Ascorbic acid supports ex vivo generation of plasmacytoid dendritic cells from circulating hematopoietic stem cells
Figures
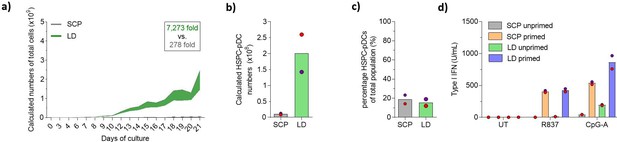
Lower cell density increases expansion during plasmacytoid dendritic cell (pDC) differentiation.
Hematopoietic stem and progenitor cells (HSPCs) were thawed and 2 × 105 cells were cultured for 21 days using the standard cultivation protocol (SCP) described previously, or with a low-density (LD) protocol (see Figure 1—figure supplement 1a and b for detailed culturing paradigms), after which HSPC-pDCs were isolated by immunomagnetic negative selection. (a) The total cumulative number of cells during culture was measured. To maintain culture format and minimize costs, a fraction of the culture was continuously discarded during passaging, which was taken into account when calculating cumulative cell numbers. (b) The number of HSPC-pDCs isolated after immunomagnetic negative selection was determined at day 21 and total cumulative number of HSPC-pDCs generated was calculated based on the fraction of cells discarded during culture. (c) Percentage of HSPC-pDCs of the total cell population at the day of isolation. (d) Isolated HSPC-pDCs were primed with type I IFN for 3 days or left unprimed, after which they were stimulated for 20 hr with agonists directed against TLR7 (R837) or TLR9 (CpG-A) and type I IFN was measured. Data shown represent mean of two cord blood donors.
-
Figure 1—source data 1
Source data related to Figure 1a-d.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig1-data1-v2.xlsx
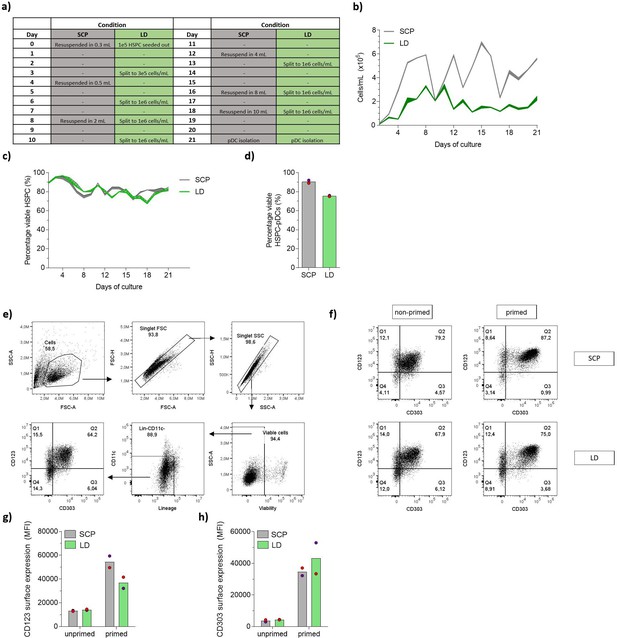
Lower hematopoietic stem and progenitor cell (HSPC) density increases expansion of HSPCs during HSPC-derived plasmacytoid dendritic cell (HSPC-pDC) differentiation.
HSPCs were thawed and 2 × 105 cells were cultured for 21 days using the standard cultivation protocol (SCP) described previously or with a low-density (LD) protocol. (a) Table showing the densities at which cells were split to during pDC differentiation. For the SCP protocol, cells were resuspended in fixed volumes disregarding cell numbers. (b) Density of cells during pDC differentiation prior to medium change. (c) Viability of isolated HSPC-pDCs. (d) Viability of cells during pDC differentiation. (e) Gating strategy used to analyze HSPC-pDCs. Sample is from non-primed HSPC-pDCs from SCP condition. Cells were first gated based on forward scatter (FSC) and side scatter (SSC) to exclude debris. Then doublets were excluded based on FSC and SSC. Next, a viability, lineage, and CD11c stain was used to gate on viable/lineageneg/CD11cneg cells for the indicated pDC-related markers. (f) Representative phenotypic analysis comparing the expression of CD123 and CD303 on non-primed and primed HSPC-pDCs from SCP or LD condition. (g, h) Surface expression of CD123 (g) and CD303 (h) on primed or non-primed HSPC-pDCs. Data shown represent mean of two cord blood donors.
