A conserved strategy for inducing appendage regeneration in moon jellyfish, Drosophila, and mice
Figures
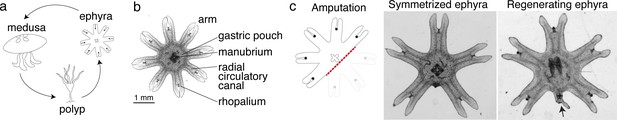
Aurelia as a system to identify factors that promote appendage regeneration.
(a) The moon jellyfish Aurelia aurita has a dimorphic life cycle, existing as sessile polyps or free-swimming medusae and ephyrae. Ephyra is the juvenile stage of medusa, a robust stage that can withstand months of starvation. In lab conditions, ephyrae mature into medusae, growing bell tissue, and reproductive organs, in 1–2 months. (b) Ephyrae have eight arms, which are swimming appendages that contract synchronously to generate axisymmetric fluid flow, which facilitates propulsion and filter feeding. The eight arms are symmetrically positioned around the stomach and the feeding organ manubrium. Extending into each arm is radial muscle (shown in Figure 2) and a circulatory canal that transports nutrients. At the end of each arm is the light- and gravity-sensing organ rhopalium. (c) In response to injury, the majority of ephyrae rapidly reorganize existing body parts and regain radial symmetry. However, performing the experiment in the natural habitat, a few ephyrae (2 out of 18) regenerated a small arm (arrow).
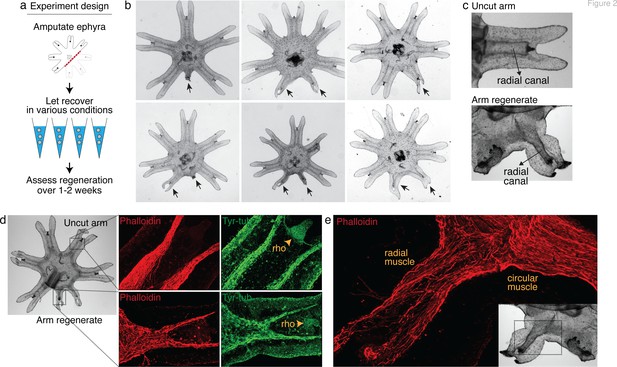
Arm regeneration in Aurelia ephyra can be induced using exogenous factors.
(a) Ephyrae were amputated (red line) across the body to remove three arms, and then let recover in various conditions. Figure 2—source data 1 tabulates the factors tested in the screen. Regeneration was assessed over 1–2 weeks until bell tissues began developing between the arms and obscured scoring. The ephyrae shown are from high-nutrient condition (see Figure 3). (b) Arm regeneration (arrows; from high food condition, see Figure 3a). (c) Radial circulatory canal in an uncut arm and is reformed in an arm regenerate. (d) Muscle (red), as indicated by phalloidin staining, and neuronal networks (green), as indicated by antibody against tyrosinated tubulin. The orange arrows indicate distal enrichment of tyrosinated-tubulin staining, which marks the sensory organ rhopalium (rho). Twenty ephyrae were examined and representative images are shown. (e) Higher magnification of the phalloidin staining shows the striated morphology of the regrown muscle in the arm regenerate (called radial muscle), which extends seamlessly from circular muscle in the body.
-
Figure 2—source data 1
This spreadsheet details the factors screened in Aurelia, rationale for screening the factors, targets of the molecular modulators tested, doses or parameters tested, estimate number of ephyrae tested, and relevant references.
- https://cdn.elifesciences.org/articles/65092/elife-65092-fig2-data1-v4.xlsx
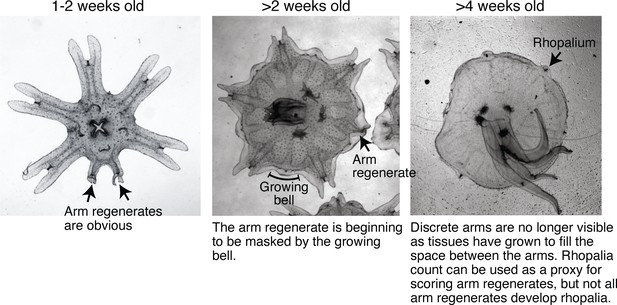
Bell growth limited the time window for assessing arm regeneration.
Ephyrae in the lab mature into full-belled medusae within ~ 4 weeks. The transition to medusa commences at 1–2 weeks after strobilation, with the onset of bell growth. Over 2–3 weeks, body tissues gradually grow and fill between the discrete arms to form a continuous bell characteristic of a medusa. Arm regeneration can be unambiguously scored in ephyrae before the bell has significantly grown. Bell growth also limited testable doses in some factors, e.g.for example, testing higher food amounts than reported here led to accelerated bell growth at a rate that did not allow enough time window to quantify regeneration.
