Shore crabs reveal novel evolutionary attributes of the mushroom body
Figures
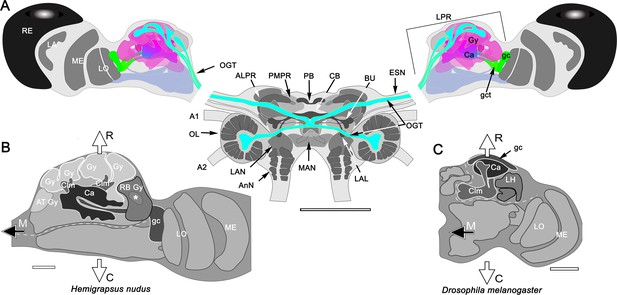
The brain of Hemigrapsus nudus and a comparison of its lateral protocerebrum with that of the fly Drosophila melanogaster.
(A) Schematic of the Hemigrapsus brain (frontal view) and major neuropils. Abbreviations: ALPR, anterior lateral protocerebrum; A1, antennular nerve; A2, antennal nerve; AnN, antenna 2 neuropil; BU, lateral bulb of the central complex; CB, central body; ESN, eyestalk nerve; LAL, lateral accessory lobe; LAN, lateral antennular lobe neuropil; LA, lamina; LO, lobula; LPR, lateral protocerebrum; MAN, median antenna 1 neuropil; MB, mushroom body; ME, medulla; OL, olfactory lobe; OGT, olfactory globular tract; PMPR, posterior medial protocerebral neuropils; PB, protocerebral bridge; RE, compound retina. The eyestalk nerve (ESN) carries all axons connecting the brain with the lateral protocerebrum. (B, C) The crab lateral protocerebrum aligned with that of Drosophila (in both, the optic lobe’s lamina is omitted) The white dashed line in H. nudus divides its rostral lateral protocerebral volume from its caudal volume; in Drosophila the dashed line divides the rostral volume of the protocerebrum from the rest of the hemi-brain. In both taxa, the filled arrow M indicates medial, and the open arrows R, C indicate, respectively, rostral and caudal. Both schematics depict a view from the ventral side of the lateral protocerebrum/ hemi-brain. Abbreviations: Ca, calyx; Clm, column; gc, globuli cells; gct, globuli cell tract; Gy, gyri; LH, lateral horn; LO, lobula complex; ME, medulla; RB, reniform body. Scale bars: A, 500 μm; B, 100 μm; C, 50 μm.
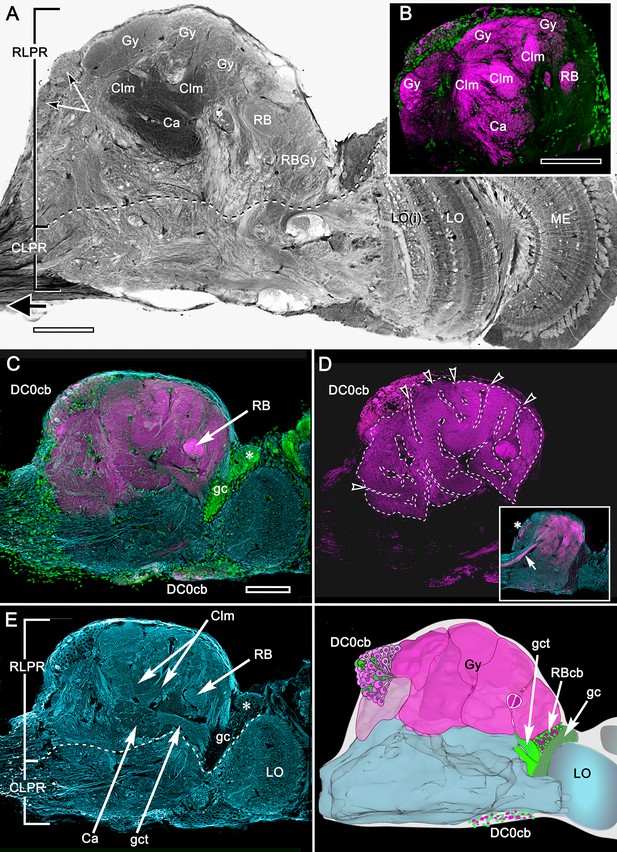
Organization of the varunid crab mushroom body and its associated gyri.
(A) Brains treated with osmium-ethyl gallate demonstrate the overall disposition of neuropils, exemplified by this section of the lateral protocerebrum from its medial border to the optic lobe medulla. Its rostral domain (RLPR) includes the mushroom body calyces (Ca), columns (Clm) and their associated gyriform neuropils (Gy). Clusters of DC0-positive neuronal cell bodies (here DC0cb) are distributed over the neuropil. Distal volumes of the RLPR are associated with the reniform body (RB; here its pedestal and a gyrus RBGy), which is situated between the mushroom body and the optic lobe, here represented by its medulla (ME) and bilayered lobula (LO, LOi). The optic lobe provides outputs to discrete neuropils comprising the caudal lateral protocerebrum (CLPR). (B–E) Anti-DC0-labeled (magenta) volumes in panel B match the corresponding membrane-dense calyces and columns and their rostral gyri in panel A. Panel C demonstrates that DC0-immunostained gyri occupy almost the entire rostral level of the RLPR, with the exception of a medial gyrus (see Figure 11). Two small cell body clusters caudally (DC0cb) lining the CLPR, near the optic lobes, include DC0-immunoreactive perikarya, as does a prominent proximal cluster of large cell bodies (DC0cb; also indicated by arrows in panel A) immediately above the entry of the eyestalk nerve. The latter cell bodies belong to neurons that extend to gyri. DC0-immunoreactive axons extending from gyri into the eye stalk nerve (inset panel D, axons indicated by arrow, cell body cluster by asterisk) suggest that some of these DC0-immunoreactive neurons may constitute efferent channels to the midbrain. The nuclear stain Syto13 (green) demonstrates the location of mushroom body globuli cells (gc) lying immediately beneath a group of slightly larger perikarya (asterisk) that supply the reniform body. In panel D, removal of anti-α tubulin and Syto13 labeling from C resolves the extent of the gyri and their many sulcus-like indentations (open arrowheads). In panel E, anti-α-tubulin (cyan) shown here alone resolves the overall fibroarchitecture of the LPR to provide correlative data with those provided by reduced silver stains (Figure 3). (F) Total Amira-generated reconstruction of a serial-sectioned, osmium-ethyl gallate treated eyestalk demonstrates the huge area of the lateral protocerebrum occupied by gyri. Superimposed are the locations of small neurons supplying the reniform body (RBcb), mushroom body globuli cells (gc) and their tract of neurites leading to the calyx (gct), here obscured by the overlying gyri. Scale bars, A, B, 100 μm; C-F, 100 μm.
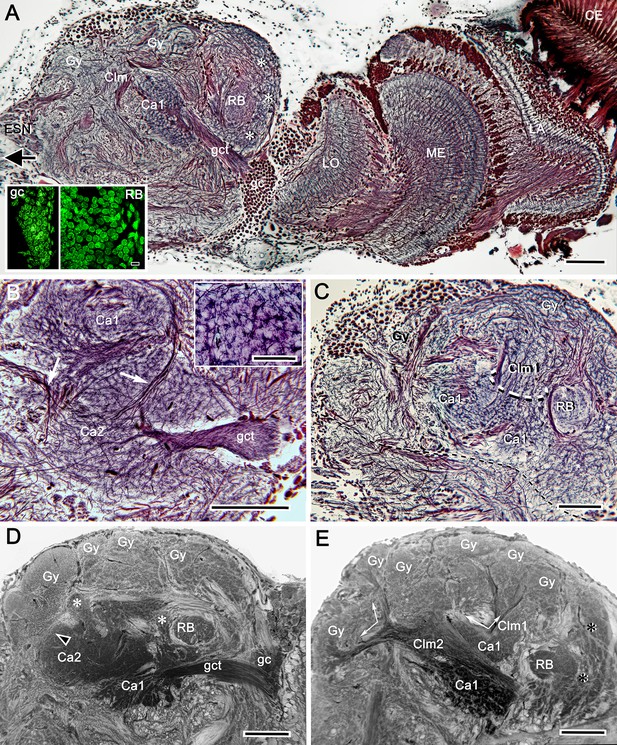
Uniquely identifiable neuropils of the mushroom bodies are resolved by reduced silver.
