Unique integrated stress response sensors regulate cancer cell susceptibility when Hsp70 activity is compromised
Figures
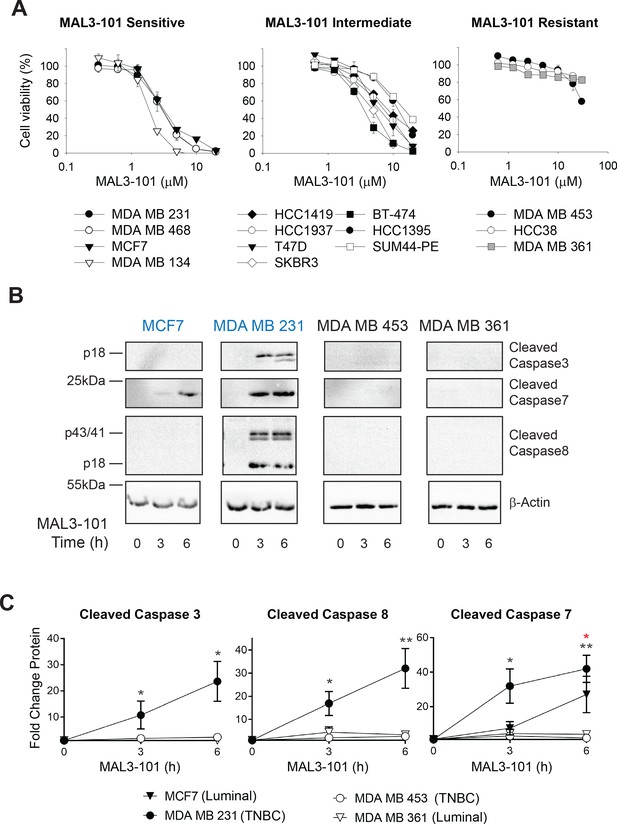
Breast cancer cell lines exhibit unique sensitivities to an Hsp70 inhibitor, MAL3-101.
(A) HER2 (diamonds), TNBC (circles), and luminal (squares and triangles) breast cancer lines were seeded into 96-well plates and treated with increasing doses of MAL3-101 for 72 hr. Viability is expressed as the average of three or more independent experiments, ± SEM. (B) MAL3-101-sensitive (MCF7 and MDA MB 231, denoted in blue) and resistant (MDA MB 453 and MDA MB 361, denoted in black) cells were treated with 12 µM MAL3-101 for the indicated times, and lysates were prepared and immunoblotted for cleaved caspase-3, caspase-7, and caspase-8. β-actin serves as a loading control. (C) The corresponding fold-increase of the indicated apoptotic markers relative to the DMSO control are plotted, ± SEM (n≥3 for cleaved caspase-3, n=3 for cleaved caspase-7, and n≥4 for cleaved caspase-8). Black asterisks correspond to statistical significance between MDA MB 231 cells (closed circle) and MDA MB 453 and MDA MB 361 (open circle and triangle, respectively), and the red asterisk represents statistical significance between MCF7 (closed triangle) and MDA MB 453 and MDA MB 361 (open circle and triangle) cells; * denotes p<0.05, ** denotes p<0.005.
-
Figure 1—source data 1
Source data for cell viability assay and apoptotic marker accumulation in Figure 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig1-data1-v3.xlsx
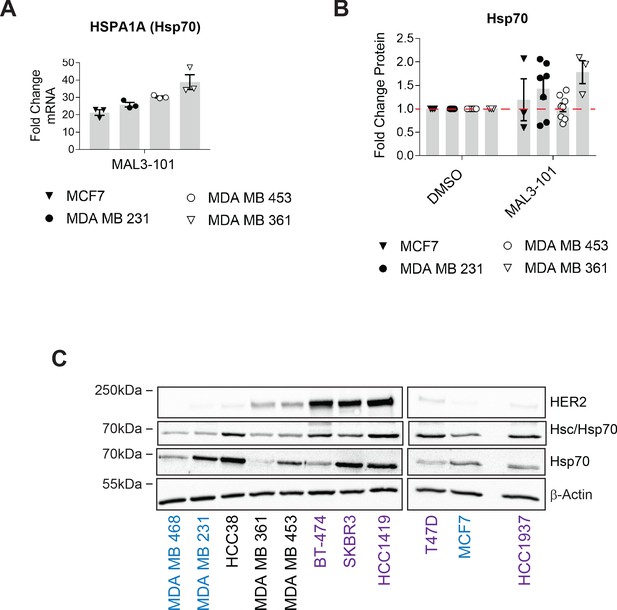
MAL3-101 sensitivity is independent of Hsp70 levels or induction.
(A) The fold change increase of the major Hsp70 gene (HSPA1A) in MAL3-101-treated cells relative to DMSO is plotted, ± SEM (n=3). MAL3-101-sensitive cells are indicated with closed symbols, while MAL3-101-resistant lines are represented with open symbols. (B) The relative fold change of Hsp70 protein levels in MAL3-101 relative to DMSO-treated conditions is plotted, ± SEM (n≥3). Symbols correspond to those indicated in part A. (C) The indicated proteins were analyzed by immunoblot in 12 breast cancer lines. Blue, purple, and back text indicates MAL3-101-sensitive, intermediate, and resistant lines, respectively. β-actin serves as a loading control.
-
Figure 1—figure supplement 1—source data 1
Raw data for Hsp70 mRNA and protein abundance.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig1-figsupp1-data1-v3.xlsx
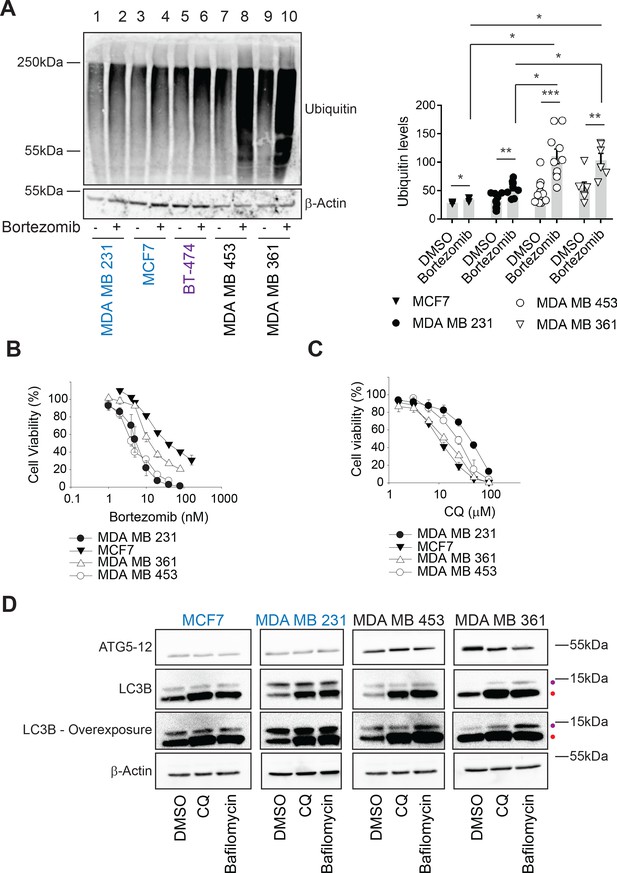
Ubiquitinated proteins are lower in MAL3-101-resistant breast cancer cells.
(A) MAL3-101 sensitive (in blue), intermediate (in purple), and resistant (in black) lines were treated with DMSO or bortezomib for 4 hr followed by immunoblot analysis to detect total levels of ubiquitinated proteins in the cell. β-actin serves as a loading control. Data for MAL3-101 sensitive and resistant lines (raw counts from imaging) are plotted in the graph to the right, ± SEM (n=8 for MDA MB 231, n=3 for MCF7, n=10 for MDA MB 453, and n=6 for MDA B 361). Normalized ubiquitin levels relative to β-actin signals are plotted in the graph. There was a ~2-fold increase in ubiquitinated protein levels in MAL3-101 resistant cells (open symbols) versus the MAL3-101-sensitive lines (closed symbols) in the presence of bortezomib. * denotes p<0.05, ** denotes p<0.005, and *** denotes p<0.0005. (B–C) MAL3-101-sensitive lines (closed symbols) and resistant cells (open symbols) were seeded into 96-well plates and treated with increasing doses of (B) bortezomib, a proteasome inhibitor, or (C) chloroquine (CQ), an autophagy inhibitor, for 72 hr. Cell viability data represent the average of three or more independent experiments, ± SEM. (D) The levels of autophagy related proteins in MAL3-101 sensitive (in blue) and resistant (in black) lines were analyzed by immunoblotting in presence or absence of CQ or bafilomycin for 6 hr. Purple and red dots respectively indicate the soluble (LC3BI) and the autophagosome-associated isoform (LC3BII) of LC3B. An overexposed image is also shown to better visualize the LC3BI and LC3BII isoforms in the cell lines.