-
Figure 1—figure supplement 1—source data 1
Source data related to Figure 1—figure supplement 1g-h.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig1-figsupp1-data1-v2.xlsx
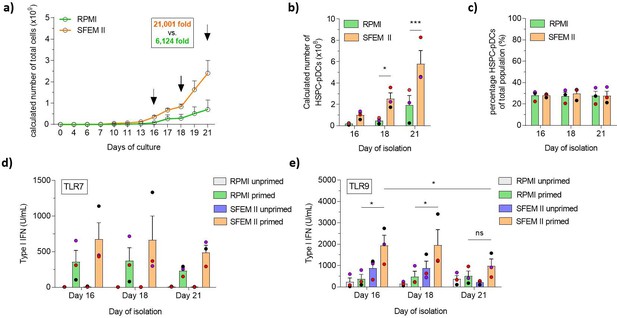
Serum-free conditions improve hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cell (HSPC-pDC) yield, and cells isolated at earlier time points retain a functional phenotype.
HSPCs were thawed and 1 × 105 cells were seeded in RPMI or SFEM II and cultured at a density of 0.5–5 × 106 cells/mL. HSPC-pDC isolation was performed after 16, 18, and 21 days of culture and HSPC-pDCs were phenotypically analyzed. (a) Calculated numbers of cells during culture. Arrows indicate days when HSPC-pDCs were isolated. (b) Numbers of isolated HSPC-pDCs. (c) Proportion HSPC-pDCs within the total population of cells at the day of isolation. (d, e) Type I interferon (IFN) responses of non-primed or primed HSPC-pDCs after activation with the TLR7 agonist R837 (c) or the TLR9 agonist CpG 2216 (d). Data shown represent ± SEM of three donors (a-c) and three donors each analyzed in technical triplicates (d-e).
-
Figure 2—source data 1
Source data related to Figure 2a-e.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig2-data1-v2.xlsx
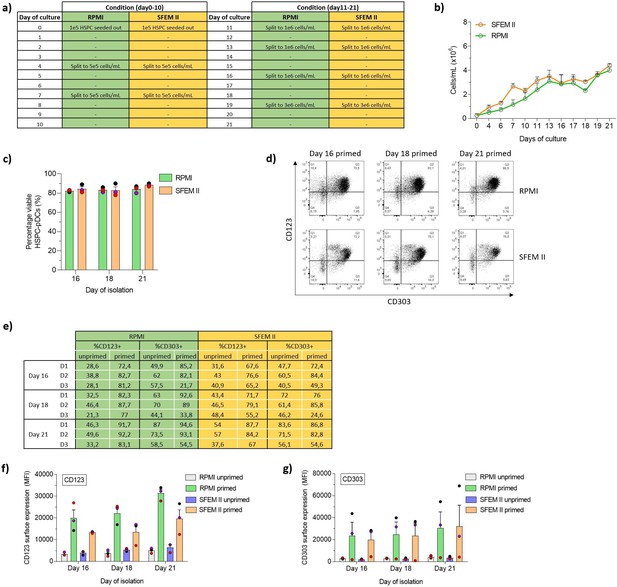
Serum-free conditions improve expansion of hematopoietic stem and progenitor cells (HSPCs) and HSPC-derived plasmacytoid dendritic cells (HSPC-pDCs) isolated at earlier time points retain a functional phenotype.
HSPCs were thawed and 1 × 105 cells were cultured in RPMI or SFEM II at a density of 0.5–5 × 106 cells/mL. HSPC-pDC isolation was performed after 16, 18, and 21 days of culture and cryopreserved. HSPC-pDCs were later thawed, primed for 3 days and subsequently phenotypically analyzed. (a) Table showing days were cells were split to a new density (between 0.5–5 × 106 cells). (b) Cell density of HSPCs during HSPC-pDC differentiation prior to medium change. (c) Viability of isolated HSPC-pDCs. (d) Representative flow cytometry plots showing primed HSPC-pDCs generated using either serum or serum-free conditions and isolation after 16, 18, and 21 days of culture. (e) Table showing the percentage of isolated pDCs expressing CD123 and CD303. (f, g) Surface expression of CD123 (f) or CD303 (g) on non-primed or primed HSPC-pDCs. Data shown represent ± SEM of three donors.