(A) Longitudinal section parallel to the rostro-caudal plane of the lateral protocerebrum demonstrating the more dorsal of the two calyces (Ca1) and its supply by the globuli cell tract (gct) from the cluster of globuli cells (gc). Columnar extensions (Clm) from the calyces reach out to overlying gyri (Gy). The reniform body is shown with three of its gyri (asterisks) and its bundled axons (RB) situated between the mushroom body and optic lobes, insets lower left compare the smallest perikarya supplying the calyces (globuli cells, gc) with the next smallest perikarya belonging to neurons of the reniform body. Abbreviations: ESN, eyestalk nerve; CE, compound retina, LA, lamina, ME, medulla; LO, lobula. (B) Adjacent calyces (Ca1, Ca2) showing their diagnostic arrangement of microglomeruli (inset upper right; see also Figure 5). Arrows indicate afferent fibers into Ca2 from nonolfactory origins. (C) The calycal origin and rostral extension of the Ca1 column (Clm1, encircled). At this level, penetration by en passant axon bundles causes the calyx to appear fragmented. (D, E) Osmium ethyl gallate-treated sections at two depths. Panel D indicates the bulk and elaboration of calycal neuropils. At this level, calyx 2 appears as the larger. The arrowhead indicates the base of column 2. Calyx 1 is shown with its globuli cell tract entering a domain denoted by lateral extensions of calyx 1. In panel E, the base of column 1 (Clm1) is shown arising from Ca1, its curvature at this level splitting it into two parts. Column (Clm2) is shown extending two branches into the gyriform layer above. Clm1 extends outwards just proximal to the pedestal of the reniform body (RB). In addition to columns, calyces provide occasional narrower finger-like extensions extending towards the gyri (asterisks in D). Scale bars, 100 μm. Inset in A, 10 μm, inset in B, 50 μm.
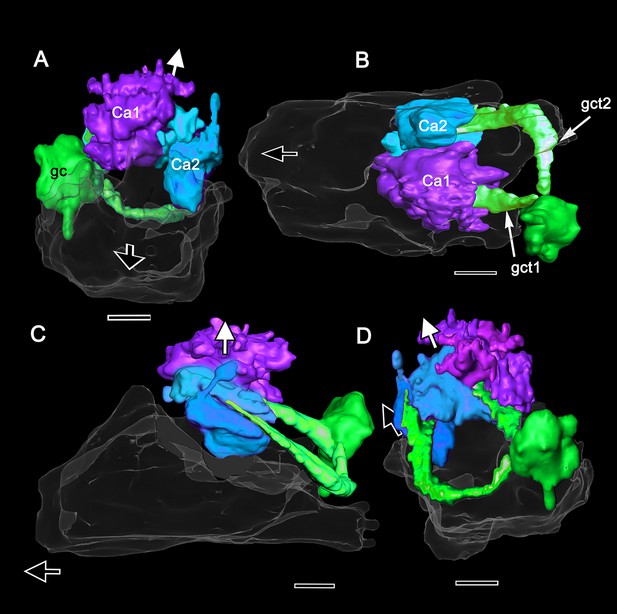
Globuli cells provide two tracts, one to each calyx as shown in this Amira reconstruction of a serially sectioned osmium-ethyl gallate-treated brain.
Two tracts composed of neurites from 22,000 to 30,000 globuli cells from a unified cluster (gc) extend to two calyces (Ca1, Ca2), Ca1 is dorsal to and larger than Ca2. (A) Viewed from above, with the medial margin of the lateral protocerebrum closest to the observer, the rostral surfaces of each calyx are highly indented with some finger-like extensions reaching upwards. (B) Viewed from beneath, with the dorsal calyx 1 closest to the observer, the more caudal levels of the calyces are seen to be layered, this distinction between caudal and rostral volumes also shown viewing into the lateral protocerebrum from its ventral surface (C). (D) 180o rotation from panel A suggests greater surface elaboration of Ca1 than Ca2.
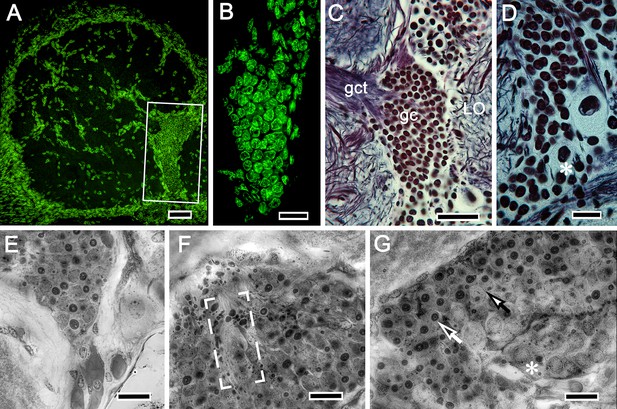
Globuli cells supplying intrinsic neurons to the mushroom bodies.
In the varunid crab, globuli cells occupy a unique location between the distal margin of the lateral protocerebrum and proximal margin of the optic lobe as shown boxed in A. (B) As is typical of globuli cells in other taxa (insects, shrimps), they are minute, here measuring less than 6 μm diameter, the next largest being reniform body perikarya (upper right in C). (D–G) Like other decapods, crabs molt and grow throughout life, their brains also increasing in size. The globuli cell cluster evidences neuroblasts (asterisk in panel D), and grouped globuli cells suggest clonal siblings (E). Ganglion mother cells are also arranged as aligned groups (F) or, as in panel (G), dividing (closed arrow) between neuroblasts (asterisk) and fully differentiated perikarya (open arrow). Scale bars, A, 50 mm; B, 20 μm; C, 50 μm; D-G, 20 μm.
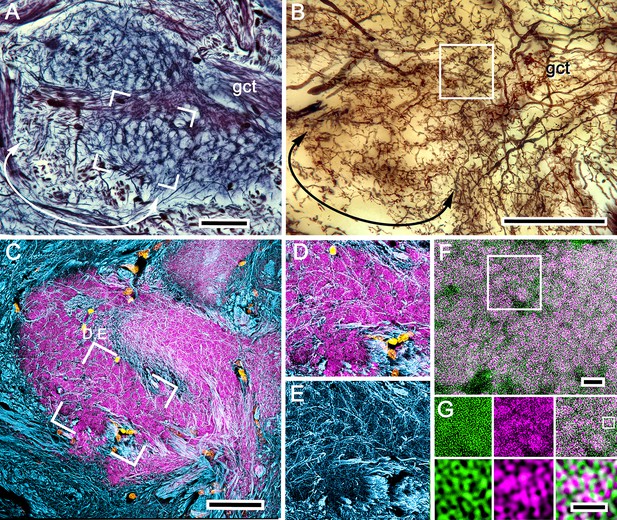
Organization of calyx 1: microglomeruli.
(A) Reduced-silver-stained calyx showing its globuli cell tract (gct) with, at a different level, many of its constituent neurites giving rise to branches that delineate the mosaic organization of microglomeruli. (B) Golgi impregnations provide examples of intrinsic neurons, the branches and fine processes of which extend through domains of the calyx that correspond to those revealed both by reduced silver and by antibodies against DC0. A subfield (curved double arrow) in panel A corresponds to the denoted branches of the intrinsic neuron in panel B. (C) Microglomeruli are specifically labeled by anti-DC0 (magenta). Microglomerular size and density in the boxed area corresponds to an equivalent microglomerular organization identified by reduced silver (boxed area in A). The mosaic is also reflected by the pattern of all intrinsic neuron processes at that level revealed by anti-α−tubulin (cyan); these enwrap DC0-positive microglomeruli (D, E). The same system of microglomeruli is resolved using combined actin/anti-synapsin (F). The area identified in a field of Golgi-impregnated intrinsic neuron processes (boxed in B) is equivalent to the area of the calyx in panel F labeled with actin/anti-synapsin demonstrating the microglomerular mosaic. The boxed area in F is shown in panel G. (G) The upper row shows actin (green) and synapsin (magenta) separately, with the superimposed images in the right panel. The boxed area in the upper right panel of G indicates a single microglomerulus. This is enlarged in the lower three panels, again showing actin and synapsin as separate images and then combined lower right to demonstrate the density of synapsin-labeled profiles, and thus direct evidence of massive convergence at a calycal microglomerulus. Scale bars, A–C, 50 μm; F, 10 μm; G, 2 μm.
