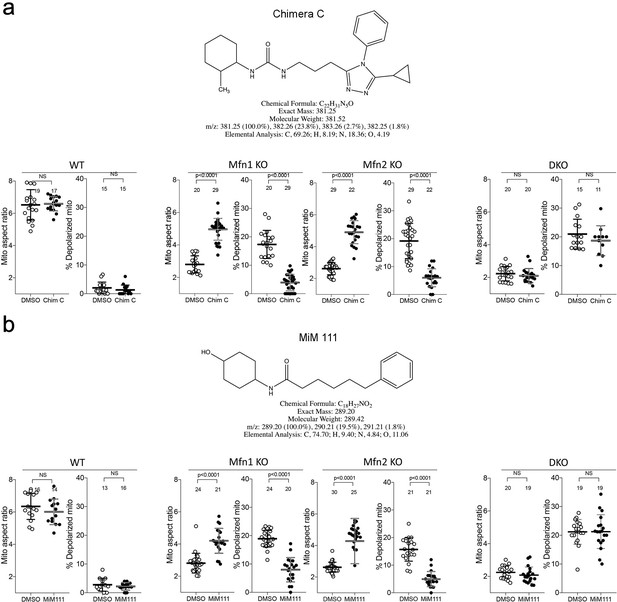
Chemical characteristics and functional profiling of mitofusin activators used in this study.
(a) Chimera C is a member of the original chemical class of mitofusin agonists described in reference 23. (b) MiM111 is the prototype of new chemical class of mitofusin activators having pharmaceutical properties suitable for in vivo use, described in reference 25. Quantitative data below each structure show the effects of each compound (100 nM, 48 hr) on mitochondrial aspect ratio (length/width; a read-out for fusion) and depolarization (indicating respiratory dysfunction) in murine embryonic fibroblasts (MEFs) having different Mfn expression profiles: Wild-type MEFs (left) with both Mfn1 and Mfn2 have normal aspect ratios, low depolarization, and are not affected by mitofusin activation. Mfn1 and Mfn2 KO MEFs (middle) expressing only Mfn2 or Mfn1, respectively, have impaired fusion with low aspect ratios, high depolarization, and both of these abnormalities improve with either mitofusin activator. Mfn double knockout MEFs (DKO, right) have no mitofusin targets, so mitochondrial fragmentation and depolarization do not respond to mitofusin activation. P values by t-test.
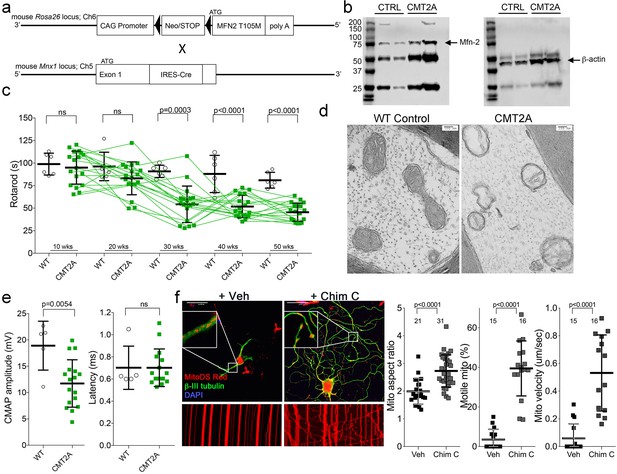
Characteristics of a neuron-specific MFN2 T105M mouse model of CMT2A.
(a) Schematic depiction Mfn2 <fs-T105M>expression strategy. (b) Immunoblot analysis of MFN2 expression in mouse sciatic nerves. (c) Serial RotaRod latency studies; CMT2A is green squares (n = 16), wild-type (WT) control is open circles (n = 6). (d) Electron micrographs of axonal mitochondrial from sciatic nerves (50 weeks). (e) Comparative neuro-electrophysiology study results of 50-week-old mice in panel c. (f) Response of CMT2A dorsal root ganglion neurons to mitofusin activation with Chimera C (100 nM, 48 hr). Top images are confocal micrographs of DRGs stained for mitochondria (red) and axons (green). Insets are higher power magnification to see mitochondrial morphology. Bottom images are kymographs showing mitochondrial (red) motility. Vertical columns are stationary mitochondria; lines transiting left to right or right to left are moving. P values are from t-test from 3 or four independent experiments.