-
Figure 2—figure supplement 1—source data 1
Source data related to Figure 2—figure supplement 1b-c and f-g.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig2-figsupp1-data1-v2.xlsx
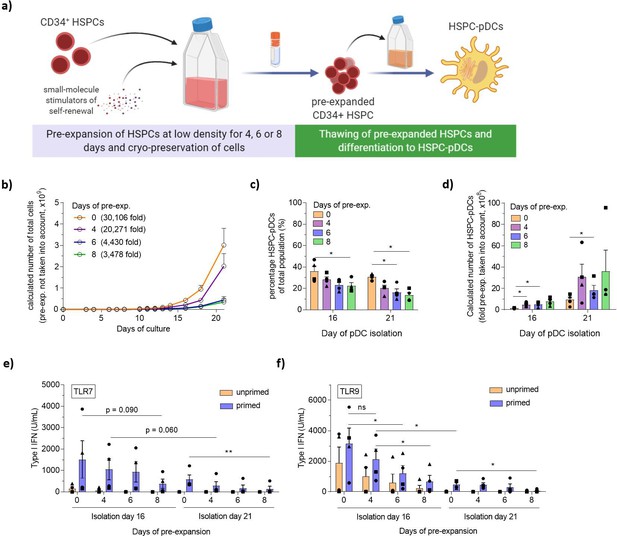
Pre-expansion of hematopoietic stem and progenitor cells (HSPCs) increases the yield of HSPC-derived plasmacytoid dendritic cells (HSPC-pDCs).
(a) Schematic representation showing generation of HSPC-pDCs from pre-expanded HSPCs. HSPCs were pre-expanded at low density (1–5 × 105 cells/mL) in SFEM II medium supplemented with UM171 for 4, 6, or 8 days and then cryopreserved. Cells were then thawed, phenotyped for CD34, and 1 × 105 HSPCs were seeded for HSPC-pDC generation. HSPC-pDCs were isolated after either 16 or 21 days of culture and phenotypically analyzed. (b) Calculated number of cells during HSPC-pDC differentiation using pre-expanded HSPCs without the pre-expansion factor taken into account (same starting cell number at differentiation). (c) Percentage of HSPC-pDCs of total population of cells. (d) Calculated number of HSPC-pDCs isolated after 16 and 21 days of culture with fold pre-expansion taken into account. (e, f) Levels of type I interferon (IFN) from HSPC-pDCs after stimulation with the TLR7 agonist R837 (e) or the TLR9 agonist CpG-2216 (f). Data shown represent ± SEM of four donors (a–d) and four donors each analyzed in technical triplicates (e, f).
-
Figure 3—source data 1
Source data related to Figure 3b-f.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig3-data1-v2.xlsx
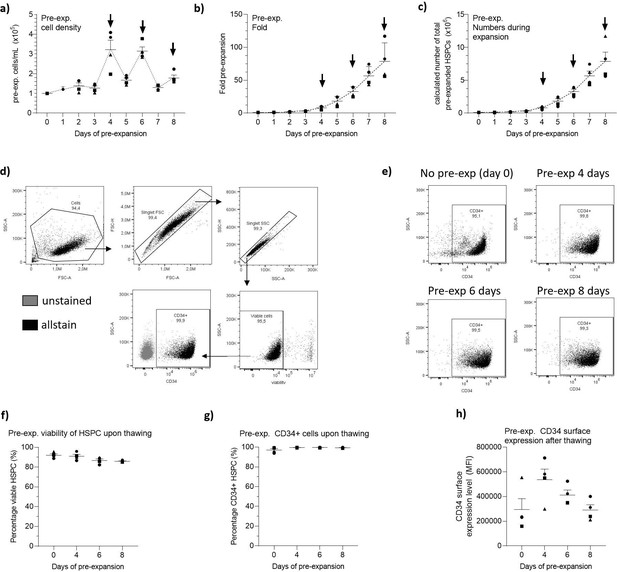
Pre-expansion of hematopoietic stem and progenitor cells (HSPCs).