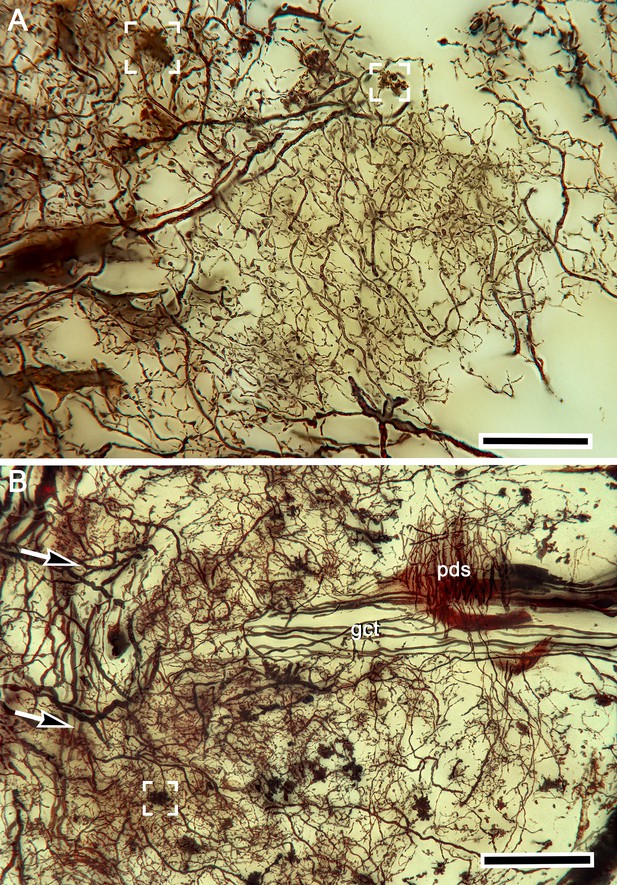
Rectilinear network organization of the calyx and afferent supply.
(A) Golgi impregnation showing regular network arrangements of intrinsic cell dendrites. Robust afferent terminals are defined by varicose specializations. A single terminal is shown in the box upper right; several convergent terminals in the box upper left. (B) The globuli cell tract (gct) extends medially beneath processes belonging to the reniform body’s pedestal (pds). Afferent fibers enter from the left (arrowed) to terminate (one boxed, lower left) amongst densely arranged intrinsic neuron dendrites extending from globuli cell neurites comprising the gct. Scale bars, A, B, 50 μm.
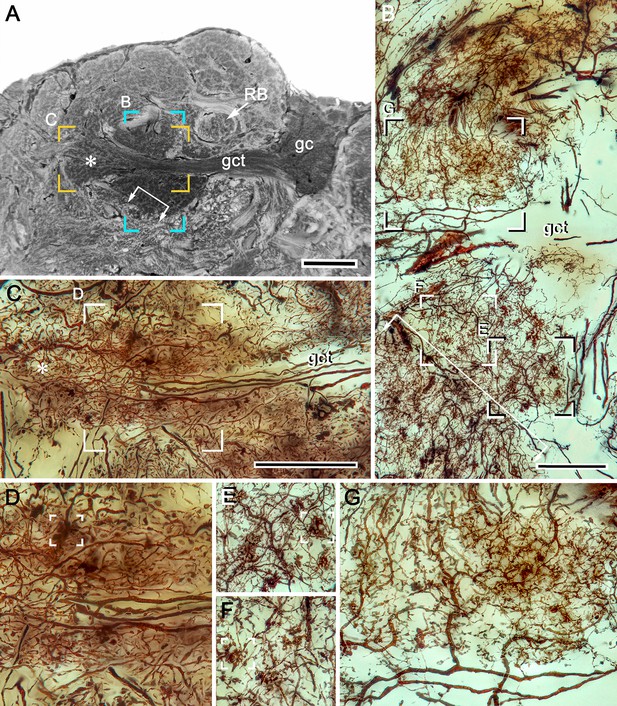
Intrinsic cell dendrites define calycal territories.
(A) Osmium-ethyl gallate resolves calycal territories matching the dendritic fields of morphologically distinct intrinsic neurons. Letters in panel A indicate panels showing corresponding locations of Golgi-impregnated dendritic fields shown in (B and C). Boxed areas in B and C are enlarged in panels (D–G) to demonstrate distinctive dendritic morphologies. The locations of the arrowed bracket and asterisk in A correspond to their locations in B (the bracket) and C (the asterisk). These further indicate the fidelity of calyx organization indicated by these two histological methods. Open boxes in panels D–F indicate different morphological specializations of afferent terminals. Abbreviations: RB, reniform body pedestal; gc, globuli cells; gct, globuli cell tract. Scale bars, A, C, 100 μm, B, 50 μm.
Fidelity of intrinsic neuron fields (A) and calycal domains revealed by osmium-ethyl gallate (B).
The equivalent of the area in panel (A) is indicated by the open box in panel (B). Corresponding loci in B and A are indicated by symbols. Boxed areas in (A) are enlarged in panels (C,D) showing rectilinear organization of intrinsic processes and their convergence at nodes corresponding to microglomeruli. RB, reniform body pedestal; gc, globuli cells; gct, globuli cell tract. Scale bars, A, B, 100 μm.
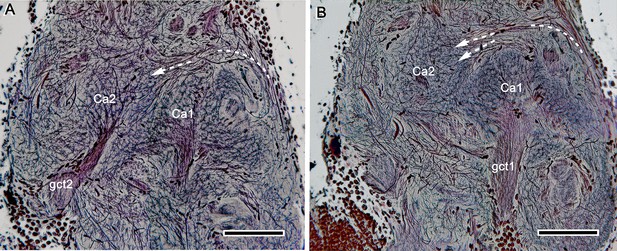
Calyx distinctions.
(A, B) Two serial silver-stained sections showing the side-by-side disposition of calyces 1, 2 (Ca1, Ca2) each supplied by its own globuli cell tract (gct1, 2). Arrowed paths indicate passage of axons extending to Ca2 from pathways (far right in both panels) from the optic lobe medulla and reniform body. Scale bars, 100 μm.
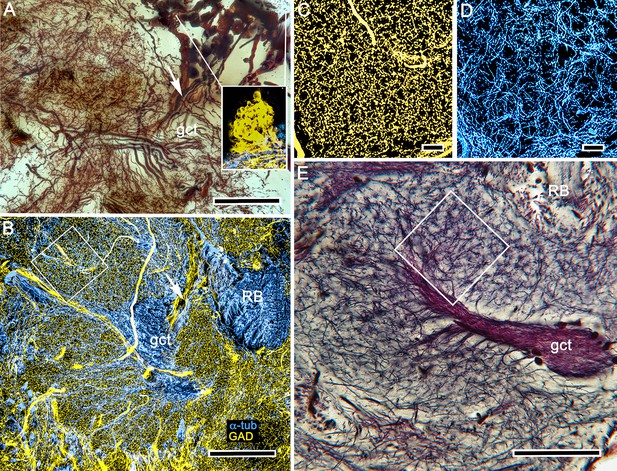
Evidence for local inhibition in the calyx by anaxonal neurons.
(A) A total of 40–50 large perikarya situated rostrally above the gyri are immunoreactive to glutamic acid decarboxylase (GAD), as shown in the right inset. These neuron cell bodies, which are here Golgi-impregnated, send stout cell body fibers (arrow) to the calyces where they provide widely branching and extremely dense processes that do not follow the patterns of the microglomerular mosaic. (B) GAD immunoreactivity (yellow) reveals their branches and finest processes spreading through every calycal domain and hence positioned to potentially interact with all the intrinsic neuron dendritic fields. (C, D) GAD immunoreactivity in an area shown by the box in panel B, and an equivalent area in panel (E) showing the mosaic of microglomeruli. This mosaic is also evident from the pattern of α-tubulin-immunoreactive (cyan) intrinsic neurons shown in D (same boxed area as in B). Scale bars, A, B, 100 μm, C, D, 10 μm, E, 50 μm.