-
Figure 2—source data 1
Source data for Figure 2.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig2-data1-v3.xlsx
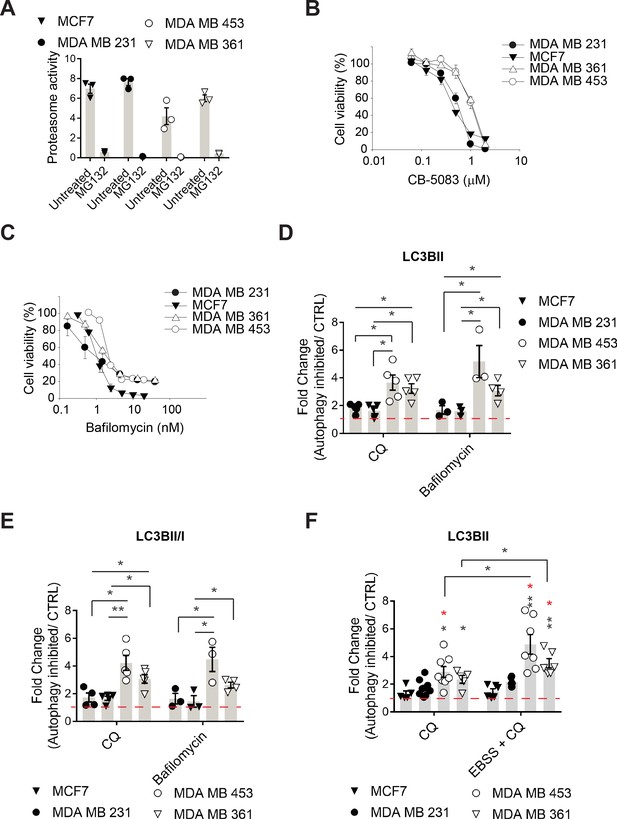
Autophagy pathway activity is higher in MAL3-101-resistant cells.
(A) Chymotrypsin-like proteasome activity was measured by monitoring AMC fluorescence after Suc-LLVY-AMC was added to cell lysates prepared from MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells. Proteasome-independent activity was established by conducting experiments in the presence of MG 132, which was negligible. Data represent averaged net proteasome activity (in pmol substrate hydrolyzed), ± SEM (n=3). (B–C) MAL3-101-sensitive (closed symbols) and resistant (open symbols) cell lines were treated with increasing doses of (B) CB-5083 (an ERAD inhibitor) or (C) bafilomycin (an autophagy inhibitor) for 72 hr, and cell viability was measured. Data represent the means of three independent experiments, ± SEM. (D–E) The levels of (D) LC3BII and (E) the LC3BII:I ratio were determined from data in Figure 2 and are expressed as the fold change relative to DMSO under the indicated conditions, ± SEM (n≥3 for bafilomycin and n≥4 for CQ). (F) LC3BII accumulation under starvation conditions (EBSS) was analyzed in MAL3-101-sensitive (closed symbols) and resistant (open symbols) cells. Black asterisks correspond to the statistical significance between MDA MB 231 cells (closed circle) and MDA MB 453 and MDA MB 361 (open circle and triangle, respectively), and red asterisks represent the statistical significance between MCF7 cells (closed triangle) and MDA MB 453 and MDA MB 361 (open circle and triangle, respectively). Data represent the fold increase relative to DMSO, ± SEM (n=3). * denotes p<0.05, ** denotes p<0.005.
-
Figure 2—figure supplement 1—source data 1
Raw data for Figure 2—figure supplement 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig2-figsupp1-data1-v3.xlsx
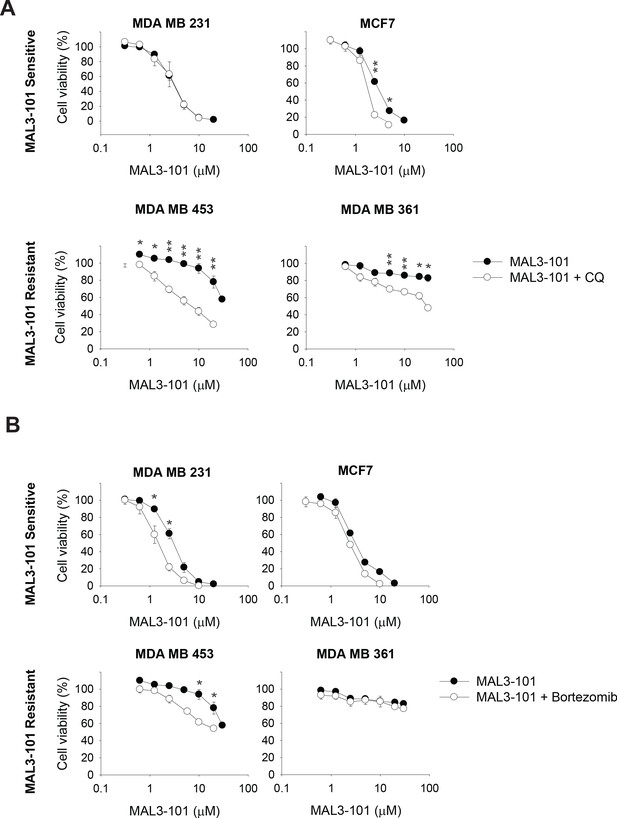
The autophagy pathway contributes to MAL3-101 resistance.
MAL3-101 sensitive and resistant lines (closed and open symbols, respectively) were treated with increasing doses of MAL3-101 in the presence or absence of a sub-lethal dose of (A) CQ or (B) bortezomib (see Table 2 and Table 3). Cell viability was detected at 72 hr, and data represent the means of three independent experiments, ± SEM. * denotes p<0.05, ** denotes p<0.005.
-
Figure 3—source data 1
Source data for cell viability assays in Figure 3.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig3-data1-v3.xlsx
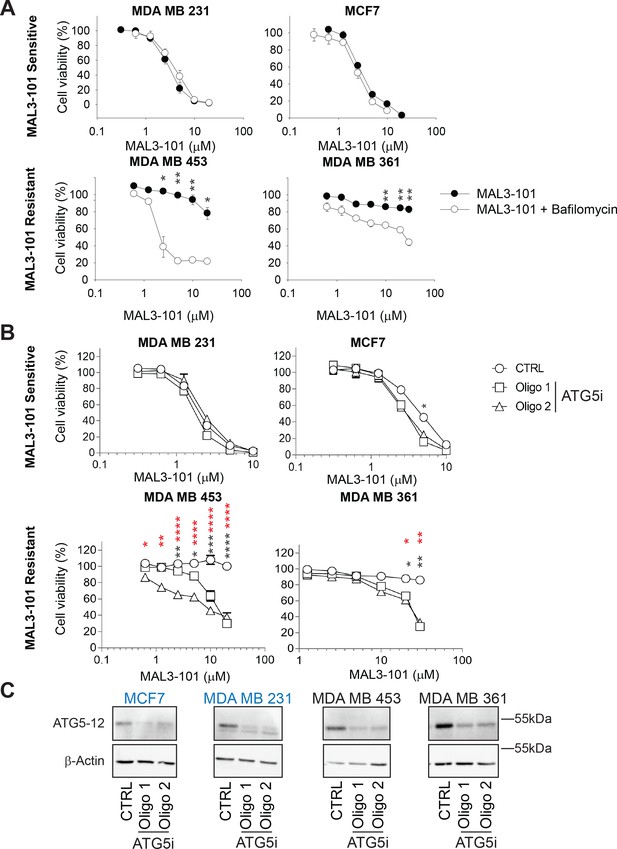
Autophagy inhibition re-sensitizes resistant cells to MAL3-101.
(A) MAL3-101 sensitive (top graphs) and resistant (bottom graphs) cells were treated with increasing doses of MAL3-101 in the presence or absence of a sub-lethal dose of bafilomycin for 72 hr (see Table 2 ), and cell viability was calculated ± SEM (n=3); * denotes p<0.05, ** denotes p<0.005. (B) MAL3-101 sensitive (top graphs) and resistant (bottom graphs) cells were transfected with a control siRNA or two different ATG5 siRNAs to block autophagy, treated with increasing doses of MAL3-101 for 48 hr, and cell viability was calculated, ± SEM (n=3). Black asterisks correspond to the statistical significance between the control (circles) and oligonucleotide 1 (squares), and red asterisks represent the statistical significance between the control (circles) and oligonucleotide 2 (triangles). * denotes p<0.05, ** denotes p<0.005, *** denotes p<0.0005 and **** denotes p<0.0001. (C) ATG5 knockdown efficiency was measured by immunoblotting ATG5-12 in MAL3-101 sensitive (in blue) and resistant (in black) cell lysates. β-actin serves as a loading control. A representative experiment (reproduced three times) is shown.