HSPCs were pre-expanded at low density (1–5 × 105 cells/mL) for 4, 6, or 8 days and cryopreserved. Cells were then thawed and phenotyped for CD34 marker expression. (a) HSPC density during pre-expansion prior to medium change. Arrows indicate points were HSPCs were cryopreserved. (b, c) Fold pre-expansion (b) and calculated number of pre-expanded cells (c). (d) Gating strategy to discriminate CD34+ cells. Cells were first gated based on FSC and SSC to exclude debris, and then doublets were excluded in regards to FSC-A and SSC-A. A viability stain was next used to gate on viable cells to analyze the expression of CD34 (allstain). (e) Phenotypic analysis of pre-expanded CD34+ HSPCs with the same gates as determined in (d). (f) Viability of cells. (g) Percentage of CD34+ cells on thawed pre-expanded HSPCs. (h) Surface expression of CD34 on pre-expanded HSPCs. Data shown represent ± SEM of four donors.
-
Figure 3—figure supplement 1—source data 1
Source data related to Figure 3—figure supplement 1a-c and f-g.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig3-figsupp1-data1-v2.xlsx
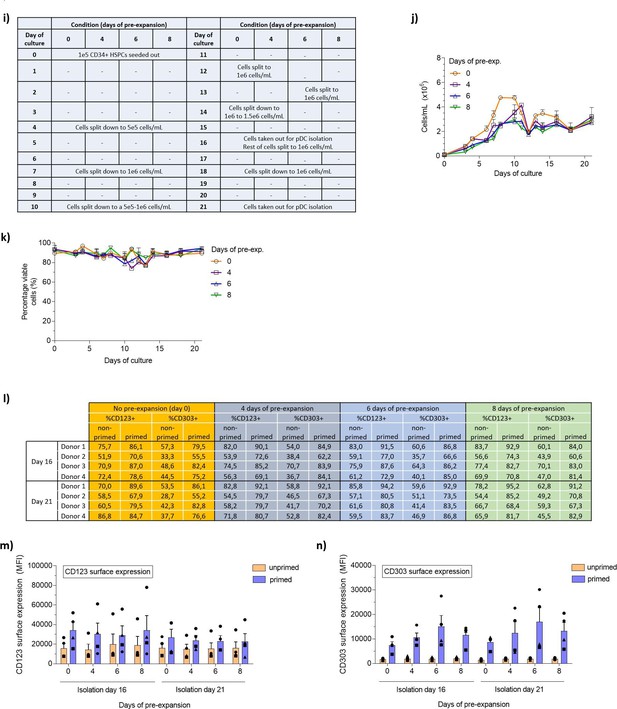
Differention of pre-expanded hematopoietic stem and progenitor cells to HSPC-derived plasmacytoid dendritic cells (HSPC-pDCs).
HSPC-pDCs from HSPCs that were pre-expanded at low density (1–5 × 105 cells/mL) for 4, 6, or 8 days. 1 × 105 HSPCs were seeded for HSPC-pDC generation. (i) Table showing when cells were split during culture. (j) Cell density during HSPC-pDC differentiation prior to medium change. (k) Viability of pre-expanded HSPCs during differentiation into HSPC-pDCs. (l) Table showing the percentage of isolated HSPC-pDCs expressing CD123 and CD303. (m) Surface expression of CD123 on primed or non-primed HSPC-pDCs from pre-expanded HSPCs. (n) Surface expression of CD303 on primed or non-primed HSPC-pDCs from pre-expanded HSPCs. Data shown represent ± SEM of four donors.
-
Figure 3—figure supplement 2—source data 1
Source data related to Figure 3—figure supplement 2m-n.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig3-figsupp2-data1-v2.xlsx
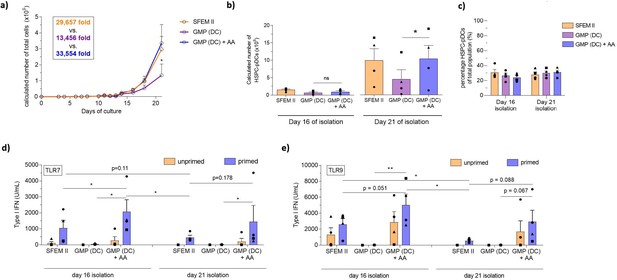
Ascorbic acid medium supplementation is required for hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cell (HSPC-pDC) generation using the current good manufacturing process (cGMP)-compliant DC medium.