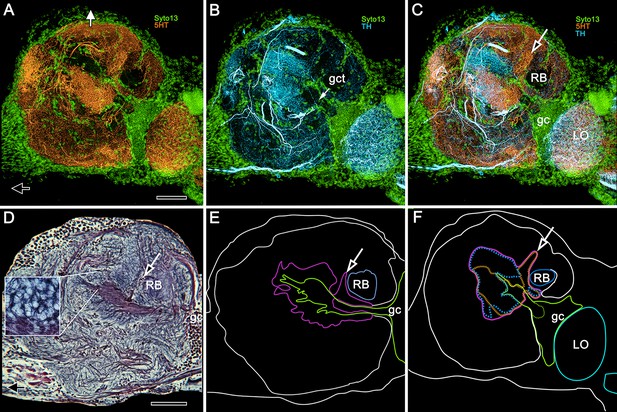
5HT and TH immunoreactivity define different calycal domains.
(A,B) Although many volumes of the lateral protocerebrum are distinguished by 5HT (panel A, 5-hydroxytryptophan, orange) immunoreactivity, the densest system of branches is in a rostral domain of calyx 1. TH (panel B, tyrosine hydroxylase, cyan) defines two domains, one partially overlapping 5HT, the other not (panel C). (D) Bodian silver-stained calyx 1 showing its distinctive microglomerular mosaic (inset). (E) Outlined in purple is the total calycal perimeter at this level of sectioning and orientation. The arrow indicates the origin of column 1. (F) Perimeter (purple) of calyx 1 in a section of the rostral lateral protocerebrum slightly rotated around its lateral-medial axis, mapping within it 5HT- and TH-immunoreactivity (as in panels A–C) also indicating the origin of column 1 (arrow, as in panel C). Abbreviations: RB, reniform body; gc, globuli cell cluster; gct, globuli cell tract; LO, lobula. Scale bars for all panels, 100 μm.
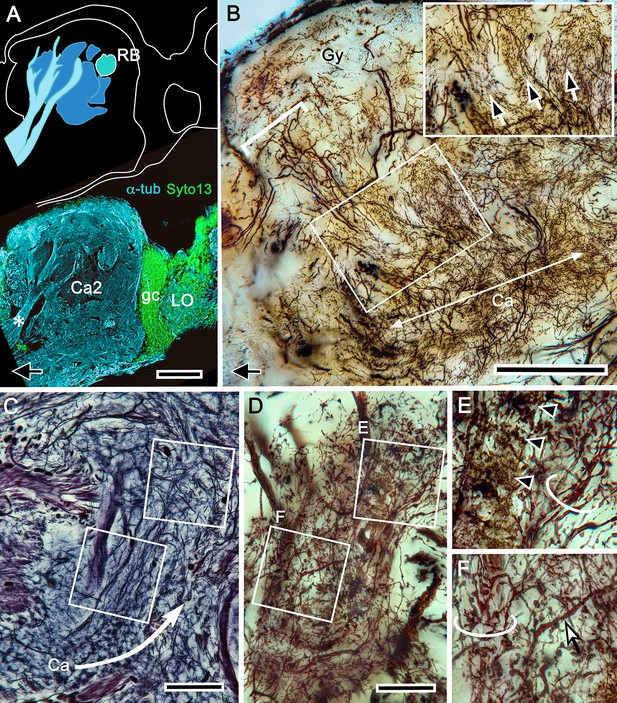
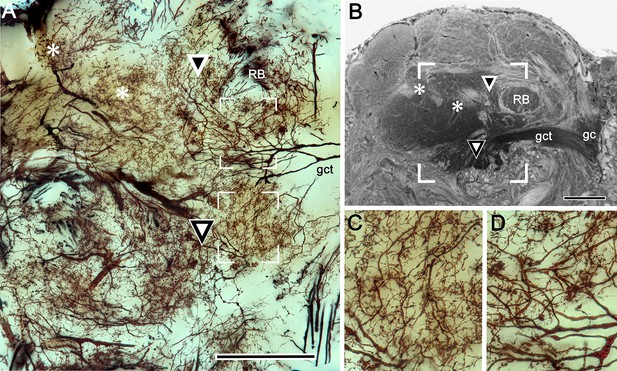
Origin of calycal extensions and columns.
(A) Anti-α−tubulin (cyan) reveals the organization of large fiber bundles and coarse neuropil but, at low magnification, shows neuropils of the calyces as almost featureless. Parts of the calyces are penetrated by incoming tributaries from the eyestalk nerve (asterisk in lower panel). (B) A corresponding Golgi-impregnated region demonstrates a system of medium-diameter branched processes that provide fine networks enwrapping the origin of columns. Some networks also extend as finger-like extensions outwards from the calyces. Panel B shows one column (bracketed) the base of which is flanked by those arrangements (enlarged in upper right inset indicated by arrows). This extensive system of processes does not derive from the globuli cell clusters, although it is clearly associated with the entire extent of both calyces, suggesting a possible role in calyx-wide modulation. (C) As shown by this reduced silver stained section, the column arising from calyx 1 is extremely elaborate, comprising at least two longitudinal divisions each defined by arrangements of intrinsic neuron processes and their branched specializations. The curved arrow in panel C indicates the continuation of intrinsic neuron microglomeruli for a short distance alongside the column. The boxed areas in panel C indicate corresponding levels identified in a Golgi-impregnated column 1 (panel D). Boxed areas in D are enlarged in panels (E, F). In panel E, ascending fibers provide short collaterals (arrowheads) across one longitudinal subdivision of the column. Ascending intrinsic processes are arranged as a tangle in two different column subdivisions (circled in panels E, F). Panel F also shows a large beaded branch (arrowed) of a mushroom body input neuron (MBIN) extending into the column. The arrow, lower left in panels A, B indicates medial. Abbreviations: RB, reniform body; Ca2, calyx 2; gc, globuli cells; LO, lobula; Gy, gyri. Scale bars, A, B, 100 μm; C, D, 50 μm.
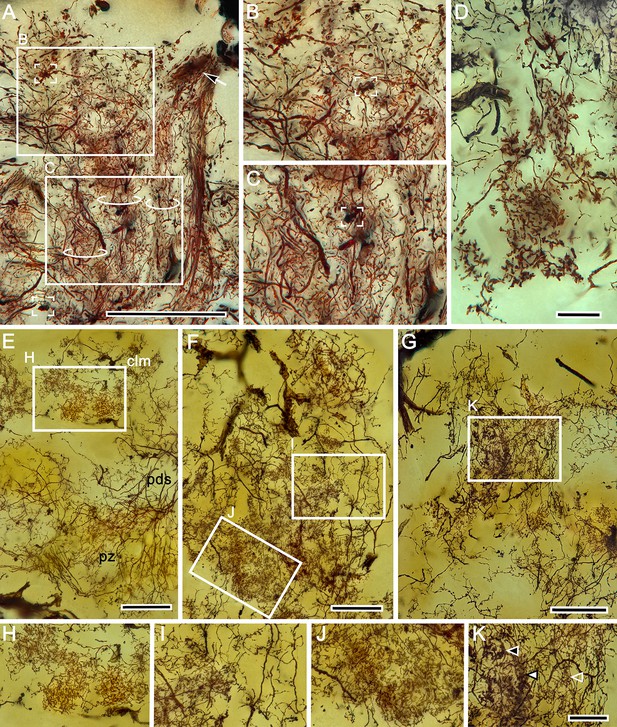
Intrinsic neuron organization in columns.