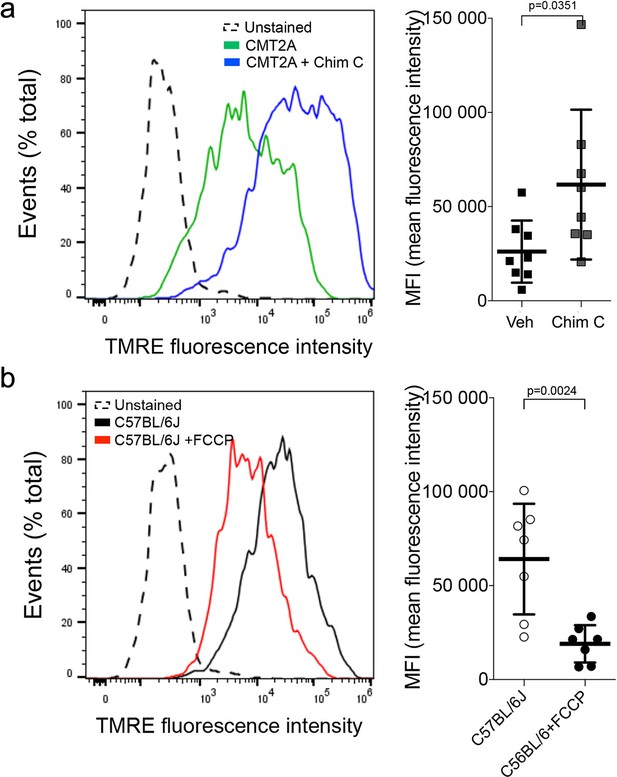
Flow cytometric profiling of mitochondrial polarization status in mouse dorsal root ganglion (DRG) neurons.
(a) DRGs from CMT2A MFN2 T105M mice. Left is representative mitochondrial gating by TMRE fluorescence from a single study; green is vehicle; blue is Chimera C (100 nM, 48 hr). Right graph shows geometric mean fluorescence intensity (MFI) for eight experiments. TMRE fluorescence is inversely proportional to depolarization; increased fluorescence indicates respiratory fitness. (b) Control studies as in a. using DRGs from normal C57BL/6J mice. FCCP is a depolarizing agent used as a positive control. P values by t-test.
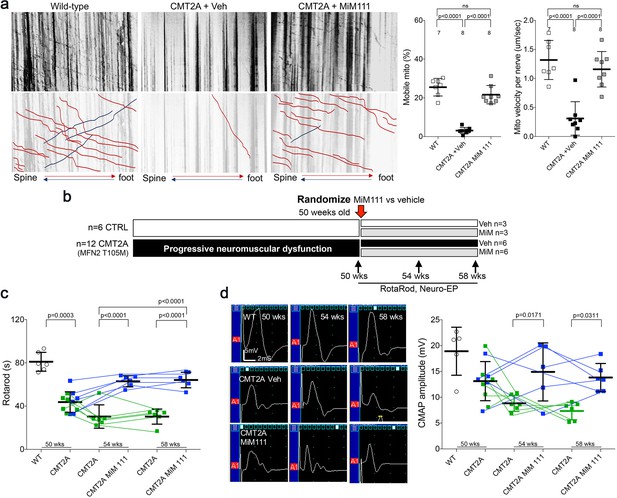
Mitofusin activation reverses neuromuscular dysfunction in MFN2 T105M mice.
(a) Ex vivo mitochondrial motility in CMT2A mouse sciatic nerve axons 4 hr after intramuscular administration of mitofusin activator MiM111 or vehicle. Top panel is kymographs. Bottom panel emphasizes motile mitochondria with red and blue lines transiting antegrade or retrograde, respectively. (Note, mitochondrial transport in ex vivo sciatic nerves favors the antegrade [spine to foot] direction because mitochondria are recruited to the site of nerve injury at the distal amputation site [Zhou et al., 2016]). (b) Experimental design to evaluate efficacy of MiM111 in late murine CMT2A. (c) RotaRod latency in vehicle- (green) and MiM111-treated (blue) MFN2 T105M mice. (d) Neuroelectrophysiology studies: (left) representative CMAP tracings; (right) quantitative data. Each symbol in c and d is one mouse. P values from ANOVA. WT control values are open circles in panels c and d; complete WT control data are in Figure 3—figure supplement 2.