HSPCs were thawed and 1 × 105 cells were seeded in SFEM II, the cGMP-compliant medium DC medium (GMP [DC]), or DC medium supplemented with ascorbic acid (GMP [DC] + AA). For all conditions, cells were kept at a density of 0.5–5 × 106 cells/mL throughout culture. HSPC-pDCs were isolated after 16 and 21 days of culture and phenotypically analyzed. (a) Calculated number of total cells during HSPC-pDC differentiation. (b) Calculated number of HSPC-pDCs after isolation at 16 days or 21 days of culture. (c) Percentage of HSPC-pDCs of total cells. (d, e) Type I interferon (IFN) response of IFN primed or unprimed HSPC-pDCs isolated after 16 or 21 days of culture after activation with the TLR7 agonist R837 (d) or the TLR9 agonist CpG-2216 (e). Data shown represent ± SEM of four donors (a–c) and ± SEM of four donors each analyzed in technical triplicates (d, e).
-
Figure 4—source data 1
Source data related to Figure 4a-e.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig4-data1-v2.xlsx
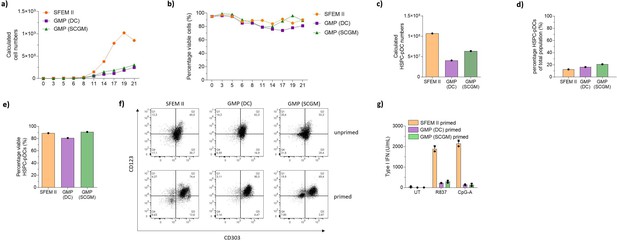
cGMP compliant mediums fails to produce functional hematopoeitic stem and progenitor cell-derived plasmacytoid dendritic cells (HSPC-pDCs).
(a–g) 1 × 105 HSPCs were cultured in SFEM II, the current good manufacturing process (cGMP)-compliant DC medium (GMP [DC]), or the cGMP-compliant SCGM (GMP [SCGM]). For all conditions, cells were kept at a density of 0.5–5 × 106 cells/mL throughout culture. HSPC-pDCs were isolated after 21 days of culture and phenotypically and functionally analyzed. (a) Calculated number of cells during HSPC-pDC differentiation. (b) Viability of cells during HSPC-pDC differentiation. (c) Calculated number of isolated HSPC-pDCs after 21 days of culture. (d) Percentage of HSPC-pDCs of total cells. (e) Viability of isolated HSPC-pDCs. (f) Representative flow cytometry plots showing the expression of CD123 versus CD303 on lin-CD11c- cells for HSPC-pDCs isolated at day 16 from the different culture conditions. (g) Type I interferon (IFN) response of HSPC-pDCs after stimulation with the TLR7 agonist R837 or the TLR9 agonist CpG-2216.Data shown are for one donor (a–f), ± SD of technical replicates from one donor (g).
-
Figure 4—figure supplement 1—source data 1
Source data related to Figure 4-figure supplement 1a-e and g.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig4-figsupp1-data1-v2.xlsx
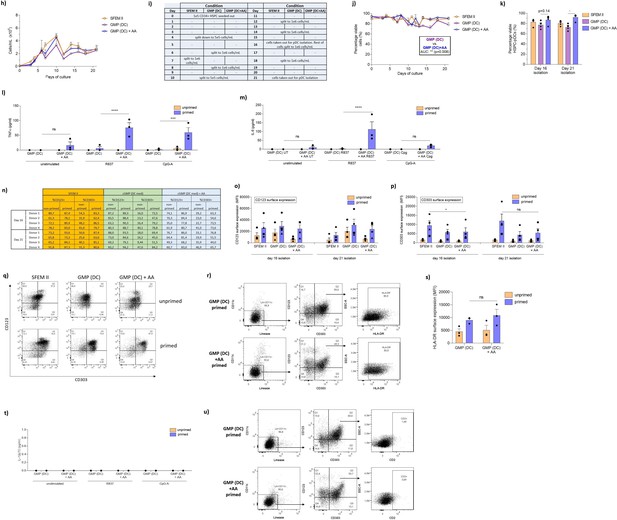
Ascorbic acid is required for generation of functional hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cells (HSPC-pDCs) with DC medium.