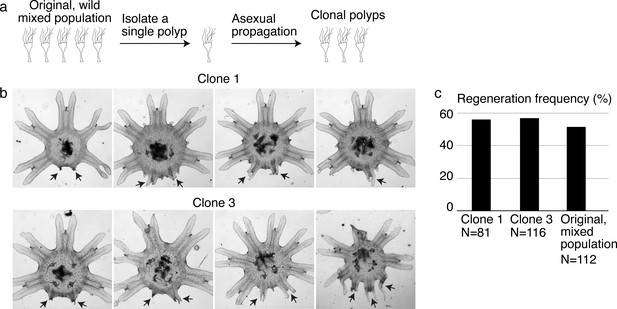
Variable extent of regeneration was observed in clonal lines.
(a) To develop genetically clonal lines, single polyps were isolated and settled onto tissue culture dishes. Within 1–3 months, with daily feeding of enriched brine shrimps, each dish was re-populated with polyps asexually budding from the single parental polyp. (b) Regeneration induction with high food was performed in two clonal lines. Arrows indicate arm regenerates. (c) Regeneration frequency in the clonal and original mixed populations was measured in the same experiment. Experiments in the main text were performed in clone 3.
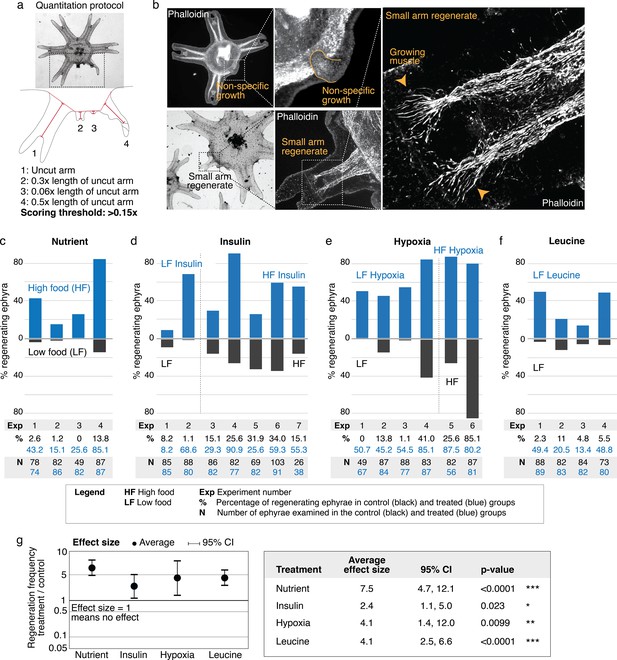
Nutrient level, insulin, hypoxia, and leucine increased regeneration frequency in Aurelia.
(a) An ephyra is regenerating if it has at least one growth from the cut site with a length greater than 0.15 of the uncut arm length. The uncut arm length was determined in each ephyra by measuring three uncut arms and taking the average. Lappets, the distal paired flaps, were excluded in the length measurement because their shapes tend to vary across ephyrae. The measurements were performed in ImageJ. (b) The threshold 0.15 was chosen to balance excluding non-specific growths that show no morphological structures (e.g., as shown, lack of phalloidin-stained structures) and retaining rudimentary arms that show morphological structures, including radial muscle sometime with growing ends (shown, phalloidin stained). (c–f) In each experiment, treated (blue) and control (gray) ephyrae came from the same strobilation. (c) Regeneration frequency in lower amount of food (LF) and higher amount of food (HF). The designation ‘high’ and ‘low’ is for simplicity, and does not presume the nutrient level in the wild. If we were to speculate, the LF amount is likely closer to typical nutrient level in the wild, based on two lines of evidence. First, regeneration frequency in LF is comparable to that observed in the natural habitat experiment. Second, in many of the wild populations studied, ephyrae mature to medusae over 1–3 months (Lucas, 2001), comparable to the growth rate in LF (by contrast, ephyrae in HF mature to medusae over 3–4 weeks). (d) Regeneration frequency in 500 nM insulin. (e) Regeneration frequency in ASW with reduced oxygen. (f) Ephyrae recovering in low food, with or without 100 mM L-leucine. (g) The effect size of a treatment was computed from the ratio between regeneration frequency in treated and control group within an experiment, that is, the metric risk ratio RR; RR=1 means the treatment has no effect (Borenstein et al., 2009). The statistical significance and reproducibility of a treatment were assessed by analyzing the effect size across experiments using the meta-analysis package, metafor (Viechtbauer, 2010), in R with statistical coefficients based on normal distribution. See Materials and methods for details. A treatment was deemed reproducible if the 95% confidence intervals (95% CIs) of RR exclude 1. The p-value evaluates the null hypothesis that the estimate RR is 1. Reproducibility and statistical significance of each treatment were verified using another common size effect metric, odds ratio (Figure 3—figure supplement 3).
-
Figure 3—source code 1
R codes.
- https://cdn.elifesciences.org/articles/65092/elife-65092-fig3-code1-v4.zip
-
Figure 3—source data 1
This spreadsheet contains the raw data of Figure 3 and its figure supplements.