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig3-figsupp1-data1-v3.xlsx
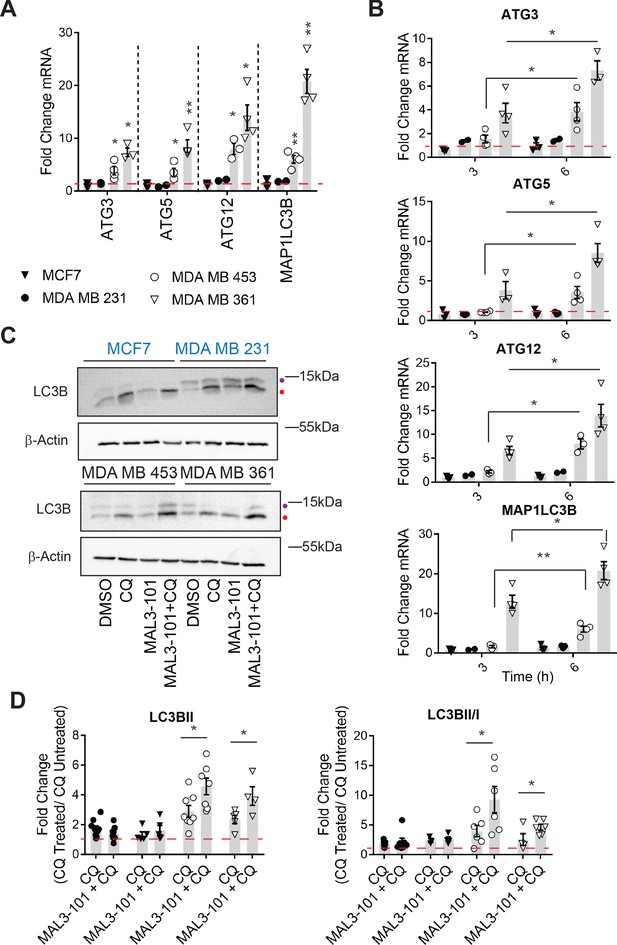
Hsp70 inhibition induces higher levels of autophagy in MAL3-101-resistant cells.
(A–B) The expression of autophagy-associated genes relative to β-actin was detected by qPCR in the presence of DMSO or 12 µM MAL3-101 for 3 and/or 6 hr in sensitive (MCF7 and MDA MB 231, closed symbols) and resistant (MDA MB 453 and MDA MB 361, open symbols) cells. The mean fold change of the indicated mRNAs is shown, ± SEM (n≥3). * denotes p<0.05, ** denotes p<0.005. (C) MAL3-101 sensitive (in blue) and resistant (in back) cells were treated for 6 hr with 12 µM MAL3-101 and CQ was added during the last 2 hr of the treatment to assess autophagic flux; that is, ‘CQ’ indicates a 4 hr treatment with DMSO plus a 2 hr acute treatment with CQ. Aliquots of cell lysates were resolved by SDS-PAGE and immunoblotted for LC3B. LC3BI is indicated by a purple dot, and the autophagosome associated form LC3BII is highlighted with a red dot. β-actin serves as a loading control. (D) The fold change in LC3BII levels and the LC3BII/LC3BI ratio between chloroquine (CQ)-treated cells relative to untreated cells was calculated, ± SEM (n≥4). Symbols are defined in panel A. * denotes p<0.05. MAL3-101-sensitive cells are represented with closed symbols, while open symbols indicate MAL3-101-resistant lines.
-
Figure 4—source data 1
Raw data for Figure 4.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig4-data1-v3.xlsx
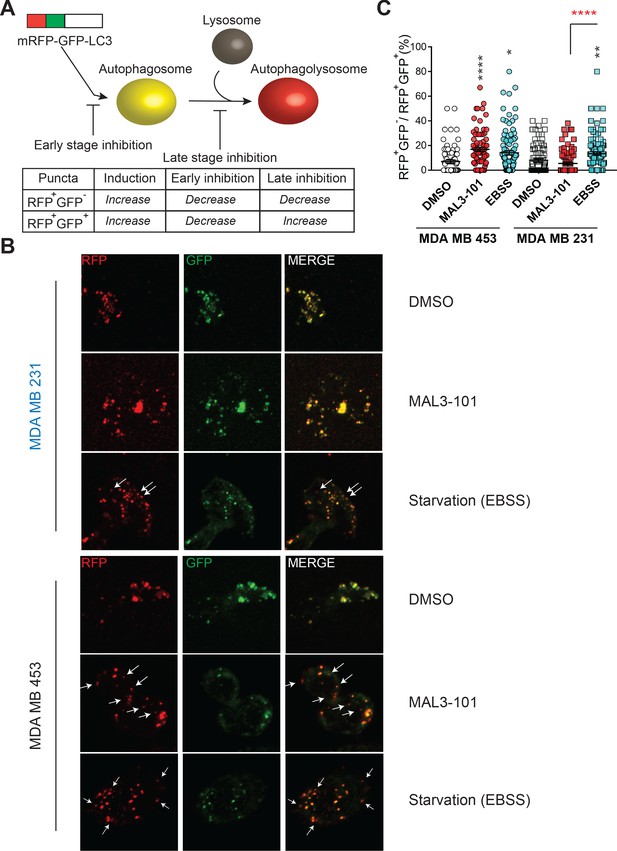
Hsp70 inhibition alters autophagic flux in MAL3-101-resistant cells.
(A) The mRFP-GFP-LC3 reporter monitors autophagic flux. RFP+GFP+ and RFP+GFP- puncta distinguish autophagosomes and autophagolysosomes, thereby allowing for measurements of different stages in the autophagy pathway. (B) Confocal images of MAL3-101 sensitive (MDA MB 231, in blue) and resistant (MDA MB 453, in black) clones stably expressing mRFP-GFP-LC3B were treated with 5 µM MAL3-101 or EBSS (starvation media), as a positive control, for 22 hr. White arrows indicate LC3B-positive (acidic) compartments (i.e. RFP+GFP- puncta). (C) The percentage of RFP+GFP- on RFP+GFP+ puncta per cell is reported for MAL3-101 sensitive (squares) and resistant (circles) lines treated as indicated in B, ± SEM (n≥80). Statistically significant differences between DMSO and cells incubated in starvation media (EBSS) or with MAL3-101 are indicated by black asterisks. Red asterisks represent the statistical significance between the MAL3-101 and EBSS samples. * denotes p<0.05, ** denotes p<0.005, and **** denotes p<0.0001.
-
Figure 5—source data 1
Fluorescent puncta quantification.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig5-data1-v3.xlsx
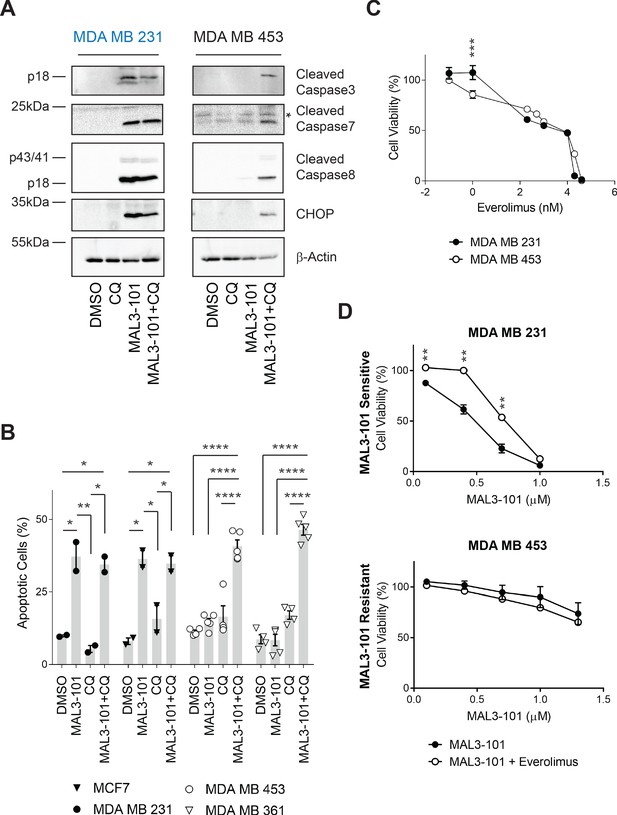
Combined inhibition of autophagy and Hsp70 induces apoptosis in MAL3-101 resistant cells.
(A) MAL3-101 sensitive (in blue) and resistant (in black) cells were treated with 12 µM MAL3-101 for 6 hr and CQ was added during the last 2 hr of the treatment to block autophagy. Lysates prepared from the treated cells were immunoblotted for cleaved caspase-3, caspase-8, and caspase-7, as well as CHOP to monitor apoptosis. β-actin serves as a loading control. * indicates a non-specific band present in the cleaved caspase-7 immunoblot. (B) Cells were treated as in panel A before aliquots were removed and stained for annexin-V and PI. The sum of the annexin-V positive and the PI and annexin-V double positive cells is represented in the graph and indicated as a percentage of apoptotic cells. MAL3-101-sensitive cells are indicated with closed symbols and MAL3-101-resistant cells are represented by open symbols. The means of independent experiments, ± SEM, are indicated (n=5 for MDA MB 453 and MDA MB 361 cells, and n=2 for MCF7 and MDA MB 231 cells, 50,000 cells were analyzed for each sample). * denotes p<0.05, ** denotes p<0.005, *** denotes p<0.0005, and **** denotes p<0.0001. (C) MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells were treated with increasing doses of everolimus (an mTOR inhibitor) for 72 hr, and cell viability was measured. Data represent the means of three or more independent experiments, ± SEM; * denotes p<0.05. (D) MAL3-101 sensitive and resistant lines (closed and open symbols, respectively) were treated with increasing doses of MAL3-101 in the presence or absence of 2 µM everolimus, as indicated. Cell viability was detected after 72 hr, and data represent the means of two or more independent experiments, ± SEM. * denotes p<0.05, ** denotes p<0.005. Log10 concentration of everolimus (C) or MAL3-101 (D) are represented on the x-axis.