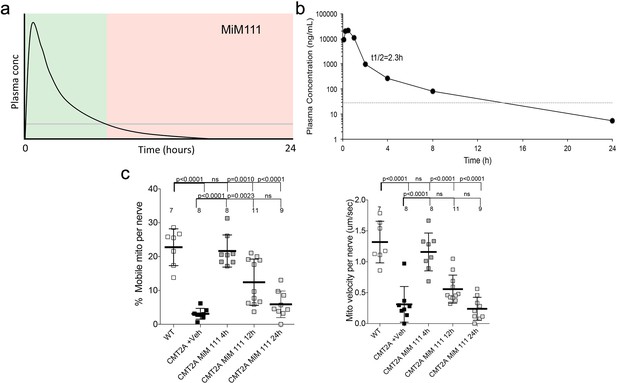
In vivo pharmacokinetics and target engagement of MiM111 administered intramuscularly.
(a) Predicted temporal relationship between plasma MiM111 concentration and peripheral nerve mitochondria activation based on published data (reference 25). Green indicates postulated therapeutic levels; pink indicates postulated sub-therapeutic levels. (b) In vivo plasma concentrations of MiM111 after a single dose of 30 mg/kg administered intramuscularly (means of 2 mice). (c) Time-dependent MiM111 target engagement measured as the increase in proportions (left) and velocity (right) of mobile mitochondria in sciatic nerve axons of CMT2A MFN2 T105M mice. Each point represents a single neuronal axon, from 2 or three mice per time point. WT is normal. P values by ANOVA.
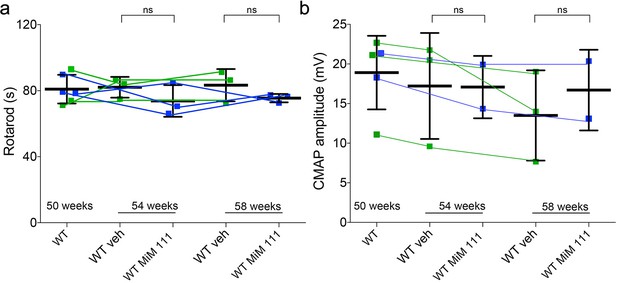
Effects of MiM111 on neuromuscular function in control mice.
(a) RotaRod latency. (b) Neuroelectrophysiologic CMAP amplitude. Each point is a mouse; green is vehicle (n = 3), blue is MiM111 30 mg/kg IM once daily (n = 3). There were no differences (t-test).
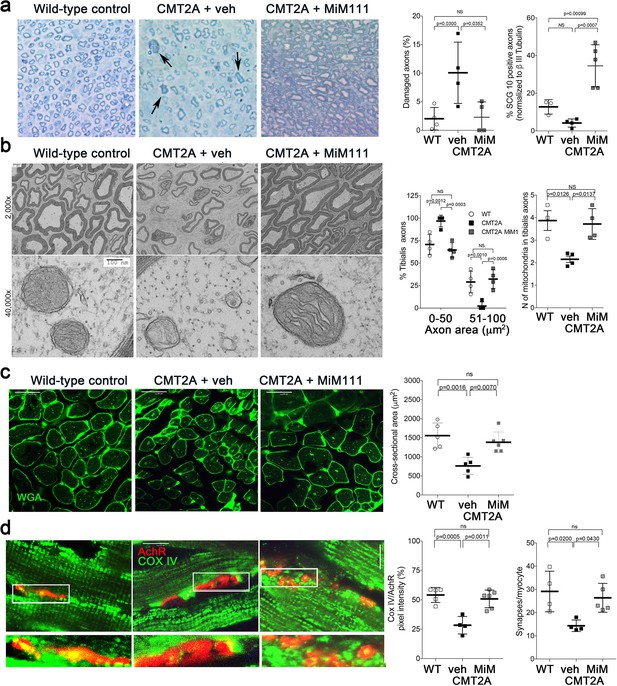
Mitofusin activation reverses histopathological findings in MFN2 T105M mice.