- https://cdn.elifesciences.org/articles/65092/elife-65092-fig3-data1-v4.xlsx
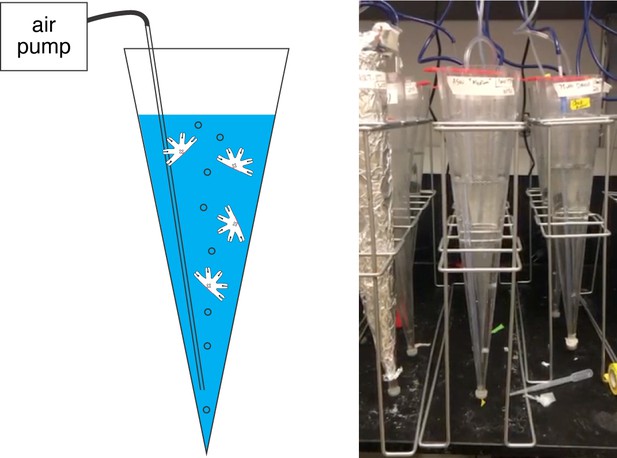
Water current is a permissive requirement for arm regeneration induction.
Various physical environments for the ephyrae recovering from injury were tested, for example, shallow versus deep water, seawater with varying salinity, cold versus warm temperature, light versus dark, stagnant water versus current, generating water current through various means, including shaking or rotating to generate turbulent mixing and as shown here air bubbling a conical tube to generate vertical current (shown here). While symmetrization occurred robustly in all conditions, consistent induction of regeneration only occurred in the presence of columnar water current. The experiments presented in this study were performed in the ‘bubbler cone‘ setup, where a 1 L sand settling cone was repurposed into an aquarium and connected to an air pump (Tetra Whisper 100) to generate a gentle current of ~1 bubble/s (Video 2). Each cone housed 30 ephyrae in 500 mL ASW or treated ASW, refreshed weekly, to avoid crowding and fouling. In the bubbler cone, ephyrae continually move along water current, either the upward bubble-generated current or the downward gravity-generated current. The conical geometry helps to minimize stagnant spots, where the ephyrae could get stuck.

Conservation of insulin receptor and HIFα in Aurelia.
Phylogenies of insulin receptor (a) and HIFα (b) genes were constructed using the maximum likelihood inference computed with the IQ-TREE stochastic algorithm (Nguyen et al., 2015), and visualized using ITOL (https://itol.embl.de/upload.cgi). These simple trees are not meant to be comprehensive, but a verification of the genes annotated as insulin-like protein receptor (ILPR) and HIFα in the Aurelia gene models by testing conservation with their known counterparts in other organisms. IQ-TREE parameters: Insulin receptor consensus tree is constructed from 1000 bootstrap trees; log-likelihood of consensus tree is –45,374.0; the Robinson-Foulds distance between ML and consensus tree is 0. HIFα consensus tree is constructed from 1000 bootstrap trees; log-likelihood of consensus tree is –24,414.4; the Robinson-Foulds distance between ML and consensus tree is 0.
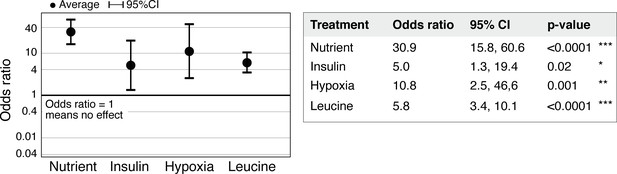
Statistical significance of regeneration induction in Aurelia assessed using odds ratio (OR).
In addition to RR analysis presented in Figure 3g, another common measure of effect size is the OR (Borenstein et al., 2009). OR compares the odds of outcome in the presence versus absence of treatment (Materials and methods). Analysis of OR across experiments was performed using the metafor package (Viechtbauer, 2010) in R with statistical coefficients based on normal distribution (Materials and methods). A treatment is reproducible if the 95% confidence intervals (95% CIs) exclude 1. The p-value evaluates the null hypothesis that the estimate OR is 1.
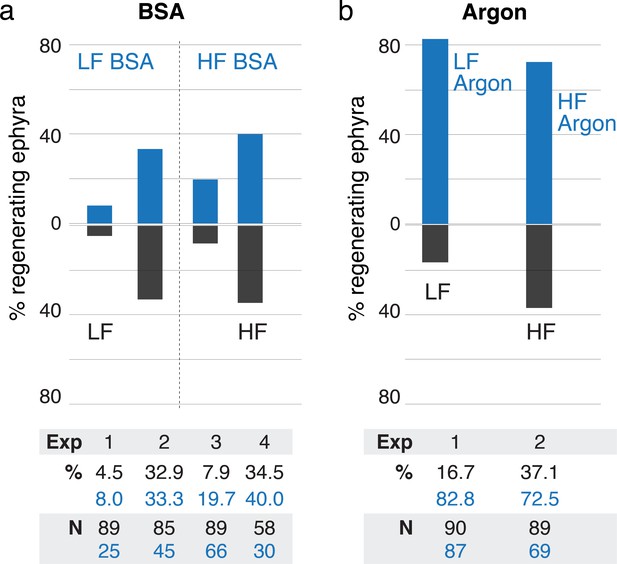
Treatments using bovine serum albumin (BSA) and argon.