(h–o) HSPC-pDCs were generated using either SFEM II, the cGMP-compliant medium DC medium (GMP [DC]), or DC medium supplemented with ascorbic acid (GMP [DC] + AA). For all conditions, cells were kept at a density of 0.5–5 × 106 cells/mL throughout culture. HSPC-pDCs were isolated after 16 and 21 days of culture and phenotypically analyzed. (h) Density of cells throughout pDC differentiation prior to medium change. (i) Table showing the days where cells were split to a new density (0.5–2 × 106 cells/mL). (j) Viability of cells during HSPC-pDC differentiation. (k) Viability of HSPC-pDCs isolated after 16 or 21 days of culture. (l, m) Unprimed or primed HSPC-pDCs generated using GMP (DC) or GMP (DC) + AA were activated with TLR7 or TLR9 agonists. 20 hr later, supernatants were collected and levels of TNFα (l) or IL6 (m) were analyzed. (n) Table showing the percentage of isolated HSPC-pDCs expressing CD123 and CD303. (o, p) Surface expression levels of CD123 (o) or CD303 (p) for primed or non-primed HSPC-pDCs. (q) Representative flow cytometry plots showing the expression of CD123 versus CD303 on lin-CD11c- cells for HSPC-pDCs isolated at day 16 from the different culture conditions. (r–u) HSPC-pDCs were generated using either the DC medium (GMP [DC]) or the GMP (DC) supplemented with ascorbic acid (GMP [DC] + AA). (r) Representative flow plot showing expression of HLA-DR on primed HSPC-pDCs. (s) Surface expression levels of HLA-DR on IFN primed and unprimed HSPC-pDCs. (t) IL-12 response of unprimed or IFN-primed HSPC-pDCs upon stimulation with the TLR7 agonist R837 or the TLR9 agonist CpG-A. (u) Representative flow cytometry plots showing expression of CD2 on primed HSPC-pDCs. Data shown ± SEM of four donors (h-k and n–q), ± SEM of three donors (l, m and r–u).
-
Figure 4—figure supplement 2—source data 1
Source data related to Figure 4—figure supplement 2h, j-m,- o-p and s-t.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig4-figsupp2-data1-v2.xlsx
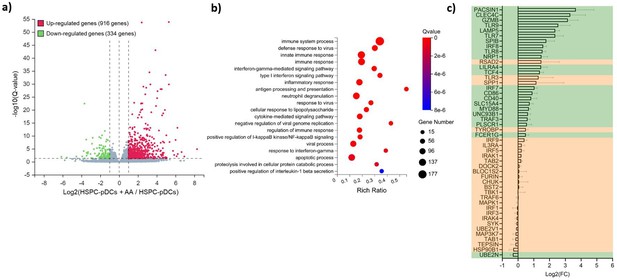
RNA-seq profile of hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cells (HSPC-pDCs) generated with ascorbic acid (AA) shows upregulation of multiple genes related to pDC function.
HSPCs were thawed and 1 × 105 cells were seeded in DC medium with or without supplementation of AA. Following 16 days of differentiation, pDCs were isolated by immunomagnetic depletion of non-pDCs, and HSPCs-pDCs were then primed for 72 hr with IFN, after which RNA was extracted and subjected to RNA-seq. (a) Volcano plot showing differentially expressed genes in the HSPC-pDCs generated with AA compared to HSPC-pDCs generated without AA. (b) Gene ontology bubble chart displaying the 20 most enriched biological processes for the differentially expressed genes in HSPC-pDCs generated with AA. The x-axis shows the enrichment ratio (rich ratio), which is the ratio between the number of differentially expressed genes within the biological process and the number of total genes annotated in that process. The size of the bubble represents the number of differentially expressed genes within the process, and the color represents the statistical significance of the enrichment. (c) Table showing differential expression of genes related to pDC development and function following addition of AA to the culture medium. The axis indicates log2 fold changes (log2FC) of gene expression in HSPC-pDCs generated with AA relative to cells generated without AA. Data shown are for three donors. Genes highlighted in green indicate significantly differentially expressed (Q-value below 0.05).