-
Figure 6—source data 1
Cell viability and apoptotic cell quantification data.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig6-data1-v3.xlsx
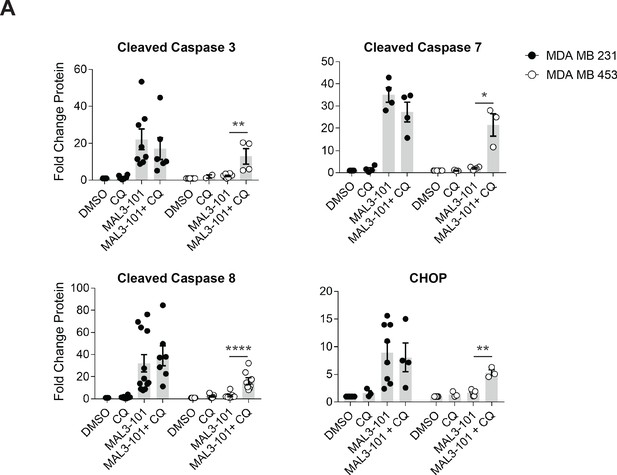
MAL3-101 induces apoptosis when autophagy is impaired in resistant cells.
The fold increase of the indicated apoptotic markers in MAL3-101 sensitive (closed circle) and resistant (open circle) cell lines relative to DMSO are plotted, ± SEM (cleaved caspase-3, n≥4; cleaved caspase-7 and CHOP, n≥3; cleaved caspase-8, n≥7). * denotes p<0.05, ** denotes p<0.005, and **** denotes p<0.0001.
-
Figure 6—figure supplement 1—source data 1
Raw data for Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig6-figsupp1-data1-v3.xlsx
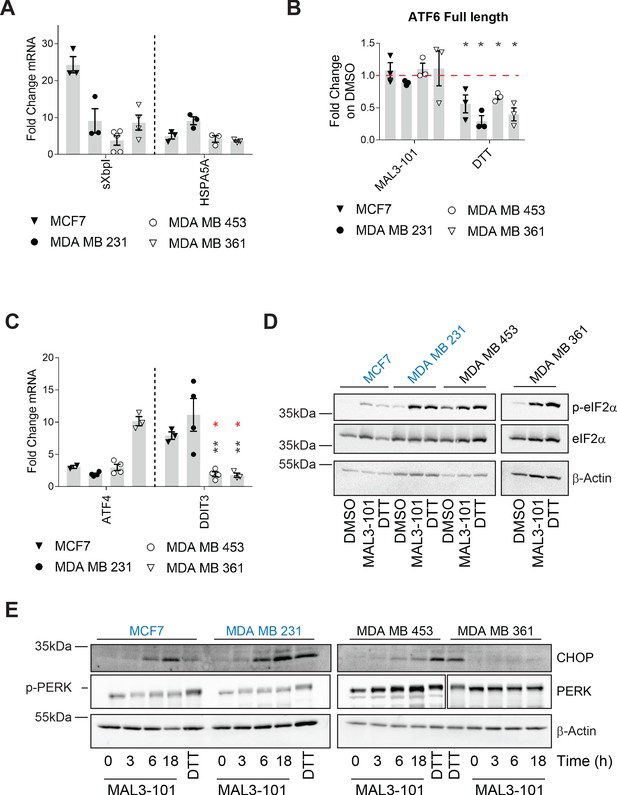
Hsp70 inhibition induces the UPR and ISR in breast cancer cells.
The relative fold change of (A) spliced Xbp1 (sXbpI) and HSPA5A (BiP) mRNA levels relative to β-actin, (B) full length ATF6 protein, and (C) the ATF4 and DDIT3 (CHOP) transcripts are shown. In part B, asterisks represent p<0.05, and in part C, black asterisks correspond to p<0.05 between the sensitive (MDA MB 231) (closed circles) and resistant (MDA MB 453 and MDA MB 361) cell lines (open symbols). Red asterisks represent p<0.05 between MCF7 (open triangles) and MDA MB 453 and MDA MB 361 cells (open symbols). In all cases, cells were treated with MAL3-101 for 6 hr. In parts A and C, the bars represent the amounts of the indicated transcripts in treated cells relative to the DMSO control. In part B, a 2 hr DTT treatment was used as a positive control. Note that the levels of induction by DTT and MAL3-101 are similar. Data depict ± SEM (n≥3). (D) Immunoblots for eIF2α and its phosphorylated isoform in MAL3-101 sensitive (in blue) and resistant (in black) cell lines treated with 12 μM MAL3-101 or DMSO for 6 hr are shown. (E) MAL3-101 sensitive (in blue) and resistant (in black) cells were treated with 12 μM MAL3-101 or DMSO for the indicated time and lysates were immunoblotted for CHOP and PERK expression. In part D and E, a 2 hr DTT treatment was used as a positive control and β-actin serves as a loading control.
-
Figure 7—source data 1
Source data for Figure 7.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig7-data1-v3.xlsx
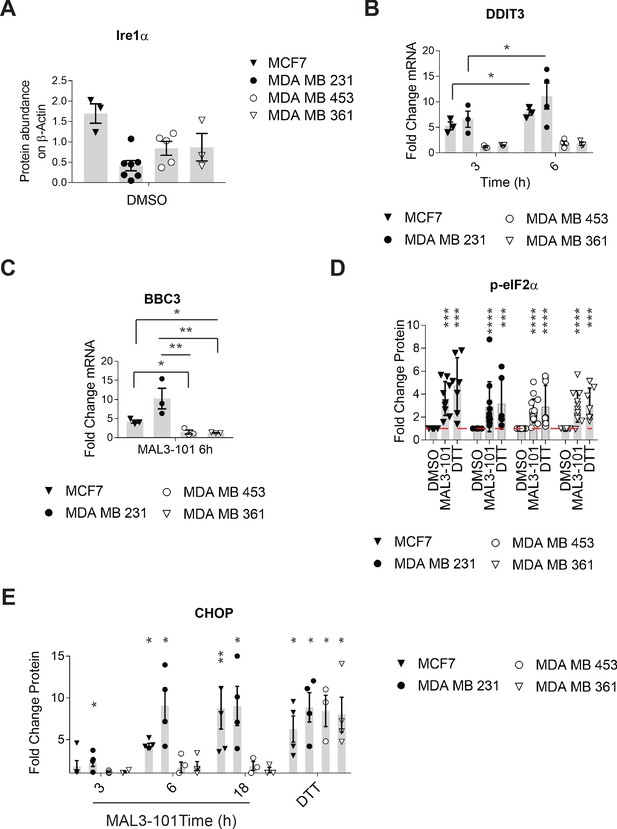
eIF2α is phosphorylated in response to MAL3-101.
(A) The relative steady state levels of the Ire1α protein in MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells are depicted, ± SEM (n≥3). (B) MAL3-101 sensitive (closed symbols) and resistant (open symbols) lines were treated for the indicated times with MAL3-101, and DDIT3 (CHOP) transcript levels were measured and are reported as the mean fold increase relative to DMSO, ± SEM (n≥3); * denotes p<0.05. (C) MAL3-101 sensitive (closed symbols) and resistant (open symbols) lines were treated for the indicated times with MAL3-101, and BBC3 (PUMA) transcript levels were measured and are reported as the mean fold increase relative to DMSO. Data depict ± SEM (n=3). * denotes p<0.05 and ** denotes p<0.005. (D) MAL3-101 sensitive (closed symbols) and resistant (open symbols) lines were treated for the indicated times with MAL3-101, and p-eIF2α proteins levels were measured and are reported as the mean fold increase relative to DMSO, ± SEM (n≥7). A 2 hr DTT treatment was used as positive control. *** denotes p<0.0005, and **** denotes p<0.0001. (E) MAL3-101 sensitive (closed symbols) and resistant (open symbols) lines were treated for the indicated times with MAL3-101, and CHOP proteins levels were measured and are reported as the mean fold increase relative to DMSO, ± SEM (n≥3). DTT treatment was again used as positive control, as above. * denotes p<0.05, ** denotes p<0.005.