(a) Treatment with 500 nM BSA did not produce significant effect in regeneration frequency (95% CI [0.9, 1.9-fold change], p=0.20). (b) Reducing oxygen using argon flow increased regeneration frequency (95% CI [1.99, 3.3-fold change], p<10–4). Effect size was computed using the metric risk ratio (see Materials and methods). LF is low food, HF is high food, Exp is Experiment ID, % is percentage of regenerating ephyra in control (black) and treated (blue), and N is the number of ephyrae in the control (black) and treated (blue) group.
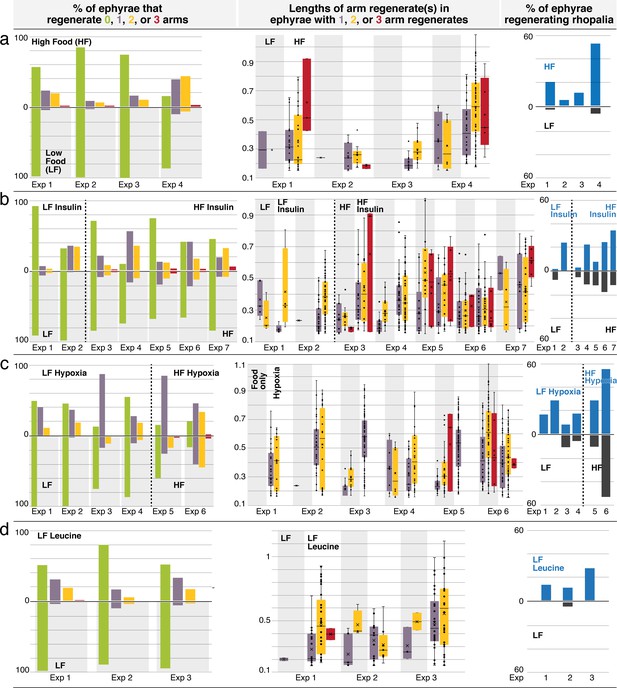
Regeneration phenotypes.
(a) In high amount of nutrients, (b) insulin, (c) hypoxia, and (d) L-leucine. For each treatment. Left: The percentage of ephyrae that regenerates zero (green), one (purple), two (yellow), or three arms (red). Middle: The length(s) of arm regenerate(s) in ephyrae that regenerate one arm (purple), two arms (yellow), and three arms (red)—normalized to the average length of uncut arms in the same ephyra. For ephyrae with multiple arm regenerates, lengths of all arms were measured and plotted individually. Boxplot: median (line), average (cross), first and third quartiles (the box), 5th and 95th percentile (whiskers), and individual data points (black circles). Right: Percentage of ephyrae that reform rhopalia in control (gray) and treated (blue) groups.
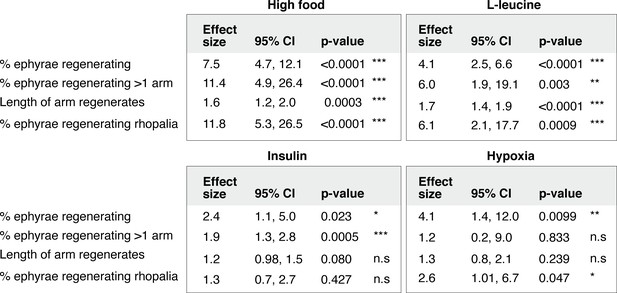
Statistical analysis of the regeneration phenotypes in high amount of nutrients, insulin, hypoxia, and L-leucine.
For frequency measurements, the effect size of a treatment compares the probability of an outcome in treated versus control group (i.e., risk ratio, Materials and methods). For length measurement, the effect size of a treatment compares the proportionate change that results from the treatment (i.e., response ratio, Materials and methods). Analysis of effect size across experiments was performed using the metafor package15 in R with statistical coefficients based on normal distribution (Materials and methods). A treatment is reproducible if the 95% confidence intervals (95% CIs) exclude 1. The p-value evaluates the null hypothesis that the estimate effect size is 1 (i.e., no effect).
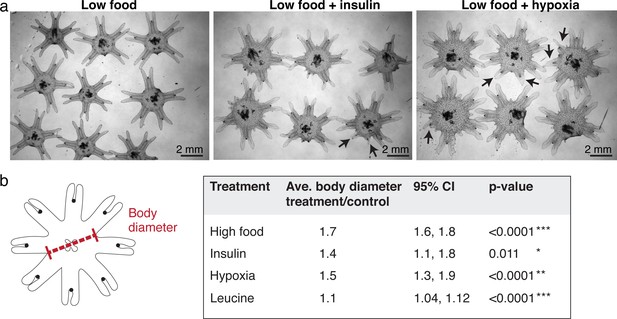
Ephyrae in high food, insulin, or hypoxia, and L-leucine tend to be bigger in size.
(a) Representative images of ephyrae growing in low food, 500 nM insulin, and hypoxia. Black arrows indicate regenerating arms. (b) Effect size analysis of the body size increase was performed using the metafor package (Viechtbauer, 2010) in R (Materials and methods). A treatment is reproducible if the 95% confidence intervals (95% CIs) exclude 1. The p-value evaluates the hypothesis that there is no effect.