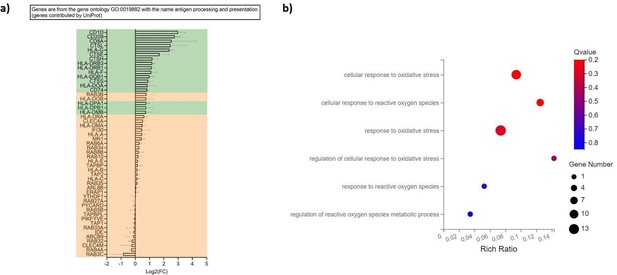
Ascorbic acid upregulates genes associated with antigen presentation.
Hematopoietic stem and progenitor cells (HSPCs) were thawed and 1 × 105 cells were seeded in DC medium with or without supplementation of ascorbic acid. Following 16 days of differentiation, plasmacytoid dendritic cells (pDCs) were isolated by immunomagnetic depletion of non-pDCs, and HSPCs-pDCs were then primed for 72 hr with IFN‐β/γ, after which RNA was extracted and subjected to RNA-seq. (a) Table showing differential expression of genes related to the gene ontology ‘antigen processing and presentation’. The x-axis indicates log2 fold changes (log2FC) of gene expression in HSPC-pDCs generated with ascorbic acid relative to cells generated without ascorbic acid. Genes highlighted in green indicate significant differential expression (Q-value below 0.05). (b) Gene ontology bubble chart displaying gene ontologies for biological processes relating to reactive oxygen species and oxidative stress for the differentially expressed genes in HSPC-pDCs generated with ascorbic acid. The x-axis shows the enrichment ratio (rich ratio), which is the ratio between the number of differentially expressed genes within the biological process and the number of total genes annotated in that process. The size of the bubble represents the number of differentially expressed genes within the process, and the color represents the statistical significance of the enrichment. All data shown are for three donors.
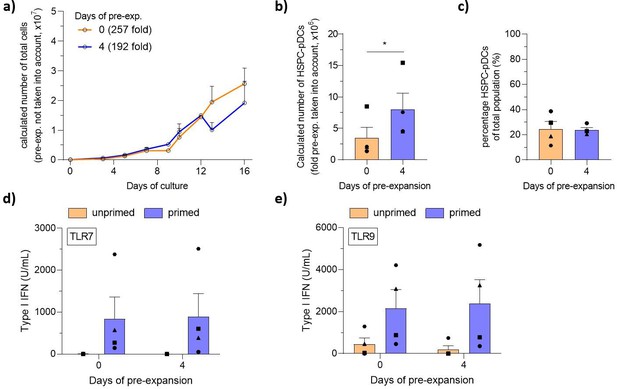
Generation of hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cells from circulating HSPCs (cHSPCs) from peripheral whole blood using optimized current good manufacturing process (cGMP)-compliant medium.
cHSPCs were pre-expanded for 4 days at low density (1–5 × 105 cells/mL) in cGMP-compliant medium (SCGM) supplemented with UM171 and then cryopreserved. Subsequently, cells were thawed, phenotyped for CD34, and 1 × 105 cHSPCs were seeded for HSPC-pDC generation. HSPC-pDCs were isolated after 16 days of culture and phenotypically analyzed. (a) Calculated number of cells during HSPC-pDC differentiation using pre-expanded HSPCs (without the pre-expansion factor taken into account). (b) Calculated number of HSPC-pDCs upon isolation of HSPC-pDCs at 16 days of culture (with fold pre-expansion taken into account). (c) Percentage of HSPC-pDCs of the total population of cells. (d, e) Levels of type I IFN upon stimulation of HSPC-pDCs with the TLR7 agonist R837 (d) or the TLR9 agonist CpG-2216 (e). Data shown represent ± SEM of four donors (a–c) and four donors each analyzed in technical triplicates (d, e).