-
Figure 7—figure supplement 1—source data 1
UPR marker quanficiation raw data.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig7-figsupp1-data1-v3.xlsx
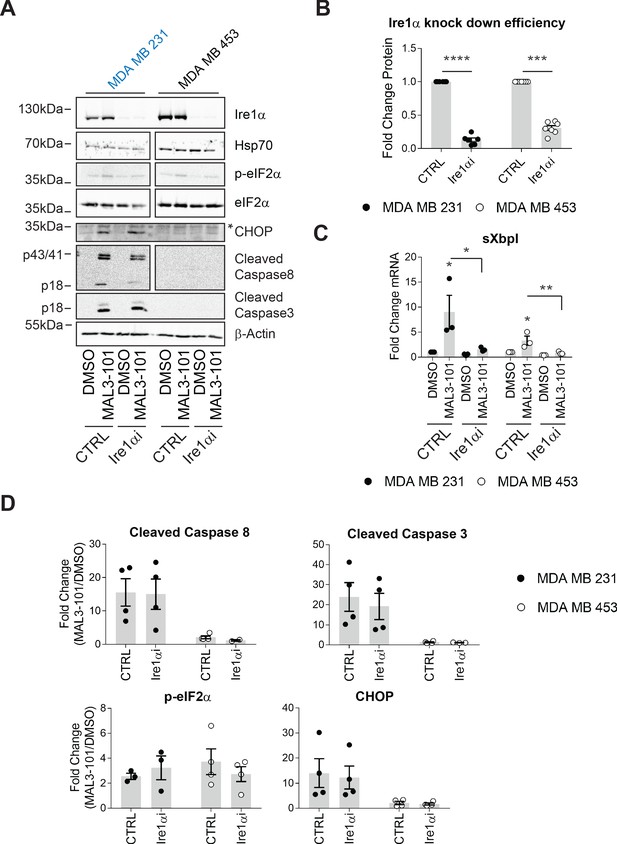
The MAL3-101 response is Ire1-independent.
(A) MAL3-101-sensitive cells (in blue) and MAL3-101-resistant cells (in black) were transfected with a control or an Ire1α-targeted siRNA. Forty-eight hr after transfection, the cells were treated with 12 μM MAL3-101 or DMSO for 6 hr, and lysates were immunoblotted for the indicated proteins. * indicates a nonspecific band in the CHOP immunoblot. β-actin served as a loading control. (B) Ire1α knockdown efficiency was measured by immunoblot in MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells and the fold decrease is shown compared to the siRNA control, ± SEM (n=7). *** denotes p<0.0005 and **** denotes p<0.00001. (C) The means, ± SEM, of the relative level of sXbpI message were calculated after qPCR in control MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells transfected with a control siRNA or an siRNA directed against Ire1α, which were then treated with 12 µM MAL3-101 or DMSO for 6 hr. * denotes p<0.05 and ** denotes p<0.005. (D) The means of the corresponding fold increase in MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells of the indicated apoptotic markers and p-eIF2α were obtained in control and Ire1α siRNA transfected cells which were then treated with MAL3-101 or DMSO for 6 hr, ± SEM (n=4).
-
Figure 8—source data 1
Source data for Figure 8.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig8-data1-v3.xlsx
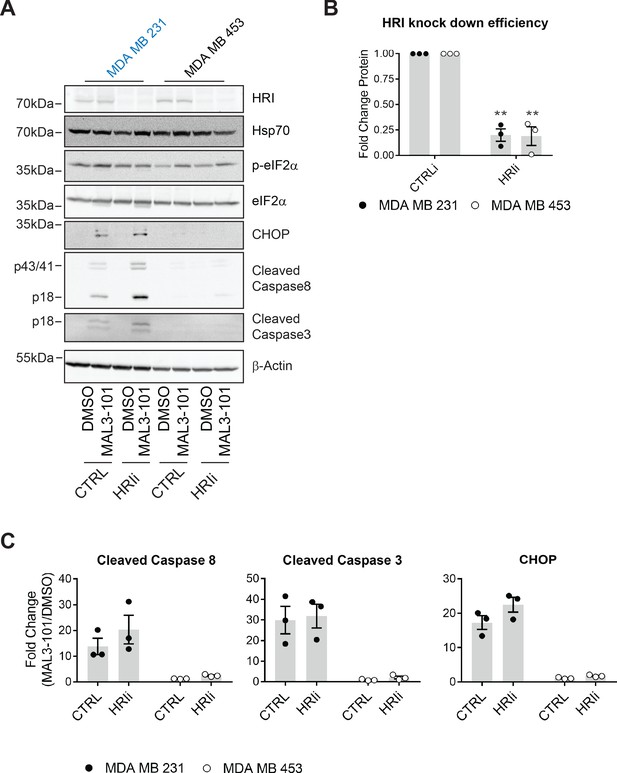
HRI is largely dispensable for the survival of breast cancer cells challenged with MAL3-101.
(A) MAL3-101 sensitive (in blue) and resistant (in black) lines were transfected with a control siRNA or an siRNA oligo directed against HRI, and 72 hr post transfection cells were treated with DMSO or 12 μM MAL3-101 for 6 hr. Lysates were prepared for immunoblot analysis. β-actin serves as a loading control. (B) HRI knockdown efficiency was measured by immunoblot 72 hr post siRNA transfection in MAL3-101 sensitive (MDA MB 231, closed circles) and resistant (MDA MB 453, open circles) cell lines. The relative fold change of HRI protein levels in knockdown to control samples is plotted ± SEM. ** denotes p<0.005. (C) The corresponding fold increase of cleaved caspase-8, cleaved caspase-3, and CHOP in MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells was measured after treatment with control, or HRI siRNAs. The fold change is shown relative to the DMSO control, ± SEM (n=3).
-
Figure 9—source data 1
Source data for Figure 9.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig9-data1-v3.xlsx
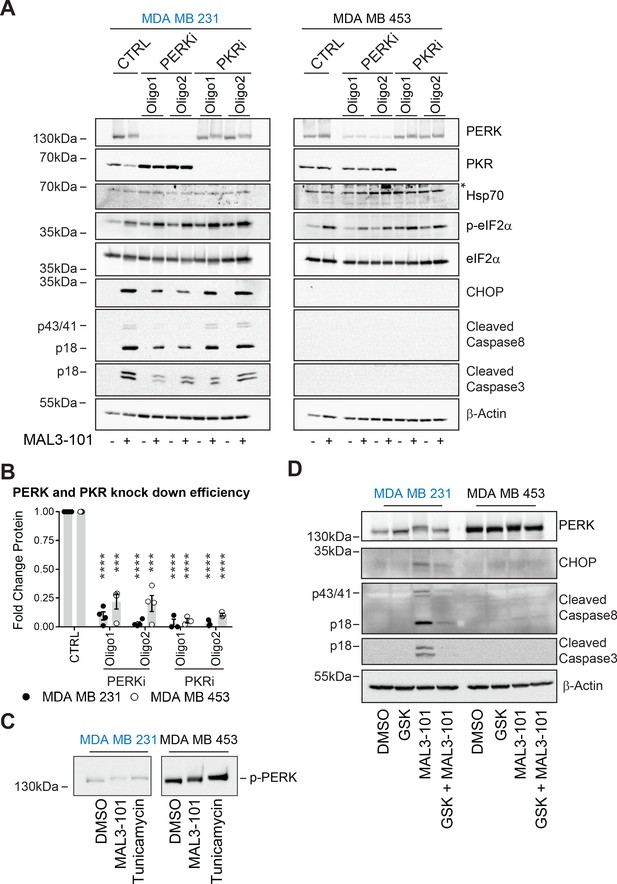
PERK is required for apoptosis induction in Hsp70 inhibitor sensitive cells.
(A) MAL3-101 sensitive (in blue) and resistant (in black) cells lines were transfected with a control siRNA or two different siRNAs that were directed against PERK or PKR. 72 hr post transfection cells were treated with DMSO or 12 μM MAL3-101 for 6 hr and lysates were prepared for immunoblot analysis. β-actin serves as a loading control, and the asterisk denotes a non-specific band in the Hsp70 blot. (B) PERK and PKR knockdown efficiency were measured by immunoblot 72 hr post siRNA transfection in MAL3-101 sensitive (MDA MB 231, closed circles) and resistant (MDA MB 453, open circles) cell lines. The relative fold change of PERK and PKR protein levels in knockdown to control samples is plotted ± SEM. *** denotes p<0.0005 and **** denotes p<0.00001. (C) MAL3-101 sensitive and resistant cells were treated with 12 μM MAL3-101, DMSO for 6 hr or with 10 μg/μl tunicamycin for 3 hr. PERK mobility was analyzed by immunoblot. (D) MAL3-101 sensitive (in blue) and resistant (in black) cells lines pre-treated with GSK-2606414 (GSK, 2 μM) for 2 hr prior to addition of DMSO or 12 μM MAL3-101 for 6 hr. Lysates were prepared for immunoblot analysis. β-actin serves as a loading control.