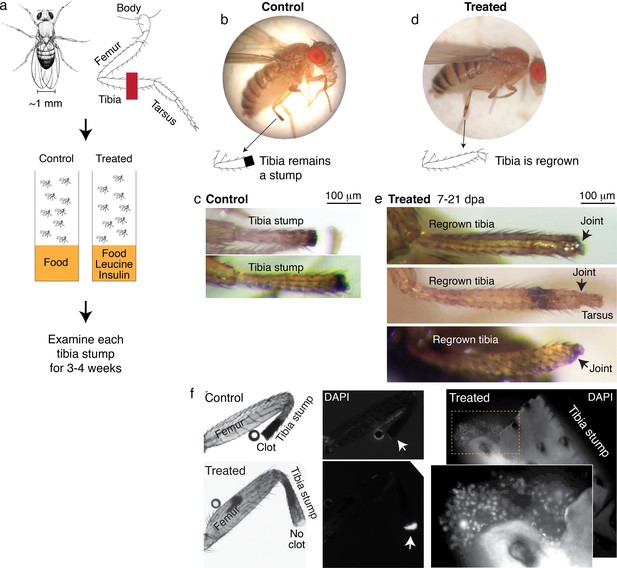
Leucine and insulin induced regeneration in Drosophila limb.
(a) Adult Drosophila have jointed limbs, with rigid segments connected by flexible joints. Amputation was performed across the tibial segment. After amputation, flies were housed in vials containing standard lab food (control) or standard lab food added with 1.7 mM L-Leucine, 1.7 mM L-Glutamine, and 33 μg/ml insulin (treated). Doses were determined through observing the highest order of magnitude dose of amino acid that could be fed to flies over a prolonged period without shortening their lifespan. The flies were examined at ~1, 3, 7, 14, and 21 days post-amputation (dpa). (b) A control fly, imaged at 7 dpa. (c) Control tibia stumps, imaged at 1–3 dpa. (d) A treated fly, imaged at 7 dpa. (e) At 7–21 dpa, regrown tibias, which culminate in joints, were observed in the treated population. (f) Tibia stumps at 3–14 dpa were dissected, fixed, and mounted in Vectashield mounting medium with DAPI. Samples from 14 dpa are shown here. Insect cuticle is not dissected to restrict DAPI penetrance only to the distal tip. Clotted tips of control tibia stumps did not stain with DAPI (10 out of 10), whereas unclotted tips of treated tibia stumps were stained with DAPI (14 out of 16). Higher-resolution confocal image of an unclotted treated tibia stump at 14 dpa.
Drosophila drawing in (a) by Ashley Smart and used with permission.
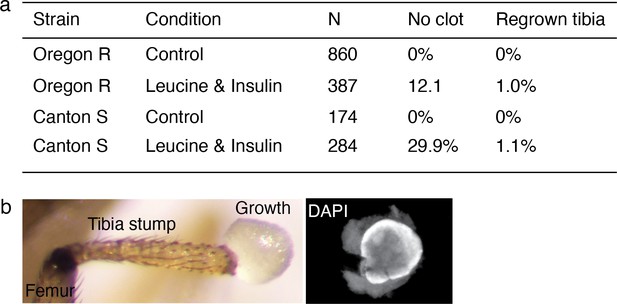
Regenerative response induced in Drosophila was observed across wild-type strain.
(a) Summary of the bulk experiment data in Oregon R and Canton S wild-type strains. (b) Few flies showed non-patterned outgrowth from the amputation site (3 out of 387 in Oregon R, 9 out of 284 in Canton S).
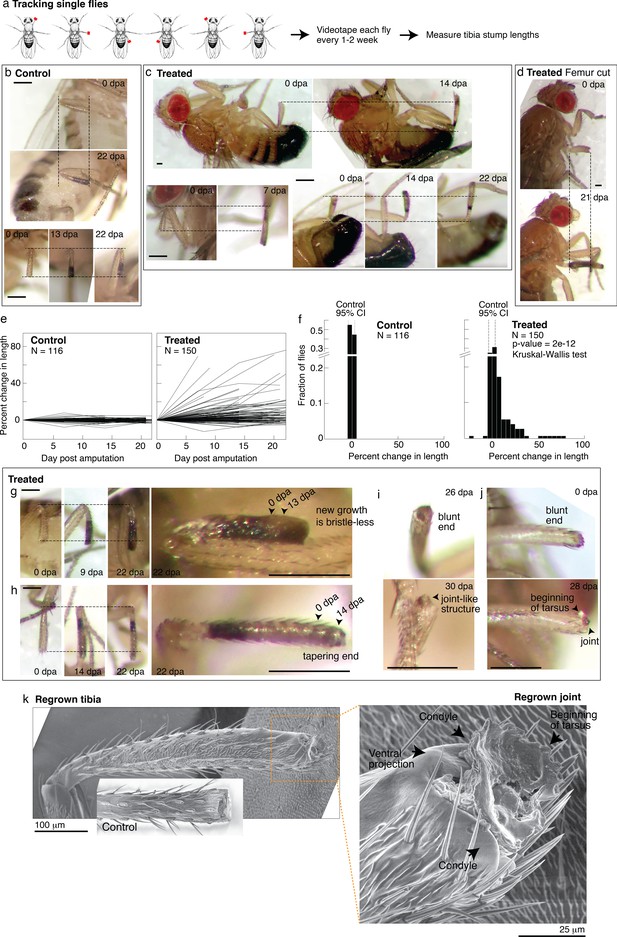
Tracking regenerative response in individual flies.