(A) Golgi impregnation of a column originating from calyx 2. Three parallel but distinct ensembles of intrinsic neuron processes (circled) all splay outwards immediately beneath the overlying gyriform neuropil, which is here unstained. Details of these ensembles shown in (B, C) demonstrate internal complexity. Of note is the presence of bouton-like terminals (two indicated by box brackets) suggesting either afferent supply from the eyestalk nerve or reciprocal inputs from rostral gyri. A cascade arrangement of processes extending parallel to the column from a more rostral level (arrow) may indicate recurrent afferent supply from the gyri. (D–G) Columns also contain what appear to be intrinsic elements (D) that do not originate from the calyces, as occurs in the mushroom bodies of certain insects (see Discussion). (E) There is a clear distinction between the arrangement of processes (enlarged in H) in the columns (clm) and the more distributed arrangements in the proximal zone (pz) of the reniform body indicated by its pedestal (pds). (F) Further complexity is demonstrated by parallel arrangements of intrinsic neurons and their collaterals suggestive of local circuits (examples indicated in boxed areas in panel F, enlarged in I, J). (G) Dense arrangements near the column’s terminal expansion reveal claw-like specializations each with its particular morphology, within parallel subdivisions of the column (indicated by arrowheads in K). Scale bars, A, 100 μm; E–G, 25 μm; D, H-K, 20 μm.
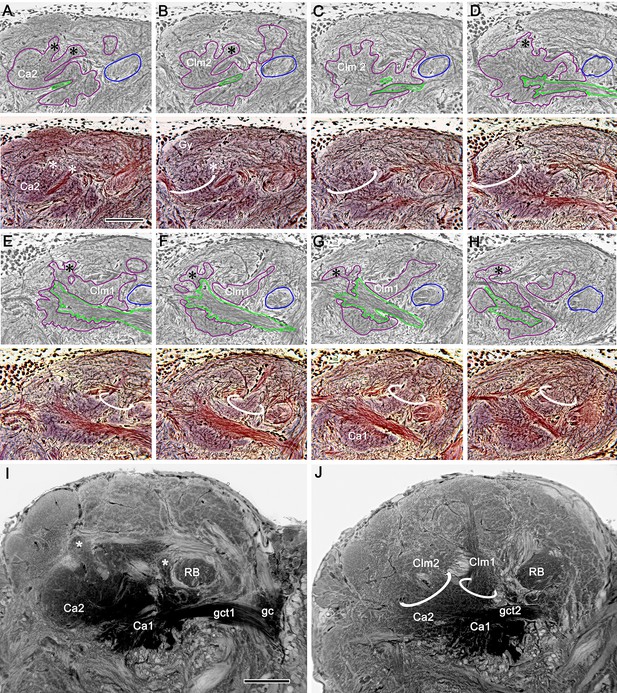
The interface between columns and gyri.
(A–H) Paired half-tone/color micrographs of selected levels of a silver-stained, serially sectioned RLPR (medial to the left, rostral upwards). Green outlines in panels A-D indicate parts of the globuli cell tract (gct2) to calyx 2 (Ca2); in E-H, parts of the gct1 to calyx 1 (Ca1). Blue outlines indicate cross sections of the reniform body pedestal (RB); magenta outlines indicate mushroom bodies (calyces and their columns). Identification of the calyces is enabled by their color and microglomeruli as in Ca2, (lower panel A). As in osmium-ethyl gallate sections, the two calyces are too close to identify separately unless viewed downwards from the rostral surface of the LPR. Columns (ringed) and smaller finger-like extensions (asterisks) extend outwards to the gyri. Column 2 (Clm2) from calyx 2 (Ca2) is shown in panels A-D. Panels E-H illustrate the origin and width of Clm1 from Ca1. Panels I and J show corresponding arrangements revealed by osmium-ethyl gallate. Scale bars, 100 μm.
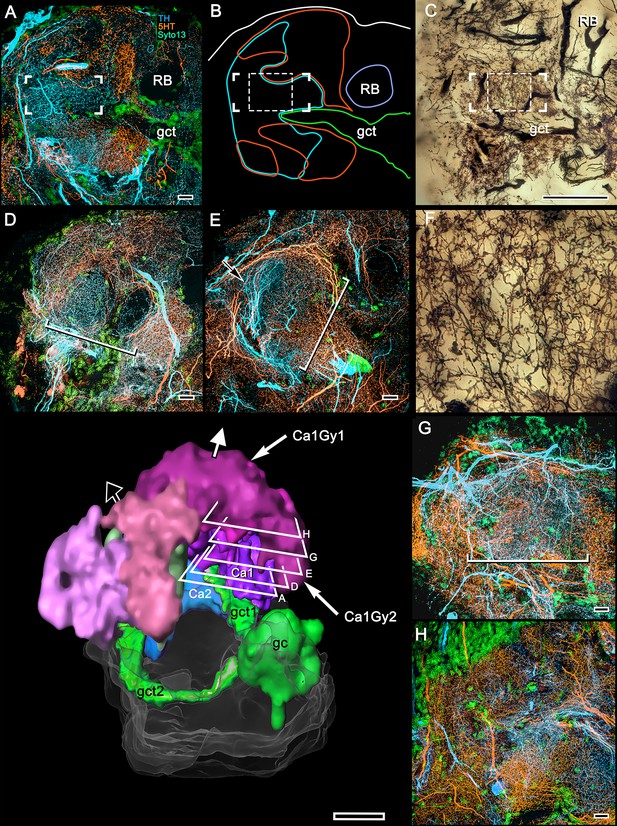
5HT and TH immunoreactivity resolve different levels in the calyx 1 column.
Looking downwards through the rostral surface of the LPR, this sequence of confocal slices (A,D,E,G,H) starts at the column’s origin from calyx 1 (panel A) then follows its outward passage rostrally to the level at which the column merges with the overlying gyri (panels G, H). (B) Shows outlines of 5HT- (orange) and TH-immunoreactive (cyan) fields at level A in relation to the globuli cell tract (gct, green) and the cross section of the reniform body’s pedestal (RB). The dendritic field of a TH-immunoreactive cell is corner-bracketed in A,B and a smaller portion of it is denoted by a dashed square in C. (C) Golgi preparation of dendrites in area equivalent to panels A,B. Bracketed and dashed areas in (B,C) show that at this level, networks of intrinsic neurons have given way to more loosely defined reticulations. (F) Enlargement of dashed square in (C). At the level of panel A, 5HT- and TH-immunoreactive fields are segregated across the origin of the column and continue to be, at least until the level shown in panel (E), suggesting 5HT- and TH-immunoreactive fields are restricted to the column’s longitudinal subdivisions. TH-immunoreactive fields can be noticeably clustered within a small area of the column’s cross section (arrow in E) as are MBONs in Drosophila mushroom bodies. The width of the column (long brackets in panels D, E, G) increases gradually through layers (D, E, and G) (see brackets), and in the gyrus (H) its borders are no longer distinguishable. The 3D reconstruction (lower left) shows a view looking into the lateral protocerebrum from its confluence with the optic lobe, its medial axis indicated by the black arrow and the rostral axis by the white arrow pointing upwards. Confocal levels are indicated as well as the two gyri (Ca1Gy1, Ca1Gy2) associated with the Ca1 column and the globuli cell tracts to Ca1 and Ca2 from the globuli cell cluster (gc). Other abbreviations: gct, globuli cell tract; RB, pedestal of the reniform body. Scale bars, A-E, G, H, 10 μm; C and 3D reconstruction, 100 μm.
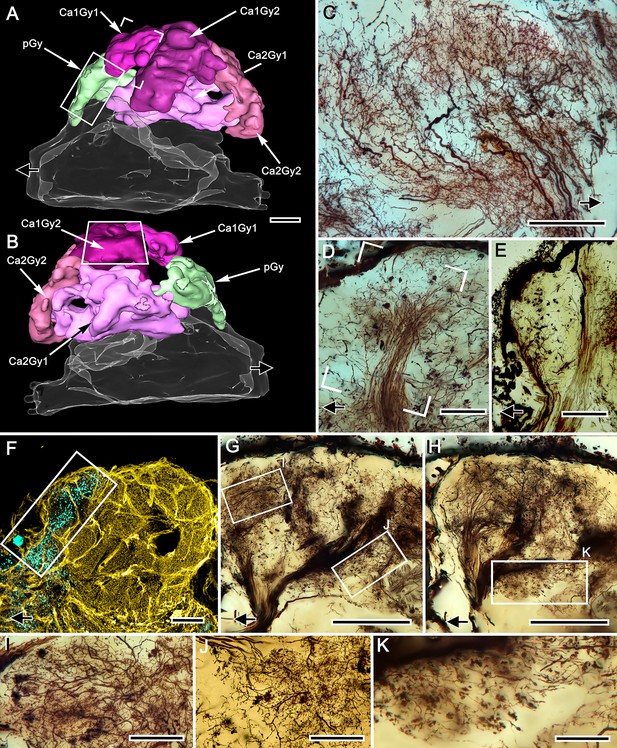
Gyriform neuropils: afferent and efferent organization.