(a) Toluidine blue stained sections of mouse mid tibial nerves. Arrows show blue-stained damaged axons in CMT2A mice. Quantitative group data for damaged axons and SCG10-regenerating axons (see Figure 4—figure supplement 1) are on the right. (b) Electron micrographs of mid-tibial nerve axons from CMT2A mouse study groups after 8 weeks of therapy. Note heterogeneity in axon size (top images; left graph) and mitochondrial abnormalities (bottom images, right graph). (c) Wheat germ agglutinin (WGA) labeled sections of tibialis anterior muscle and quantitative myocyte cross sectional area. (d) Confocal micrographs of neuromuscular junctions to show mitochondrial occupancy yellow organelles within red synapses (also see Figure 4—figure supplement 1). Each symbol represents results from one mouse. Data are means ± SD; p values are 1- or 2-way ANOVA.
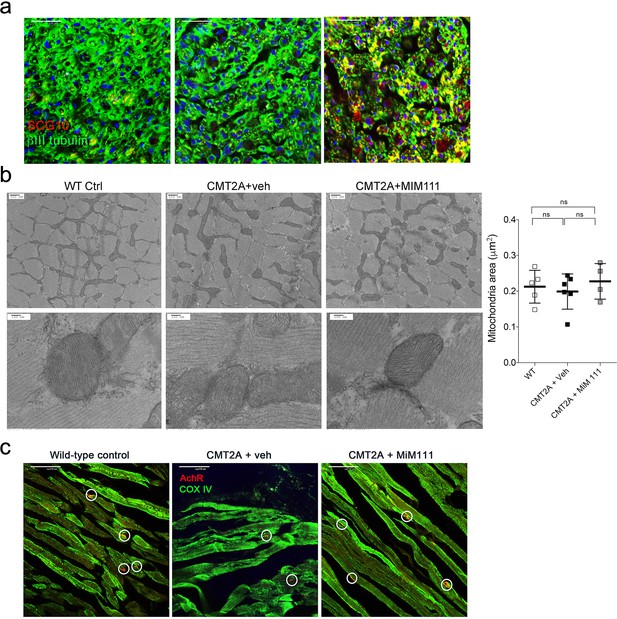
Effects of MiM111 on neuromuscular integrity in CMT2A MFN2 T105M mice.
(a) Labeling of regenerating neurons with SCG10 (red). (b) Mitochondria in tibialis muscles of CMT2A mice are normal. Mitochondrial area is quantified from group data on the right. (c) Neuromuscular junctional synapses in tibialis muscle of CMT2A MFN2 T105M mice. Acetylcholine receptor (AchR; red) labels terminal neuron synapses (encircled); green is mitochondrial cytochrome C oxidase IV. Quantitative data for panels a and c are in Figure 4a and d, respectively. P values by ANOVA.
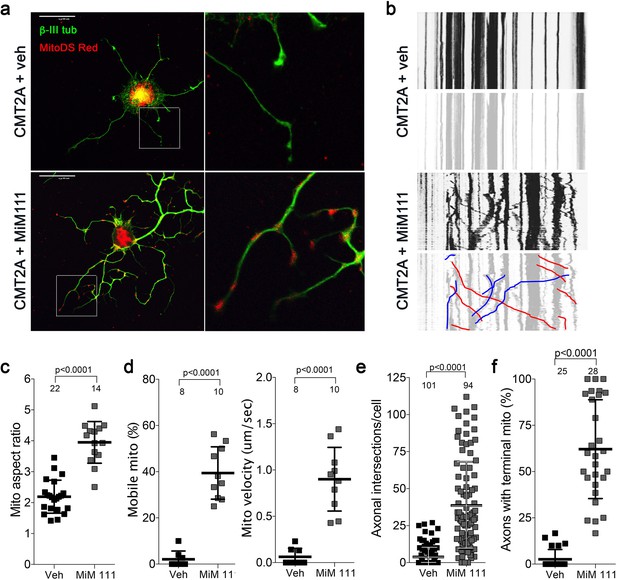
Mitofusin activation reverses mitochondrial pathology and stimulates growth of CMT2A dorsal root ganglion neurons in vitro.