-
Figure 10—source data 1
PERK and PKR raw quantification.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig10-data1-v3.xlsx
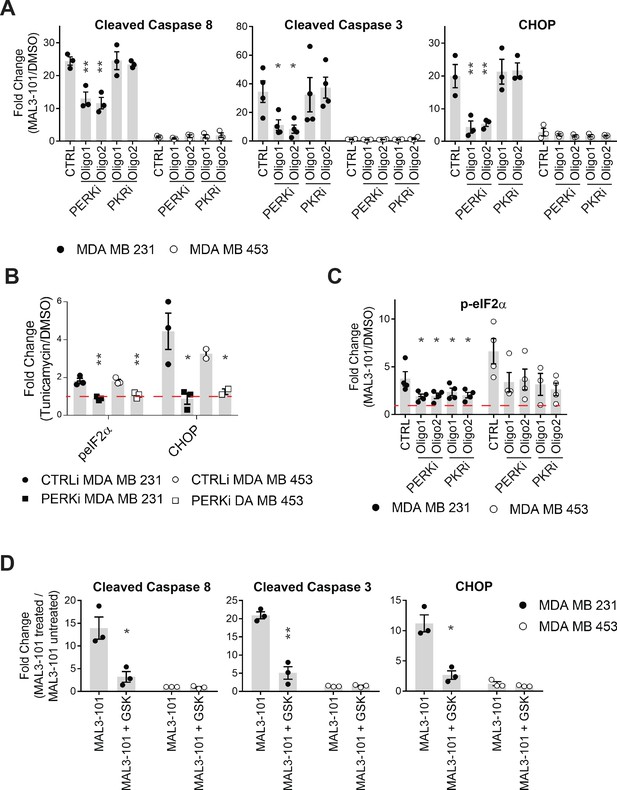
PERK knockdown reduces apoptosis.
(A–C) The corresponding fold increase of (A) cleaved caspase-8, cleaved caspase-3, CHOP and (C) p-eIF2α in the same cells were measured after treatment with control, PKR, or PERK siRNAs. The fold change is shown relative to the DMSO control, ± SEM (n=3 for CHOP and cleaved caspase-8, and n=4 for p-eIF2α and cleaved caspase-3). * denotes p<0.05, ** denotes p<0.005. (B) The fold increase of CHOP and p-eIF2α in MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells was measured after treatment with control (circles), or PERK (squares) siRNAs in the presence or absence of 10 µg/mL tunicamycin for 3 hr. The fold change is shown relative to the DMSO control, ± SEM (n=3 for p-eIF2α for both MDA MB 231 and MDA MB 453 cells and n=3 and n=2 for CHOP in MDA MB231 and MDA MB 453 cells, respectively). * denotes p<0.05, ** denotes p<0.005. (D) The corresponding fold increase of cleaved caspase-8, cleaved caspase-3, and CHOP in MAL3-101 sensitive (closed symbols) and resistant (open symbols) cells was measured after treatment with a vehicle control or GSK-2606414 (GSK) in the presence or absence of MAL3-101. The fold change is shown relative to the DMSO control, ± SEM (n=3). * denotes p<0.05, ** denotes p<0.005.
-
Figure 10—figure supplement 1—source data 1
Source data for Figure 10—figure supplement 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig10-figsupp1-data1-v3.xlsx
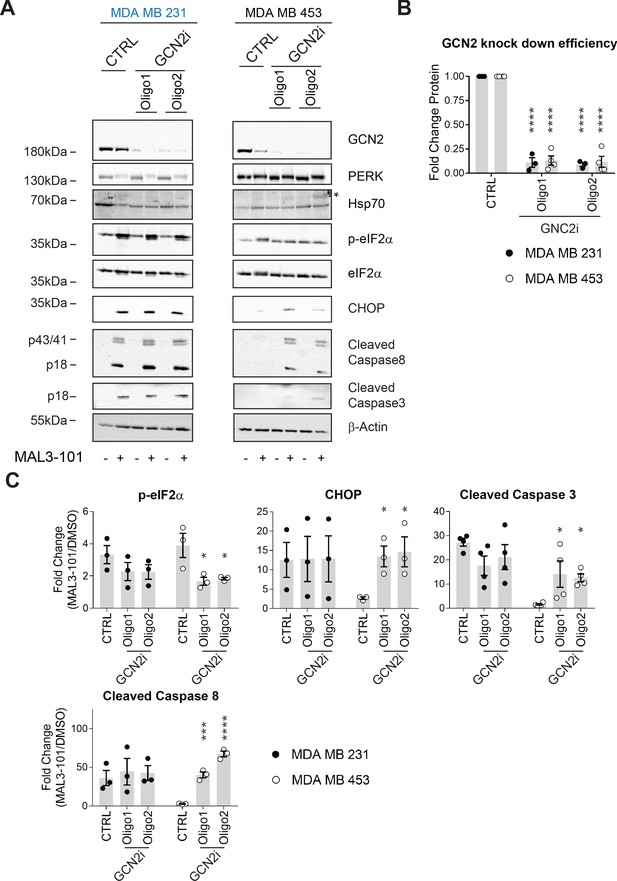
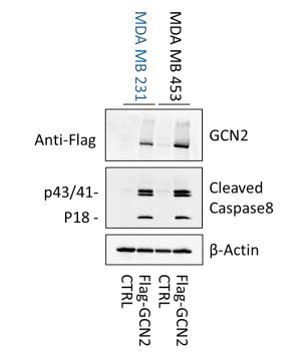
GCN2 is required for resistant cell survival when challenged with MAL3-101.
(A) MAL3-101 sensitive (MDA MB 231, in blue) and resistant (MDA MB 453, in black) cells were transfected with a control siRNA or two different siRNAs directed against GCN2, and 72 hr post transfection cells were treated with DMSO or 12 μM MAL3-101 for 6 hr. Lysates were immunoblotted to detect the indicated proteins. A non-specific band in the Hsp70 immunoblot is indicated with an asterisk (*), and β-actin served as a loading control. (B) GCN2 knockdown efficiency was measured by immunoblot 72 hr post siRNA transfection in MAL3-101-sensitive (MDA MB 231, closed circles) and MAL3-101-resistant (MDA MB 453, open circles) lines. The relative GCN2 fold change in knockdown to control samples is plotted, ± SEM (n≥3); **** denotes p<0.00001. (C) The corresponding fold increase of the indicated apoptotic markers and p-eIF2α in MAL3-101-sensitive (MDA MB 231, closed symbols) and MAL3-101-resistant (MDA MB 453, open symbols) cells treated with a control or siRNAs directed against GCN2 and treated with DMSO or MAL3-101 is shown, ± SEM (n≥3) * denotes p<0.05, *** denotes p<0.0005 and **** denotes p<0.00001.
-
Figure 11—source data 1
Source data for Figure 11.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig11-data1-v3.xlsx
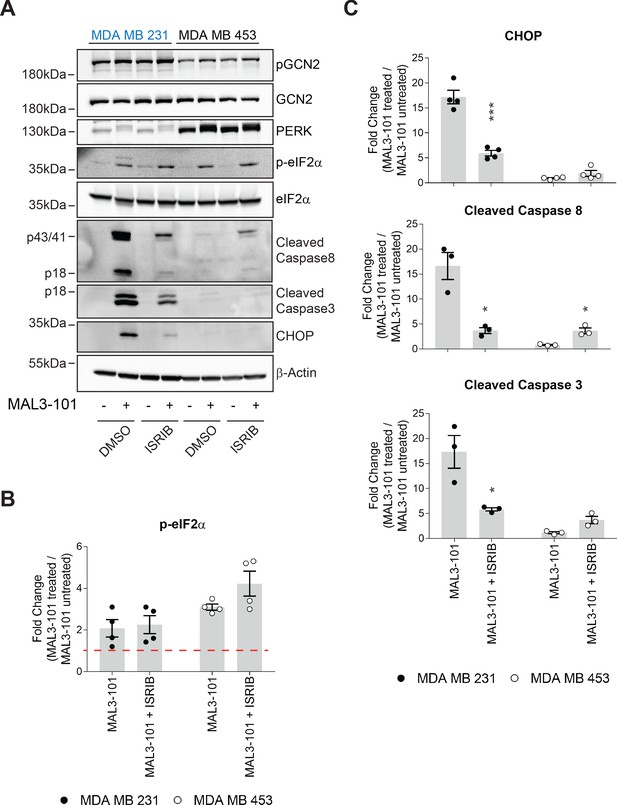
ISR orchestrates a cell survival response when Hsp70 is inhibited.
(A) MAL3-101 sensitive (MDA MB 231, in blue) and resistant (MDA MB 453, in black) cells were treated with 10 µM ISRIB for 10 hr and 15 µM MAL3-101 or DMSO was present during the last 6 hr of the treatment. Lysates prepared from the treated cells were immunoblotted to detect the indicated proteins. β-actin serves as a loading control. (B–C) The corresponding fold increase of (B) p-eIF2α and (C) cleaved caspase 3, cleaved caspase 8, and CHOP in MAL3-101-sensitive (MDA MB 231, closed symbols) and MAL3-101-resistant (MDA MB 453, open symbols) cells treated with or not with ISRIB in the presence or absence of MAL3-101 is shown, ± SEM (n=3 for cleaved caspase 3 and caspase8; n=4 for CHOP and p-eIF2α). Data represent the fold increase relative to DMSO. * denotes p<0.05, and *** denotes p<0.0005.