(a) To track the regenerative response in individual flies, flies were amputated in different limbs (fore, mid, and hindlimb) and housed in small groups such that in any given vial, each fly was uniquely identifiable by sex and amputated limb. Each fly was imaged immediately after amputation and 1–3 additional times over the course of 2–4 weeks. (b–d) Representative time lapse from control (b) and treated flies (c–d). dpa: days post-amputation. For each fly, time-lapse images are shown at the same magnification. Scale bars: 250 μm. (e) Change in tibia stump length over time. (f) Distribution of change in tibia stump length in control and treated flies. Percent change in length is the difference between the length at the final time point and 0 dpa, relative to the length at 0 dpa. CI: confidence interval. Statistical difference between control and treated distributions was evaluated using the nonparametric Kruskal-Wallis test. The p-value tests the null hypothesis that the data are drawn from the same distribution. We confirmed that the probability of observing growth in each condition was not biased by differences in the initial length of tibia stump (Figure 5—figure supplement 1b). (g–i) For each fly, time-lapse images are shown at the same magnification. Scale bars: 250 μm. (g) In this treated tibia stump, the new growth showed different pigmentation and no sensory bristles. (h) In this treated tibia stump, the segment was reformed as suggested by the tapering end. (i) In this treated tibia stump, the segment was reformed and a joint-like structure grew. (j) In this treated tibia stump, amputation was performed just before the joint. After 4 weeks, a new joint-like structure appeared, from which tissues of the next segment (tarsus) started growing. (k) Fly with a regrown tibia at 21 dpa was mounted onto an environmental SEM with a copper stub. Inset shows a clotted tibia stump from a control fly, with the discoloration at the end corresponding to the clot. Magnification of the regenerated joint: the arrows denote the two condyles and additional ventral projection.
Drosophila drawing in (a) is by Ashley Smart and used with permission.
-
Figure 5—source code 1
Drosophila code.
- https://cdn.elifesciences.org/articles/65092/elife-65092-fig5-code1-v4.zip
-
Figure 5—source data 1
This spreadsheet contains the raw data of Figure 5 and its figure supplements.
- https://cdn.elifesciences.org/articles/65092/elife-65092-fig5-data1-v4.xlsx
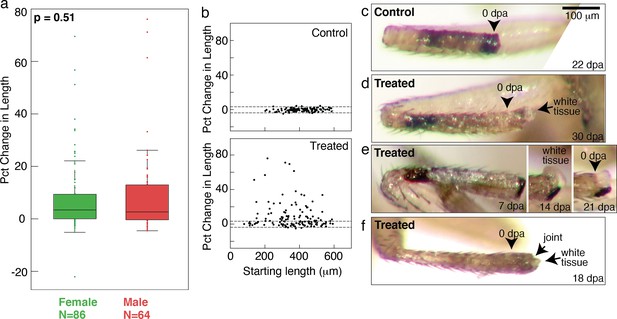
Tracking regenerative response in single flies.
(a) No sex-based differences were observed in the regenerative response. Distributions of changes in stump lengths in male and female flies are not statistically different (p=0.48, nonparametric Kruskal-Wallis test). (b) Growth was observed from proximal (short tibia stump) or distal (long tibia stump) amputation across tibia. (c) A control tibia stump. (d–f) Treated tibia stumps showing white tissues growing from the end.
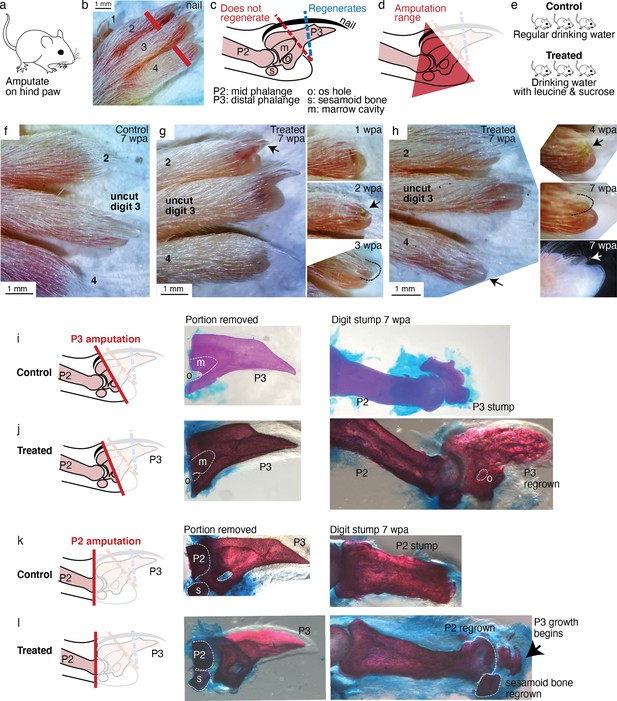
Leucine and sucrose induced regeneration in adult mouse digit.