(a) Confocal micrographs of CMT2A mouse DRGs cultured for 48 hr with MiM111 or its vehicle. Note greater neuronal process length and branching in MiM111-treated neuron. Exploded insets (right) show neuronal process termini. Mitochondria express mitoDS Red; neuronal processes stained for β-III tubulin are green. (b) Kymographs of mitochondrial motility in neuronal processes of live DRGs from studies shown in (a). Top panel is raw data. Bottom panels emphasize motile mitochondria with red and blue lines transiting left to right or right to left, respectively. (c-f) Quantitative group data demonstrating effect of MiM111 on CMT2A DRG mitochondrial aspect ratio (c), motility (d, e), neuronal process length and branching (e), and proportion of neuronal process termini containing mitochondria (f).
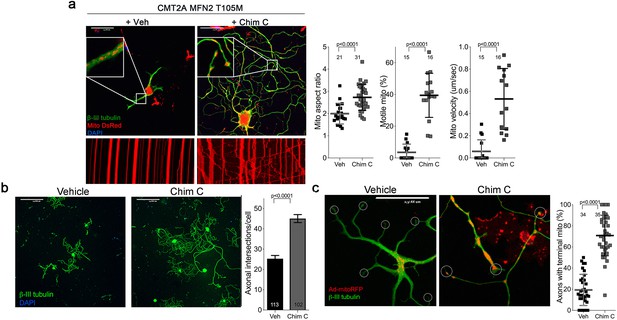
Mitofusin activation with Chimera C reverses mitochondrial pathology and stimulates growth of CMT2A dorsal root ganglion neurons in vitro.
(a) CMT2A mouse DRGs cultured for 48 hr with Chimera C (100 nM) or its vehicle (Me2SO4). Exploded insets show mitochondria expressing mitoDS Red; neuronal processes stained for β-III tubulin are green. Kymographs (below) show mitochondrial motility in live cell studies; quantitative group data for aspect ratio and motility are to the right. (b) Neuronal process length and branching in CMT2A DRG neurons cultured with vehicle or Chimera C. Quantitative group data are to the right. Note greater length and branching of neuronal processes in MiM111-treated neuron. Mitochondria are expressing mitoDS Red. (c). Proportion of neuronal process termini (encircled) containing mitochondria (red) in vehicle- or Chimera C-treated CMT2A DRGs. P values by t-test.
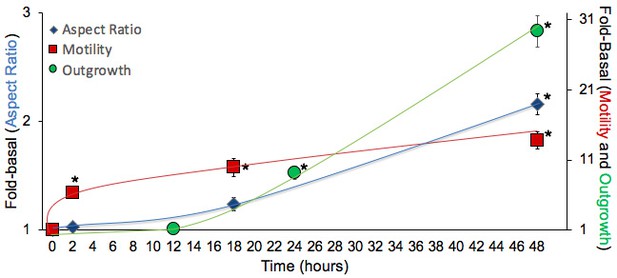
Time course studies of DRG mitochondria responses to mitofusin activation after aspiration axotomy.
Studies were performed as in Figure 5. *P < 0.05 vs. basal (ANOVA). Three independent studies were performed for each endpoint; each symbol represents the mean value for all three biological replicates, each of which is the average of approximately 3 (mitochondrial motility in different neurons), 20 (outgrowth of different cell neuronal processes), or 300 (aspect ratio of individual mitochondria), technical replicates. The increase in mitochondrial motility (red) was rapid, being half-maximal after 2 hr. The increase in neuron outgrowth (green) was significant after 24 hr. Increased mitochondrial aspect ratio (blue), reflecting the morphological consequences of enhanced fusion, was only significant after 48 hr.
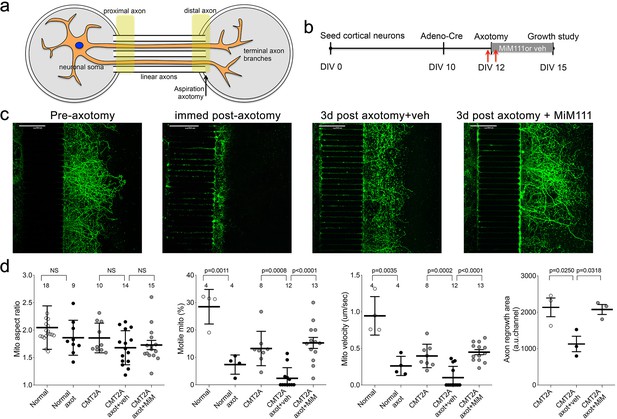
Mitofusin activation stimulates post-axotomy regrowth of CMT2A cortical neurons in vitro.