(A, B). Amira-generated 3D reconstructions of the gyri and their calyces. Black arrows indicate the direction of the eyestalk nerve; likewise in panels C–H. (C) Ensemble of efferent dendrites in gyrus 2 associated with calyx 1 (Ca1Gy2). The represented area is boxed in panel B. (D) Afferent tributary from the olfactory globular tract (olfactory projection neurons) supplying gyrus Ca1Gy1 neuropil (open rectangle in panel A). (E) Smooth oval presynaptic specializations denote the terminals of another olfactory globular tract tributary that exclusively supplies the most proximal gyrus (pGy, rectangle in panel A), which has no obvious connection to the mushroom body. (F) Double-labeling with anti–GAD (yellow) and anti-allatostatin (cyan) of the lateral protocerebrum resolves allatostatin immunoreactivity in the proximal gyrus (rectangle in A and F), which shows little to no GAD immunoreactivity. (G, H) Stacked z-axis projections of Golgi-impregnated tracts, imaged through two successive 50 μm sections showing massive supply by afferent neurons. These reach levels of the lateral protocerebrum that include those at and well beneath the gyriform layer (box I, panel G), including levels of the calyces (box J in panel G and box K in panel H). Notably, many efferent neurons relaying information from gyri (panel H) send their downstream axons from the lateral protocerebrum into these tracts. (I–K) Enlargements from regions indicated in G, H, but at sample thicknesses of less than 20 μm to isolate details obscured in flattened optical sections. Scale bars, A–C, F–H, 100 μm; D,E, 50 μm; I–K, 50 μm.
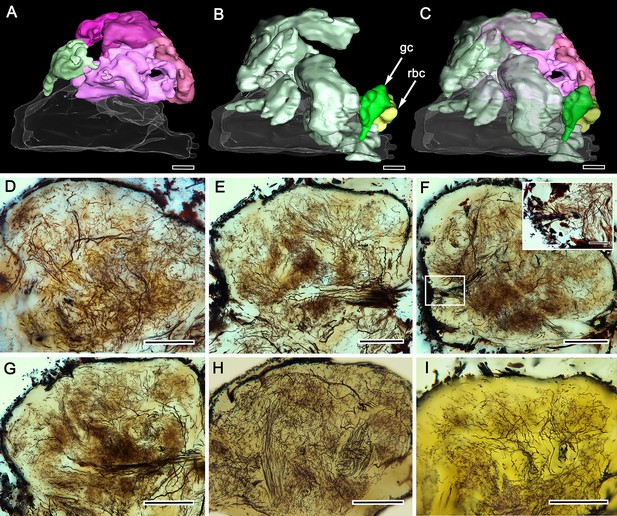
Perikaryal distributions and gyrus neurons.
(A–C) Amira-generated 3D reconstruction of the gyri in panel A with the proximal gyrus (light green) above the medial root of the eyestalk nerve. Panel B shows extensive patches of neuronal perikarya, with the globuli cell cluster (gc, dark green) and reniform body cluster (rbc, yellow) situated at the distal margin of the RLPR. Merged images in panel C show the relative dispositions of cell body clusters and gyri. (D–I) Stitched reconstructions of Golgi mass impregnations resolve top (rostral)-down (F, H), lateral (E, G, I) and sub-gyrus (D) views of neuropils. The boxed area in F includes cell bodies of a lateral patch, enlarged in the upper right inset. Scale bars, A-I, 100 μm, inset to panel F, 25 μm.
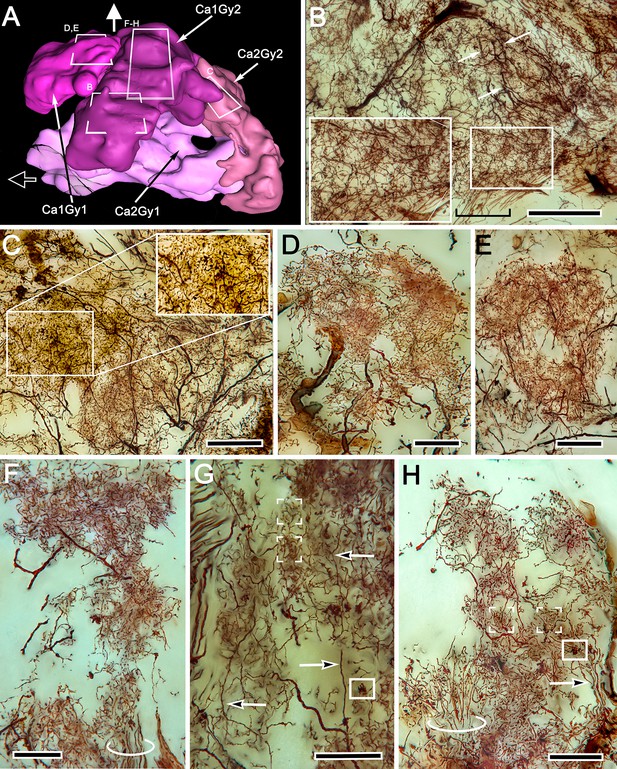
Gyrus neuropils and columns: local circuit neurons.
(A) 3D reconstruction of gyri associated with calyx 1 and calyx 2, showing approximate locations of networks shown in B–H. Open arrow indicates medial, white arrow indicates rostral. (B) Dense network supplied by ascending processes (boxed area is enlarged on left), ascribed to Ca1Gy2 derived from column 1. Dendrites of an efferent neuron (arrows) spread through this layer. (C) Efferent neuron dendrites overlap a broad field of dense terminal specializations. Gnarled bouton-like specializations (boxed and enlarged on right) show that terminals from the eyestalk nerve enter this level. (D–H) Dense and elaborately branched elements interpreted as local interneurons (D, E) and intrinsic elements of the Ca1 column (F–H). Ascending processes (circled in F,H and arrowed in G,H) are further evidence of local circuits. Dense groups of converging processes (box brackets in G,H) correspond in size to afferent boutons (boxed in G,H). Scale bars, B, C, 100 μm; D-H, 50 μm.
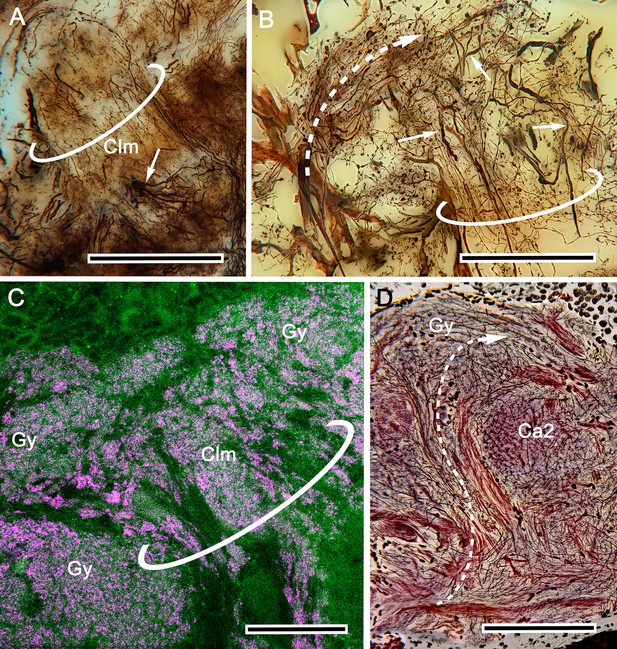
Column-gyrus interface organization.
(A) Terminus of column 2 from calyx 2 (Clm, circled). The column is notable for its many extremely slender intrinsic processes. Also, visible in this preparation are branches of extrinsic (efferent) neurons extending across the column (arrow). (B) Terminus of column 2 (circled) with bundled intrinsic processes and the stouter profiles of efferent neurons (arrowed) ascending from the column into overlying gyral neuropil. This superficial level also receives terminals decorated with small bead-like specializations provided by bundled small-diameter axons from the eyestalk nerve (curved arrow). (C) Synapsin (magenta)-actin (green) labeling showing at the terminus of a column (circled) its longitudinal divisions denoted by various sizes and densities of synaptic sites across its width. Similar variation is resolved in flanking neuropil of the gyrus (Gy). (D) Reduced silver demonstrates bundles (dashed arrow) from the caudal lateral protocerebrum ascending outside a column into gyri, demonstrating that multimodal convergence occurs at many levels in the lateral protocerebrum. Part of Ca2 is also visible at this level. Scale bars, A, D, 100 μm; B, 50 μm; C, 25 μm.