(a–b) Amputation was performed on hindpaws of adult (3–6 months old) mice, on digits 2 and 4, proximal to the nail. (c) Schematic of the distal phalange (P3) and middle phalange (P2). Amputations that remove <30% of P3 (blue line) regenerate, whereas amputations that remove >60% of P3 (red line) do not regenerate. Amputations in the intermediate region can occasionally show partial regenerative response. (d) Amputations in this study were performed within the red-shaded triangle. (e) Amputated mice were given regular drinking water (control) or drinking water supplemented with 1.5% L-leucine, 1.5% L-glutamine, and 4–10% w/v sucrose (2 exps with 4%, 3 exps with 10%). Drinking water, control and treated, was refreshed weekly. (f) A representative paw from the control group. The amputated digits 2 and 4 simply healed the wound and did not regrow the distal phalange. (g) In this treated mouse, digit 2 (arrow) regrew the distal phalange and nail. Insets on the right show the digit at earlier time points. At week 1, the amputation site still appeared inflamed. At week 3, the beginning of the nail appears (arrow). At week 3, a clear nail plate was observed. (h) In this treated mouse, digit 4 (arrow) regrew and began to show nail reformation by week 4 (top inset, see arrow), that turns into a clear nail plate by week 7 (middle inset), as can be seen more clearly from the side-view darkfield image (bottom inset). (i–l) Whole-mount skeletal staining. Dissected digits were stained with Alizarin red, an anionic dye that highly localizes to the bone. Left panels show illustration of the amputation plane, middle panels show skeletal staining of the portions removed, and right panels show skeletal staining of the digit stumps 7 weeks after amputation.
-
Figure 6—source data 1
This spreadsheet contains the raw data of the mouse digit phenotype in Figure 6 and its figure supplements.
The spreadsheet documents the raw image filename, amputation type, and phenotype scoring.
- https://cdn.elifesciences.org/articles/65092/elife-65092-fig6-data1-v4.xlsx
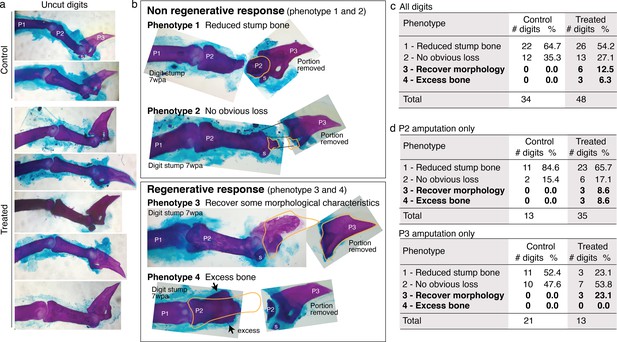
Mouse digit phenotypes.
Whole-mount skeletal staining was performed with Alizarin red. wpa: week post-amputation, P1: phalange 1, P2: phalange 2, P3: phalange 3, s: sesamoid bone. (a) Skeletal staining of unamputated digits (digit 3) from control and treated groups show no obvious differences in uncut digits due to the treatment. (b) Skeletal staining of digits stumps at 7 wpa and the original portion removed from the digits. Some digit stumps show no change or appear to have undergone histolysis (Chamberlain et al., 2017) resulting in reduced bone mass (phenotypes 1 and 2). Some digit stumps show regenerative response, either recovery of some morphological characteristics (phenotype 3, detailed more in Figure 6—figure supplement 2) or excess, ectopic bone mass (Phenotype 4). We erred on the conservative side in scoring phenotype 3 and 4; when in doubt, digits were classified into phenotype 1 or 2. (c–e) Phenotype counts in all digits (c), in digits amputated across P2 (d), and in digits amputated across P3 or joint (e).
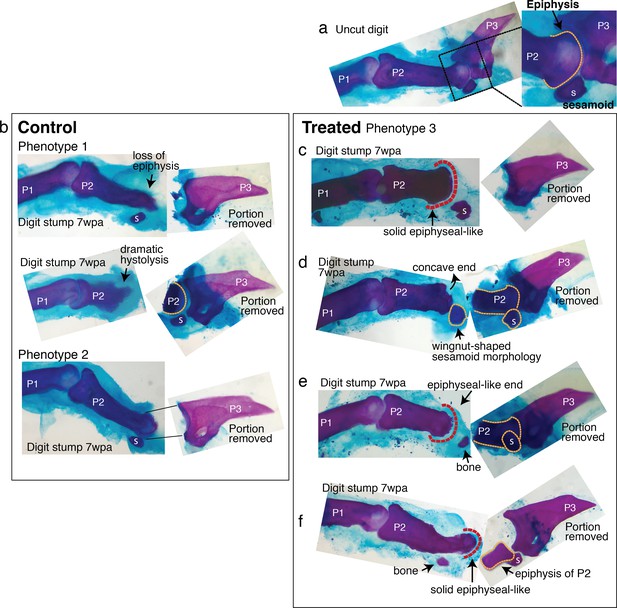
Regenerative response observed in mouse digi.