(a) Schematic depiction of microfluidic platform. Yellow areas show proximal axon where mitochondrial motility was measured and distal axon where mitochondrial aspect ratio was measured. (b) Experimental design. DIV is days in vitro. Red arrows are times of pre- and immediate post-axotomy mitochondrial studies. (c) Representative images of CMT2A neuron terminal branches at different times relative to aspiration axotomy. (d) Quantitative group data demonstrating effect of MiM111 on CMT2A cortical neuron mitochondrial aspect ratio (left panel), motility (middle panels), and axon length (right panel).
Tables
Characteristics and sources of human primary fibroblasts used for motor neuron reprogramming studies.
| Diseases | Mutation | Age | Sex | Passage# | Source | Fibroblast ID |
|---|---|---|---|---|---|---|
| CMT2A | MFN2 Thr105Met | 41 | F | P4-P10 | Dr. Robert H. Baloh | - |
| CMT2A | MFN2 Arg274Trp | 23 | M | P4-P10 | Dr. Barbara Zablocka | - |
| CMT2A | MFN2 His361Tyr | 41 | M | P4-P10 | Dr. Robert H. Baloh | - |
| CMT2A | MFN2 His364Trp | 28 | F | P6-P10 | Dr. Michael E. Shy | - |
| CMT1A | PMP22 DUP | 28 | F | P4-P10 | Coriell Institute | GM05167 |
| CTRL 1 | - | 68 | F | P3-P7 | NINDS | ND34769 |
| CTRL 2 | - | 71 | F | P3-P7 | NINDS | ND36320 |
| CTRL 3 | - | 55 | F | P3-P7 | NINDS | ND29510 |
| CTRL 4 | - | 66 | M | P8-P10 | NINDS | ND29178 |
| CTRL 5 | - | 72 | M | P3-P7 | NINDS | ND34770 |
| CTRL 6 | - | 55 | M | P4-P10 | NINDS | ND38530 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene Mus musculus | Mfn-2 | NCBI Gene | Gene ID: 170731 | MFN2 ENSMUSG00000029020 |
| Gene (Human) | MFN-2 | NCBI Gene | Gene ID: 9927 | MFN2 ENSG00000116688 |
| Genetic reagent (M. musculus) | Rosa-STOP-mMFN Thr105Met (T105M) mice | (C57BL/6 Gt(ROSA) 26 Sortm1 (CAG MFN2*T105M)Dple/) | The Jackson Laboratory: 025322 | C57Bl/6 |
| Genetic reagent M. musculus | HB9-Cre mice | (B6.129S1-Mnx1tm4(cre)Tmj/J) | The Jackson Laboratory : 006600 | C57Bl/6 |
| Genetic reagent M. musculus | C57BL/6J mice | C57Bl/6 | The Jackson Laboratory : 000664 | C57Bl/6 |
| Mfn2 null M. musculus | Mfn2 null MEFs | ATCC | CRL-2994 | Murine embryonic fibroblasts |
| Mfn1/Mfn2 null M. musculus | Mfn1 and Mfn2 double knock out MEFs | ATCC | CRL-2993 | Murine embryonic fibroblasts |
| Mfn1 null M. musculus | Mfn1 null MEFs | ATCC | CRL-2992 | Murine embryonic fibroblasts |
| Cell line (H. sapiens) | Dermal fibroblast (MFN2 T105M) | Dr. Robert H. Baloh (Cedars Sinai) | Female | |
| Cell line (H. sapiens) | Dermal fibroblast (MFN2 H361Y) | Dr. Robert H. Baloh (Cedars Sinai) | Male | |
| Cell line (H. sapiens) | Dermal fibroblast (MFN2 R274W) | Dr. Barbara Zablocka (Mossakowski Med Res Ctr) | PMID:28076385 | Male |
| Cell line (H. sapiens) | Dermal fibroblast (MFN2 R364W) | Dr. Michael E. Shy (University of Iowa) | Female | |
| Cell line (H. sapiens) | Dermal fibroblast (Normal) | NINDS | ND34769 | Female |
| Cell line (H. sapiens) | Dermal fibroblast (Normal) | NINDS | ND36320 | Female |
| Cell line (H. sapiens) | Dermal fibroblast (Normal) | NINDS | ND29510 | Female |