-
Figure 11—figure supplement 1—source data 1
Source data for Figure 11—figure supplement 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig11-figsupp1-data1-v3.xlsx
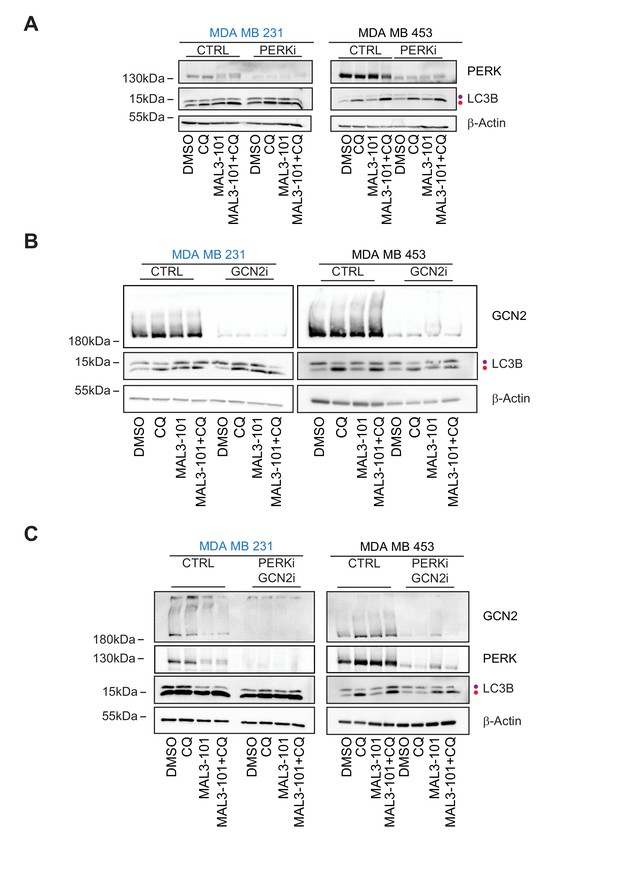
GCN2 is required to activate autophagy in MAL3-101-treated resistant cells.
(A) MAL3-101 sensitive (MDA MB 231, in blue) and resistant (MDA MB 453, in black) cells were transfected with a control siRNA or a mixture of two siRNAs against PERK, and 72 hr after transfection the cells were treated with 12 µM MAL3-101 for 6 hr and CQ was added during the last 2 hr of the treatment to block autophagy. Lysates were prepared for each condition immunoblotted for LC3B to monitor autophagy induction. (B) The same cells were used and treated as above except that a control or mixture of oligo 1 and 2 against GCN2 was used. (C) The same cells were again used and treated as above except that a control or mixture of siRNAs against both PERK and GCN2 was used. In each panel, β-actin serves as a loading control and the purple and red dot indicate the LC3BI and LC3BII isoforms, respectively.
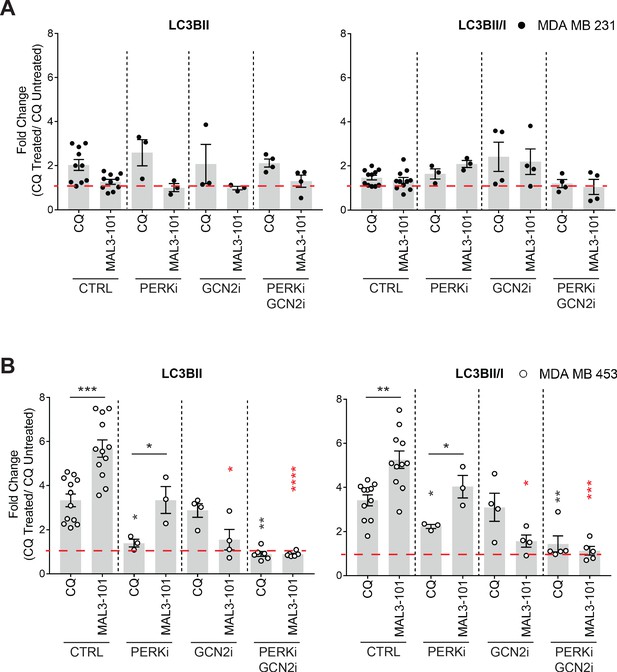
PERK and GCN2 contribute to autophagy induction in MAL3-101 resistant cells.
(A–B) LC3BII levels and the LC3BII:I ratio are reported as the fold change of CQ treated versus untreated samples, ± SEM. The accumulation of autophagy markers in control siRNA transfected cells was compared to PERK and GCN2 single knockdowns and the PERK-GCN2 double knockdown samples (n≥8 for CTRL; n=3 for PERKi, n≥3 for GCN2i, and n≥4 for GCN2i-PERKi samples). Black asterisks correspond to the statistical significance between the CQ versus the MAL3-101 ratios, and red asterisks represent the statistical significance between the MAL3-101 value in the control (CTRL) versus the GCN2i and GCN2i-PERKi cells treated with MAL3-101; * denotes p<0.05, ** denotes p<0.005, *** denotes p<0.0005 and **** denotes p<0.00001.
-
Figure 12—figure supplement 1—source data 1
LC3BII and LC3BII/I raw data for Figure 12—figure supplement 1.
- https://cdn.elifesciences.org/articles/64977/elife-64977-fig12-figsupp1-data1-v3.xlsx
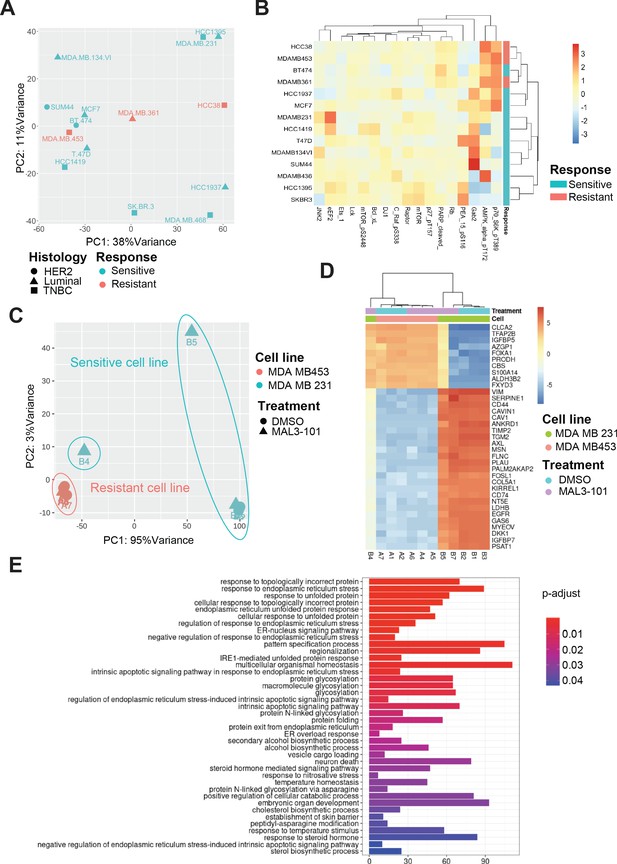
Transcriptional upregulation of the unfolded protein response is a signature of MAL3-101 sensitive cells.
(A) Principal component analysis plot of publicly available RNAseq datasets is presented for tested breast cancer cell lines. MAL3-101-sensitive cells are indicated in aqua green while resistant cell lines are indicated in light red. (B) Heatmap representation of significantly differentially expressed proteins between publicly available RPPA datasets for sensitive and resistant cells (MDA MB 231 and MDA MB 453). Wilcoxon signed-rank test between resistant and sensitive cell lines was used to determine proteins that were differentially expressed. p<0.1 was considered significant. (C) Principal component analysis plot of RNAseq performed on MAL3-101 treated (Samples A4, A5, A6, B4, B5, B7) or DMSO (Samples A1, A2, A7, B1, B2, B3) treated sensitive and resistant cells. MDA MB 453 cells (MAL3-101 resistant, sample A) are represented in light red and MDA MB 231 cells (MAL3-101 sensitive, sample B) in aqua green. (D) The top 35 differentially expressed genes detected in MAL3-101 treated and untreated cell lines (MDA MB 231 in green and MDA MB 453 in light red) are represented in a heatmap graph. (E) Gene Ontology enrichment plot of Biological Processes using significantly differential genes in MDA MB 231 cell lines with and without MAL3-101 treatment. p<0.05 was considered significant.