Six digit stumps (of total 48 examined) show regenerative response. The most dramatic two are presented in Figure 4. The remaining four are presented here. wpa: week post-amputation, P1: phalange 1, P2: phalange 2, P3: phalange 3, s: sesamoid bone. (a) An uncut digit, shown for a comparison. Magnified is the P2/P3 joint area to highlight key morphological markers: the knobby epiphyseal cap of P2 and the sesamoid bone embedded in the tendon on the flexor side of P2. (b) Digit stumps from control mice show either bone stump histolysis (top and middle, phenotype 1) and no visible changes in bone stump (bottom, phenotype 2). (c–f) Digit stumps from treated mice that show regenerative response. (c) In this digit, the amputation removed all P3 by a cut through the joint. At 7 wpa, the P2 stump is reduced, but recovered the epiphyseal-like end (red dashed line)—marked by solid curved shape, as opposed to irregularly shaped histolyzing bone. (d) In this digit, the amputation removed a significant portion of P2 and the sesamoid bone. The P2 stump does not regain an epiphyseal end (the end is concave and irregular). However, the sesamoid bone is reformed, as identified by its location on the flexor side of P2 and wingnut shape (Wirtschafter and Tsujimura, 1961) under the microscope. The recovery of sesamoid bone is non-trivial, as digit sesamoids form in juxtaposition to the condensing phalange, detaching from the phalange by formation of a cartilaginous joint (Eyal et al., 2019). (e) In this digit, the amputation removed a significant portion of P2 and the sesamoid bone. At 7 wpa, the P2 stump appears to be reforming an epiphyseal, rounded end (red dashed line). There is a small bone distal to P2, whose curvature articulates with the P2 end, but there are not enough morphological characters to identify the bone. (f) In this digit, the amputation removed the epiphyseal cap of P2 and the sesamoid bone. The P2 stump appears to have lost some mass, but reforms an epiphyseal-like end (red dashed line). There is an additional small bone located where the sesamoid bone should be, but lacks sufficient morphological characters to identify.
Videos
Arm regenerates pulse synchronously with existing arms.
This video was taken 2 weeks after amputation. The synchronous pulsing suggests functional rebuilding of neuromuscular tissues.
Experimental setup in Aurelia.
See Figure 3—figure supplement 1 for more details.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Aurelia aurita) | A. aurita sp. 1 | Gift from the Cabrillo Marine Aquarium,San Pedro, CA | Alternatively named Aurelia coerulea (Scorrano et al., 2016). | |
| Strain, strain background (Drosophila melanogaster) | OregonR | Gift from Angela Stathopolous’ lab, Caltech | RRID:BDSC_5 | |
| Strain, strain background (D. melanogaster) | CantonS | Gift from Kai Zinn lab, Caltech | RRID:BDSC_64349 | |
| Strain, strain background (Mus musculus) | CD1 | Charles River Laboratories | Strain 022 | Female, 3–6 months old RRID:IMSR_CRL:022 |
| Chemical compound, drug | L-leucine | Sigma-Aldrich | L1002 | Methyl-ester hydrochloride |
| Chemical compound, drug | L-leucine | Sigma-Aldrich | L8000 | |
| Chemical compound, drug | L-leucine | VWR | E811 | USP grade |
| Chemical compound, drug | L-glutamine | Sigma-Aldrich | G3126 | |
| Chemical compound, drug | L-glutamine | Sigma-Aldrich | G8540 | USP grade |
| Chemical compound, drug | Sucrose | Avantor | 8360 | AR ACS grade |
| Peptide, recombinant protein | Insulin | Sigma-Aldrich | I0908 | |
| Peptide, recombinant protein | Insulin | MP Biomedicals | 0219390080 | |
| Antibody | Anti-tyrosinated alpha tubulin (Rat monoclonal) | Sigma-Aldrich | MAB1864-I | (1:200) RRID:AB_2894901 |
| Antibody | Goat anti-mouse Alexa Fluor 488 (Goat polyclonal) | Life Technologies (Thermo Fisher Scientific) | A11029 | (1:200) RRID:AB_2894900 |
| Other | Alexa Fluor 555 phalloidin | Life Technologies (Thermo Fisher Scientific) | A12379 | Histological stains (1:20) |
| Other | Hoechst 33342 | Sigma-Aldrich | B2261 | Histological stains (1:10) |
| Other | Vectashield mounting medium with DAPI | Vector | H1200 | Histological stains (1:1) |
| Other | Alizarin red | Beantown Chemical | BT144735 | Histological stains, 0.005% |
| Other | Alcian blue | Across Organics | AC40046-0100 | Histological stains, 0.015% |
-
Aurelia aurita.