| Transfected construct (Human Adenovirus Type5 (dE1/E3)) | Adenovirus β-galactosidase | Vector Biolabs | Cat#: 1080 | |
| Transfected construct (Human Adenovirus Type5 (dE1/E3)) | Adenovirus Mito-Ds-Red2 | Signagen | Cat#: 12259 | |
| Transfected construct (Human Adenovirus Type5 (dE1/E3)) | Adenovirus Cre-recombinase | Vector Biolabs | Cat#: 1794 | |
| Recombinant DNA reagent | rtTA-N144 (plasmid) | Addgene | Cat#: 66810 | Lentiviral construct to transfect and express the plasmid |
| Recombinant DNA reagent | pTight-9-124-BclxL (plasmid) | Addgene | Cat#: 60857 | Lentiviral construct to transfect and express the plasmid |
| Recombinant DNA reagent | LHX3-N174 and ISL1-N174 (plasmid) | PMID:28886366 | Lentiviral construct to transfect and express the plasmid | |
| Antibody | Anti-Mfn-2 (Mouse monoclonal) | AbCAM | Cat#: ab56889 | (1:1000) |
| Antibody | Anti-COX-IV (Rabbit polyclonal) | AbCAM | Cat#: ab16056 | (1:1000) |
| Antibody | Anti-Stathmin-2 (Rabbit polyclonal) | Novus Biologicals | Cat#: NBP1-49461 | (1:1000) |
| Antibody | Anti-GAPDH (Mouse monoclonal) | AbCAM | Cat#: ab8245 | (1:3000) |
| Antibody | Anti-FSP-1 (Rabbit polyclonal) | Novus Biologicals | Cat#: NBP1-49461 | (1:400) |
| Antibody | Anti-MNX1 (Mouse monoclonal) | DSHB | Cat#: 81.5C10 | (2 µg/ml) |
| Antibody | Anti-β-tubulin III (Mouse monoclonal) | Biolegend | Cat#: 801201 | (1:200) |
| Antibody | Alexa-Fluor 488 (Goat anti-mouse) | ThermoFisher | Cat#: A11029 | (1:400) |
| Antibody | Alexa- Fluor 488 (Goat anti-rabbit) | ThermoFishe | Cat#: A11008 | (1:400) |
| Antibody | (Goat anti-rabbit IgG) | ThermoFisher | Cat#: 31460 | (1:3000) |
| Antibody | Alexa- Fluor 594 (Goat anti rabbit) | ThermoFisher | Cat#: A32740 | (1:400) |
| Antibody | (Peroxidase-conjugated anti-mouse IgG) | Cell Signaling | Cat#: 7076S | (1:3000) |
| Antibody | (α-Bungarotoxin Alexa flour 594) | ThermoFisher | Cat#: B12423 | (0.5 μg/ml) |
| Sequence-based reagent | HB9CRE Fw | The Jackson Laboratory | 006600 | CTAGGCCACAGAATTGAAAGATCT |
| Sequence-based reagent | HB9CRE Rv | The Jackson Laboratory | 006600 | GTAGGTGGAAATTCTAGCATCATCC |
| Sequence-based reagent | HB9CRE TG Fw | The Jackson Laboratory | 006600 | GCGGTCTGGCAGTAAAAACTATC |
| Sequence-based reagent | HB9CRE TG Rv | The Jackson Laboratory | 006600 | GTGAAACAGCATTGCTGTCACTT |
| Sequence-based reagent | Mfn2 T105M M Fw | The Jackson Laboratory | 025322 | GACCCCGTTACCACAGAAGA |
| Sequence-based reagent | Mfn2 T105M M Rv | The Jackson Laboratory | 025322 | AACTTTGTCCCAGAGCATGG |
| Sequence-based reagent | Mfn2 T105M Wt Fw | The Jackson Laboratory | 025322 | AAGGGAGCTGCAGTGGAGTA |
| Sequence-based reagent | Mfn2 T105M Wt Rv | The Jackson Laboratory | 025322 | CCGAAAATCTGTGGGAAGTC |
| Sequence-based reagent | MFN2 T105M Fw | This paper | PCR primers for cell line mutation validation | TTGCACTGAATAGGGCTTTG |
| Sequence-based reagent | MFN2 T105M Rv | This paper | PCR primers for cell line mutation validation | CATTCACCTCCACAGGGTG |
| Sequence-based reagent | MFN2 R274W Fw | This paper | PCR primers for cell line mutation validation | CGTGGTAGGTGTCTACAAGAAGC |