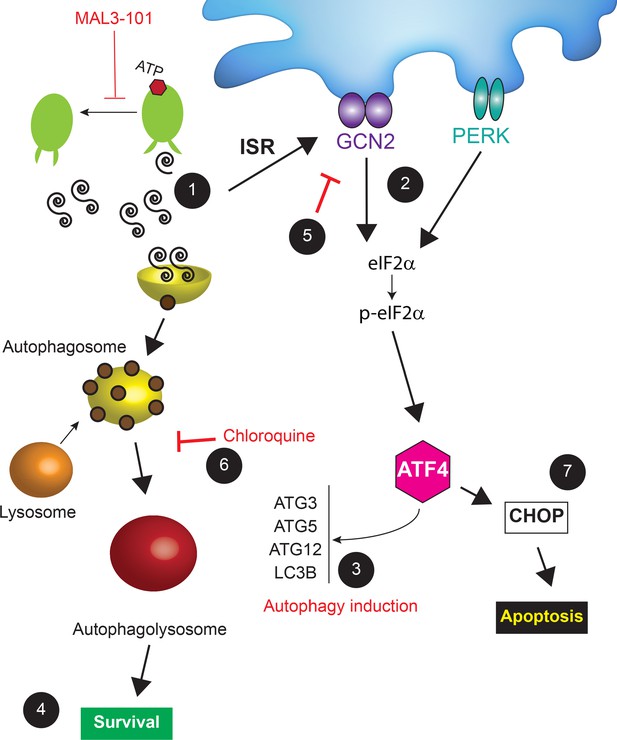
A model for cancer cell adaptation to Hsp70 inhibition.
(1) In MAL3-101 resistant and sensitive cells, Hsp70 inhibition lead to activation of the ISR due to the accumulation of misfolded proteins (only misfolded proteins in the cytoplasm are shown). (2) GCN2-mediated eIF2α phosphorylation, which can be induced by amino acid starvation (see Discussion), also induces (3) the transcription of autophagy-related genes, favoring autophagy induction and (4) protecting MAL3-101-resistant breast cancer cells. (5) If GCN2 activity is impaired or (6) autophagy is inhibited (for example by chloroquine), Hsp70 inhibition is cytotoxic so (7) CHOP accumulates and resistant cells undergo apoptosis. (7) In contrast, in MAL3-101-sensitive cells, PERK induces ATF4 and CHOP, which results in cell death.
Tables
Breast cancer cells exhibit a range of sensitivities to MAL3-101, a specific Hsp70 inhibitor.
The indicated breast cancer lines were seeded into 96-well plates and treated with increasing doses of the indicated compounds for 72 hr. Viability was measured using the CellTiter-Glo assay. IC50 values were generate using a sigmoidal nonlinear regression with SigmaPlot 11.0. ND stands for an undetermined value. MAL3-101 sensitivities of Hsp70 inhibitor resistant cells are in bold.
| Breast cancer subtype | Cell line | MAL3-101 | CQ | Bafilomycin | Bortezomib | CB-5083 | 17-AAG | VER155008 |
|---|---|---|---|---|---|---|---|---|
| TNBC | MDA MB 231 | 3.3 µM | 40 µM | 2.0 nM | 5.9 nM | 0.6 µM | 1.3 µM | 18.3 µM |
| MDA MB 468 | 3.1 µM | 30 µM | 2.0 nM | 5.2 nM | 0.8 µM | 1.5 µM | >100 µM | |
| HCC1937 | 7.5 µM | 42 µM | 5.9 nM | 5.0 nM | 1.0 µM | 5.6 nM | ND | |
| HCC1395 | 11.6 µM | 16 µM | 3.0 nM | 9.2 nM | 0.3 µM | ND | ND | |
| HCC38 | >30 µM | 60 µM | 2.7 nM | 6.0 nM | 0.5 µM | 17.6 nM | >100 µM | |
| MDA MB 453 | >30 µM | 23 µM | 2.0 nM | 4.3 nM | 1.1 µM | 13.5 nM | >100 µM | |
| Luminal | MDA MB 134 | 1.9 µM | 18 µM | 8.2 nM | 3.7 nM | ND | ND | ND |
| MCF7 | 3.0 µM | 10 µM | 1.0 nM | 40.0 nM | 0.5 µM | 63 nM | >100 µM | |
| T47D | 6.6 µM | 18 µM | 2.7 nM | 9.8 nM | ND | 1.3 µM | >100 µM | |
| BT-474 | 4.0 µM | 12 µM | 2.5 nM | 16.3 nM | ND | 15.8 nM | >100 µM | |
| SUM44-PE | 13.5 µM | 42 µM | 1.5 nM | 16.0 nM | ND | ND | ND | |
| MDA MB 361 | >30 µM | 13 µM | 1.7 nM | 13.0 nM | 1.0 µM | 25.8 nM | >100 µM | |
| HER2 | SKBR3 | 4.5 µM | 28 µM | 2.0 nM | 4.4 nM | 0.6 µM | 18.5 nM | >100 µM |
| HCC1419 | 8.2 µM | 34 µM | 1.5 nM | 7.8 nM | 0.8 µM | 54.5 nM | 100 µM |
The cell numbers and autophagy or proteasome inhibitor concentrations used for the cell viability assay in combination with increasing doses of MAL3-101 are shown.
The concentrations of bortezomib, CQ, and bafilomycin to induce no greater than 30% of cell death in each line after 72 hr treatment are shown. ND stands for undetermined value.
| Breast cancer subtype | Cell line | Number of cells/well | Everolimus | CQ | Bafilomycin | Bortezomib |
|---|---|---|---|---|---|---|
| TNBC | MDA MB 231 | 3000 | 2.0 µM | 14 µM | 0.8 nM | 4.0 nM |
| MDA MB 468 | 2500 | ND | 14 µM | 0.6 nM | 2.7 nM | |
| HCC1937 | 2000 | ND | 21 µM | 2.2 nM | 2.0 nM | |
| HCC1395 | 3000 | ND | 5.0 µM | 1.6 nM | 3.5 nM | |
| HCC38 | 3000 | ND | 33 µM | 1.0 nM | 3.0 nM | |
| MDA MB 453 | 3000 | 2.0 µM | 12 µM | 1.0 nM | 2.5 nM | |
| Luminal | MDA MB 134 | 5000 | ND | 15 µM | 6.6 nM | 2.5 nM |
| MCF7 | 1000 | ND | 7 µM | 0.5 nM | 13.0 nM | |
| T47D | 2000 | ND | 9.5 µM | 1.3 nM | ND | |
| BT-474 | 3000 | ND | 6.0 µM | 1.4 nM | 10.2 nM | |
| SUM44-PE | 8000 | ND | 25 µM | ND | 7.0 nM | |
| MDA MB 361 | 4500 | ND | 8.0 µM | 1.0 nM | 6.5 nM | |
| HER2 | SKBR3 | 2500 | ND | 13 µM | 1.0 nM | 2.5 nM |
| HCC1419 | 4000 | ND | 12 µM | 0.8 nM | 2.5 nM |
Breast cancer cells exhibit a range of sensitivities to MAL3-101 in the presence of either autophagy or proteasome inhibitors.
Cells were seeded into 96-well plates and treated with increasing doses of MAL3-101 in the presence or absence of subcritical doses of bortezomib (proteasome inhibitor), or CQ or bafilomycin (autophagy inhibitors) for 72 hr. Viability was measured using the CellTiter-Glo assay. IC50 values were generate using a sigmoidal nonlinear regression with SigmaPlot 11.0. ND stands for undetermined value. MAL3-101 resistant cells are highlighted in yellow.
| Breast cancer subtype | Cell line | MAL3-101 | MAL3-101 + CQ | MAL3-101 + Bafilomycin | MAL3-101 + Bortezomib | MAL3-101+ Everolimus |
|---|---|---|---|---|---|---|
| TNBC | MDA MB 231 | 3.3 µM | 3.3 µM | 3.4 µM | 1.5 µM | 5.1 µM |
| MDA MB 468 | 3.1 µM | 3.0 µM | 3.2 µM | 1.8 µM | ND | |
| HCC1937 | 7.5 µM | 5.1 µM | 6.4 µM | 7.1 µM | ND | |
| HCC1395 | 11.6 µM | 5.8 µM | 3.9 µM | 10.6 µM | ND | |
| HCC38 | >30 µM | 17.0 µM | 20.0 µM | >30 µM | ND | |
| MDA MB 453 | >30 µM | 10.0 µM | 6.1 µM | >30 µM | >30 µM | |
| Luminal | MDA MB 134 | 1.9 µM | 2.1 µM | 1.9 µM | 1.9 µM | ND |
| MCF7 | 3.0 µM | 1.9 µM | 2.7 µM | 3.0 µM | ND | |
| T47D | 6.6 µM | 3.1 µM | 4.2 µM | ND | ND | |
| BT-474 | 4.0 µM | 2.0 µM | 2.0 µM | 6.2 µM | ND | |
| SUM44-PE | 13.5 µM | 4.5 µM | ND | 8.0 µM | ND | |
| MDA MB 361 | >30 µM | 26 µM | 30.0 µM | >30 µM | ND | |
| HER2 | SKBR3 | 4.5 µM | 1.3 µM | 2.3 µM | 2.6 µM | ND |
| HCC1419 | 8.2 µM | 2.9 µM | 3.4 µM | 5.5 µM | ND |