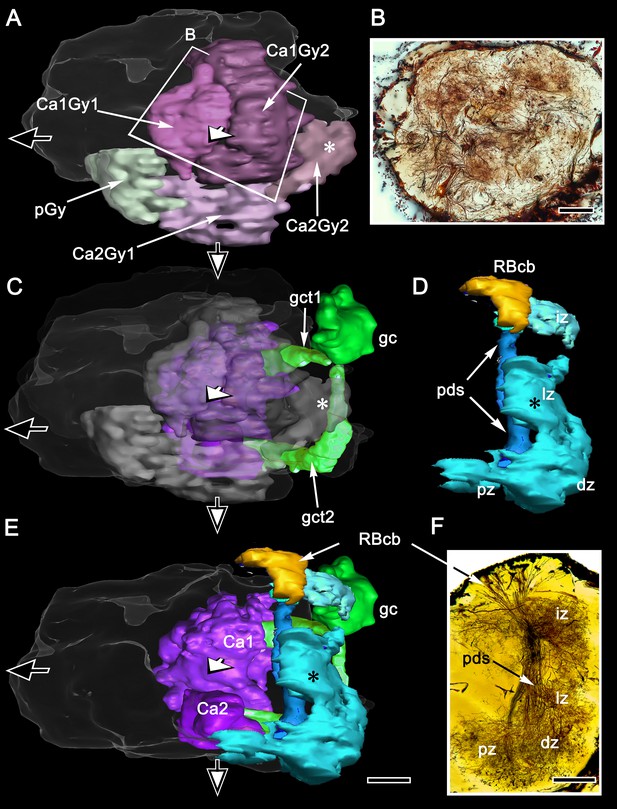
Disposition of the reniform body.
(A) 3D reconstruction of gyri from osmium-ethyl gallate specimens identifies their 2:1 relationship with the paired calyces. These can be compared to the distribution of gyrus neurons resolved by Golgi impregnations (B level indicated in A). The Golgi method impregnates a fraction of the constituent neurons, the fields of which are mostly constrained to a single gyrus. Here impregnated neurons are viewed through the lateral protocerebrum’s rostral surface. The reconstructions depict the lateral protocerebrum slightly rotated clockwise around the medial-lateral axis such that neuropils are viewed from above. In panel A (and panels C, E) the black arrow pointing left indicates medial, the white arrow pointing out of the plane of the page indicates rostral, and the filled arrow pointing downwards ventral. A simplified schematic of the relationships between the calyces and their cognate gyri is provided in Figure 13—figure supplement 2. (C) The globuli cell cluster (gc) provides two globuli cell tracts (gct1, gct2) to the two mushroom body calyces Ca1, Ca2. The lateral lobe of gyrus 2 associated with calyx 2 (Ca2Gy2, indicated by the asterisk in panel A) reaches out distally and partially fills a gap (asterisk in C) between two processes (the lateral and dorsal zones, iz and lz) originating from the reniform body’s pedestal (pds). (D) The reniform body pedestal (blue) is a dense fiber bundle originating from a dorsal cluster of small cell bodies (RBcb, yellow) at the dorsal surface of the rostral lateral protocerebrum. The reniform body’s neuropil volumes are colored cyan. The pedestal’s initial and lateral zones (iz, lz) are separated from its ventrally dividing branches, shown Golgi-impregnated in panel (F), that provide the distal and proximal zone neuropils (dz, pz). (E) Combined volumes from panels A, C, and D demonstrating the position and modest dimensions of the reniform body relative to the mushroom bodies, comprising the calyces (Ca1, Ca2), columns and associated gyri. Scale bars, A-C, B, F, 100 μm.
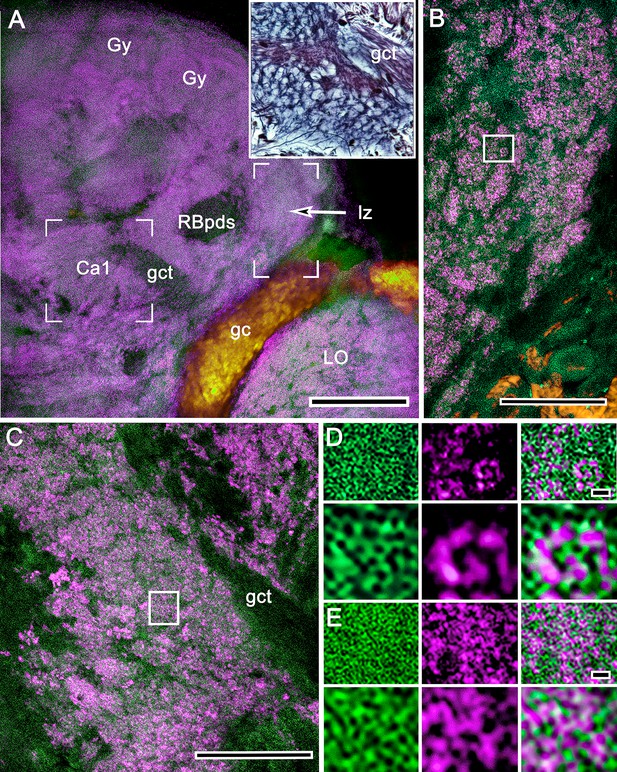
Comparison of synaptic densities associated with the calyces and reniform body.
(A) Calyx 1 (Ca1), its associated gyri (Gy), the reniform body pedestal (RBpds) and, at this level, its lateral zone (lz) are shown labeled with actin (green)/anti-synapsin (magenta). In A, the open square corresponds to a similar area of silver-stained microglomeruli (upper right inset). The same area is shown enlarged in panel C. The open rectangle in A is enlarged in B to the same scale as panel C. (B) Reniform body neuropils comprise synaptic configurations that contribute to large glomerulus-like islets. (C) Microglomeruli of the calyces are smaller and more densely packed than those of the reniform body. (D, E) Comparison of synapsin/actin contributions to glomerulus-like units of the reniform body (D) and to microglomeruli of the mushroom body calyx (E) further demonstrates major differences regarding the density of their converging presynaptic elements. Other abbreviations: LO, lobula; gc, globuli cells; gct, globuli cell tract. Scale bars, A, 100 μm; B,C, 25 μm; D, E, 1 μm.
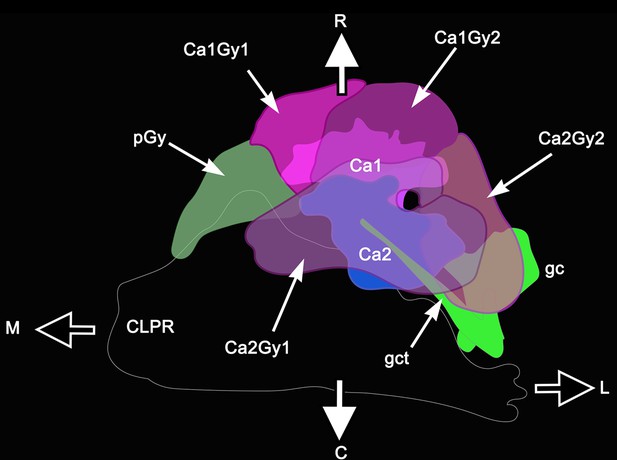
Calyx-gyri topographical relationships.
The lateral protocerebrum (minus optic lobes) is viewed from its ventral aspect. Details of the caudal lateral protocerebrum (CLPR) are omitted. Ca1, Ca2, calyces 1 and 2; Ca1Gy1, the medial of two gyri associated with Ca1; Ca1Gy2, second more lateral gyrus; Ca2Gy1, medio-ventral gyrus associated with Ca2; Ca2Gy2, second more lateral gyrus associated with Ca2. pGy, proximal gyrus. Axes: R, rostral; C, caudal; L, lateral (distal); M, medial (proximal; see also Figure 1a–c).