| Sequence-based reagent | MFN2 R274W Rv | This paper | PCR primers for cell line mutation validation | CTGGTGAGGGCTGATGAAAT |
| Sequence-based reagent | MFN2 H361Y and R364W Fw | This paper | PCR primers for cell line mutation validation | CCTGGCAGTGAAAACCAGAG |
| Sequence-based reagent | MFN2 H361Y and R364W Rv | This paper | PCR primers for cell line mutation validation | AAGGCGTGTCCTAACTGCC |
| Chemical compound, drug | Trans-MiM111 | Mitochondria in Motion, Inc | Cpd 13b in PMID:32506913 | |
| Chemical compound, drug | Chimera C | Paraza Pharma | Cpd 2 in PMID:32506913 | |
| Chemical compound, drug | Papain | Sigma | Cat#: P4762 | |
| Chemical compound, drug | Laminin | Sigma | Cat#: L2020 | |
| Chemical compound, drug | Poly-d-Lysine | Sigma | Cat#: P7886 | |
| Chemical compound, drug | Poly-ornithine | Sigma-Aldrich | Cat#: P4957 | |
| Chemical compound, drug | Fibronectin | Sigma-Aldrich | Cat#: F4759 | |
| Chemical compound, drug | Polybrene | Sigma-Aldrich | Cat#: H9268 | |
| Chemical compound, drug | Doxycycline | Sigma-Aldrich | Cat#: D9891 | |
| Chemical compound, drug | G418/Geneticin | Invitrogen | Cat#: 10131-035 | |
| Chemical compound, drug | Retinoic Acid | Sigma | Cat#: R2625 | |
| Chemical compound, drug | BDNF, NT-3, CNTF, GDNF | Peprotech | Cat#: 450-02, Cat#: 450-03, Cat#: 450-13, Cat#: 450-10 | |
| Chemical compound, drug | Dibutyryl cAMP | Sigma | Cat#: D0627 | |
| Chemical compound, drug | Valproic acid | Sigma | Cat#: 676380 | |
| Chemical compound, drug | Puromycin | Invitrogen | Cat#: A11138-03 | |
| Chemical compound, drug | Collagenase | Worthington Biochemical | Cat#: 41J12861 | |
| Chemical compound, drug | (2-Hydroxypropyl)-β-cyclodextrin | Sigma | Cat#: 332607 | |
| Chemical compound, drug | Carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone | Sigma | Cat#: C2759 | |
| Chemical compound, drug | B27 supplement | Gibco | Cat#: 17504-044 | |
| Chemical compound, drug | Insulin-transferrin-sodium selenite | Sigma | Cat#: 1884 | |
| Chemical compound, drug | Glucose | Sigma | Cat#: G5767 | |
| Chemical compound, drug | L-glutamine | Gibco | Cat#: 25030-149 | |
| Chemical compound, drug | Goat serum | Jackson Immunoresearch | Cat#: 005-000121 | |
| Chemical compound, drug | Glutaraldehyde | Electron Microscopy Science | Cat#: 16216 | |
| Chemical compound, drug | MitoTracker Green | Thermo Fisher | Cat#: M7514 | |
| Chemical compound, drug | Calcein AM | Thermo Fisher | Cat#: C3100MP | |
| Chemical compound, drug | Hoechst | Thermo Fisher | Cat#: H3570 | |
| Chemical compound, drug | MitoTracker Orange | Thermo Fisher | Cat#: M7510 | |
| Chemical compound, drug | Tetramethylrhodamine ethyl ester | Thermo Fisher | Cat#: T-669 | |
| Software, algorithm | ImageJ | C. A. Schneider | https://imagej.net/Sholl_Analysis | |
| Software, algorithm | Viasys Healthcare Nicolet Biomedical instrument with Viking Quest version 11.2 software | Middleton | Cat#: OL060954 | |
| Software, algorithm | Gallios instrument with FlowJo 10 software | Beckman Coulter | N/A | |
| Other | RotaRod | Ugo Basile | Cat#: 47650 | |
| Other | XonaChips | Xona Microfluidics | Cat#: XC450 |