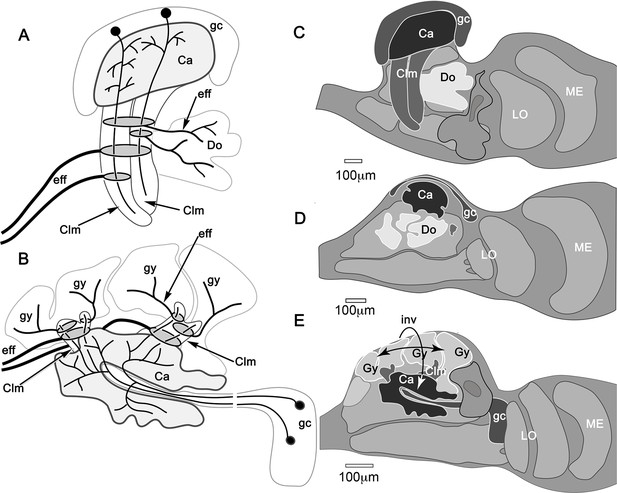
Mushroom body inversion and gyriform organization.
(A, B) Schematics of corresponding neuron ground patterns. (A) Morphological traits defining the mushroom body are consistent despite inversion. In Stomatopoda, intrinsic neuron dendrites corresponding to the Kenyon cells of insect mushroom bodies occupy specific domains (here levels) in the calyces (Ca). Their prolongations down the columns are intersected by efferent dendritic fields (shaded ovals) the axons of which (eff) extend medially (left) to the midbrain (not shown) or to domains (Do) deeper in the lateral protocerebrum. (B) In Hemigrapsus nudus, the mushroom body is inverted. Intrinsic neuron dendrites occupy characteristic planar territories in the calyces (Ca), with rostrally projecting extensions in the columns intersected by efferent neuron dendrites. As in stomatopods, axons of some efferent neurons (eff) extend medially to the superior protocerebrum of the midbrain (not shown). Other efferents supply the gyriform neuropils overlying the lateral protocerebrum (Gy). (C–E) Comparisons of mushroom bodies in Malacostraca. (C) In the stomatopod N. oerstedii, globuli cells cover the calyx; columns project caudally into the lateral protocerebrum neuropil. Neuropil domains (Do) within the lateral protocerebrum receive inputs from the lobes. (D) In the crayfish Procambarus clarkii, the lobeless mushroom body lies at the lateral protocerebrum’s rostral surface. Neuropils (Do) in the lateral protocerebrum receive its outputs. (E) In H. nudus, calyces are deeply buried in the lateral protocerebrum. Columns extend rostrally to an overlying gyriform neuropil, the entire arrangement indicating mushroom body inversion and expansion of the rostral gyri. Abbreviations: Ca, calyx; Clm, mushroom body column; Do, domains receiving mushroom body efferents; eff, efferent neurons; gc, globuli cells; Gy, gyri; LO, lobula; ME, medulla.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological sample | Hemigrapsus nudus | Friday Harbor Laboratories | N/A | n = 76 |
| Antibody | α-Tubulin (Mouse, monoclonal) | Developmental Studies Hybridoma Bank (DSHB) | CAT#: 12G10; RRID:AB_1157911 | 1:100 |
| Antibody | α-Tubulin (Rabbit, polyclonal) | Abcam | CAT#: ab15246; RRID:AB_301787 | 1:250 |
| Antibody | Synapsin (SYNORF1; Mouse, monoclonal) | Developmental Studies Hybridoma Bank (DSHB) | CAT#: 3C11; RRID:AB_528479 | 1:100 |
| Antibody | Serotonin (5HT; Rabbit, polyclonal) | ImmunoStar | CAT#: 20080; RRID:AB_572263 | 1:1000 |
| Antibody | Glutamic acid decarboxylase (GAD; Rabbit, polyclonal) | Sigma-Aldrich | CAT#: G5163; RRID:AB_477019 | 1:500 |
| Antibody | Tyrosine hydroxylase (TH; Mouse, monoclonal) | ImmunoStar | CAT#: 22941; RRID: AB_572268 | 1:250 |
| Antibody | Allatostatin (Ast7; Mouse, monoclonal) | Developmental Studies Hybridoma Bank (DSHB) | CAT#: 5F10; RRID:AB_528076 | 1:100 |
| Antibody | DC0 (Rabbit, polyclonal) | Generous gift from Dr. Daniel Kalderon (Skoulakis et al., 1993) | RRID:AB_2314293 | 1:400 |
| Antibody | AffiniPure Donkey Anti-Mouse IgG (H+L) Cy3 (polyclonal) | Jackson ImmunoResearch | CAT#: 715-165-150; RRID:AB_2340813 | 1:400 |
| Antibody | AffiniPure Donkey Anti-Rabbit IgG (H+L) Cy5 (polyclonal) | Jackson ImmunoResearch | CAT#: 711-175-152; RRID:AB_2340607 | 1:400 |
| Antibody | AffiniPure Donkey Anti-Rabbit IgG (H+L) Alexa Flour 647 (polyclonal) | Jackson ImmunoResearch | CAT#: 711-605-152; RRID:AB_2492288 | 1:400 |
| Other (serum) | Normal donkey serum | Jackson ImmunoResearch | RRID:AB_2337258 | N/A |
| Other (DNA stain) | SYTO 13 Green Fluorescent Nucleic Acid Stain | Thermo Fisher Scientific | CAT#: S7575 | 1:2000 |
| Other (Phalloidin stain) | Alexa Fluor 488 Phalloidin | Thermo Fisher Scientific | CAT#: 12379; RRID:AB_2315147 | 1:40 |
| Other (Chemical) | α-Terpineol | Sigma-Aldrich | CAT#: 432628 | N/A |
| Other (histology chemical) | Potassium dichromate | Sigma-Aldrich | CAT#: 207802 | N/A |
| Other (HPLC purified water) | HPLC Water | Sigma-Aldrich | CAT#: 270733 | N/A |
| Other (histology chemical) | Osmium tetroxide | Electron Microscopy Sciences | CAT#: 19150 | N/A |
| Other (histology chemical) | Ethyl gallate | Fisher Scientific | CAT#: G001625G | N/A |
| Other (histology chemical) | Phosphate buffer tablets | Sigma-Aldrich | CAT#:45ZE83 | N/A |
| Other (histology chemical) | Glutaraldehyde | Electron Microscopy Sciences | CAT#: 16220 | N/A |
| Other (histology chemical) | Paraformaldehyde | Electron Microscopy Sciences | CAT#: 15710 | N/A |
| Other (histology chemical) | Sodium cacodylate | Electron Microscopy Sciences | CAT#: 50-980-232 | N/A |
| Other (histology chemical) | Silver nitrate | Electron Microscopy Sciences | CAT#: 21050 | N/A |
| Other (histology chemical) | Pure copper shot | Sigma-Aldrich | CAT#: 326488 | N/A |
| Other (histology chemical) | Propylene oxide | Electron Microscopy Sciences | CAT#: 20401 | N/A |
| Other (embedding resin) | Durcupan ACM resin (4-part component kit) | Sigma-Aldrich | CAT#: 44610 | N/A |
| Other (embedding medium) | Paraplast Plus | Sigma-Aldrich | CA#: 76258 | N/A |
| Other (mounting medium) | Permount mounting medium | Fisher Scientific | CAT#: SP15-100 | N/A |
| Other (mounting medium) | Entellan | Sigma-Aldrich | CAT#: 1.07961 | N/A |
| Software, algorithm | Photoshop CC | Adobe Inc | N/A | N/A |
| Software, algorithm | Helicon Focus | Helicon Soft | N/A | N/A |
| Software, algorithm | Zen System Software | Zeiss | N/A | N/A |
| Software, algorithm | TrakEM2 | Cardona et al., 2012 | N/A | N/A |
| Software, algorithm | Amira 2019.4 | Thermo Fisher | N/A | N/A |
| Other (microscope) | LSM 5 Pascal confocal microscope | Zeiss | N/A | N/A |
| Other (microscope) | Orthoplan light microscope | Leitz | N/A | N/A |