-
Figure 6—source data 1
Source data related to Figure 6a-e.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig6-data1-v2.xlsx
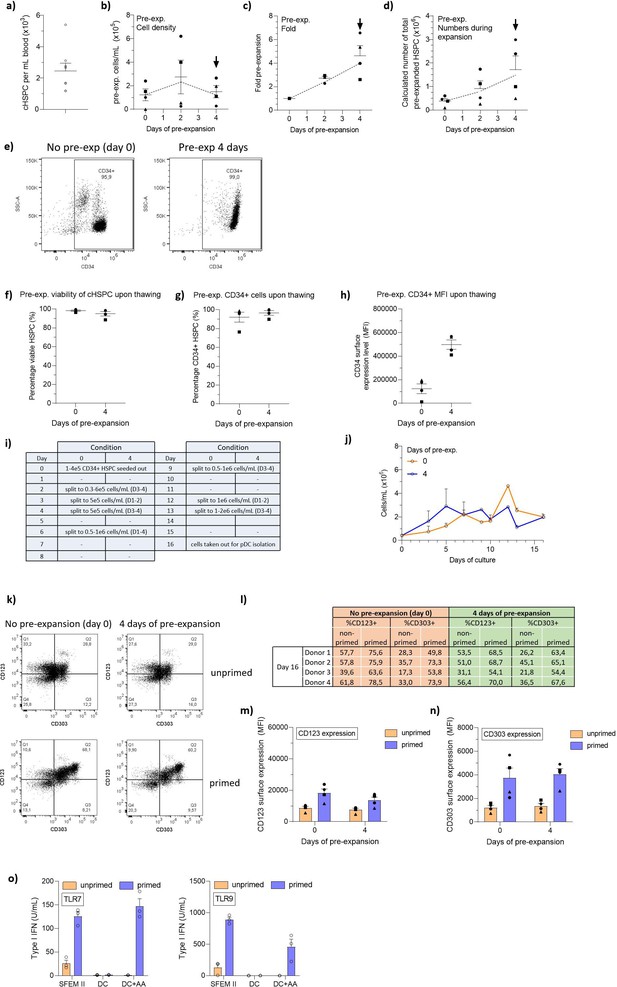
Differentiation of circulating CD34+ hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cells (cHSPC-pDCs) from cHSPCs using optimized current good manufacturing process (cGMP)-compliant conditions.
Circulating CD34+ HSPCs (cHSPCs) were isolated from buffy coats and cells were either directly cryopreserved or pre-expanded at low density (1–5 × 105 cells/mL) for 4 days and then cryopreserved. Cells were then thawed, phenotyped, and 1 × 105 HSPCs were seeded for HSPC-pDC generation. (a) Numbers of cHSPCs that can be isolated per mL of blood (assuming 450 mL blood per buffy coat) for eight healthy donors. (b) HSPC density during pre-expansion prior to medium change. Arrows indicate time points when HSPCs were cryopreserved. (c) Fold pre-expansion of cHSPCs. (d) Calculated number of pre-expanded cHSPCs. (e) Representative flow cytometry plots showing the expression of CD34 on freshly isolated cHSPCs or cHSPCs after pre-expansion for 4 days. (f) Viability of cells. (g) Percentage of CD34+ cells. (h) CD34 surface expression of cells. (i) Table showing when cells were split during differentiation of cHSPCs into cHSPC-pDCs. (j) Density of cells during cHSPC-pDC differentiation prior to medium change. (k) Representative flow cytometry plots showing expression of CD123 versus CD303 on lin-CD11c- cells for HSPC-pDCs from the different culture conditions. (l) Table showing the percentage of isolated HSPC-pDCs expressing CD123 and CD303. (m) Surface expression of CD123 on primed or non-primed HSPC-pDCs. (n) Surface expression of CD303 on primed or non-primed HSPC-pDCs. (o) Type I interferon (IFN) response of HSPC-pDCs generated from cHSPCs using either SFEM II medium, DC medium, or DC medium supplemented with ascorbic acid (AA). HSPC-pDCs were activated with either the TLR7 agonist R837 or the TLR9 agonist CpG-2216. Data shown represent ± SEM of eight (a) and four (b–n) donors, and one donor analyzed in technical triplicates (o).
-
Figure 6—figure supplement 1—source data 1
Source data related to Figure 6—figure supplement 1a-d, f-j, m-o.
- https://cdn.elifesciences.org/articles/65528/elife-65528-fig6-figsupp1-data1-v2.xlsx
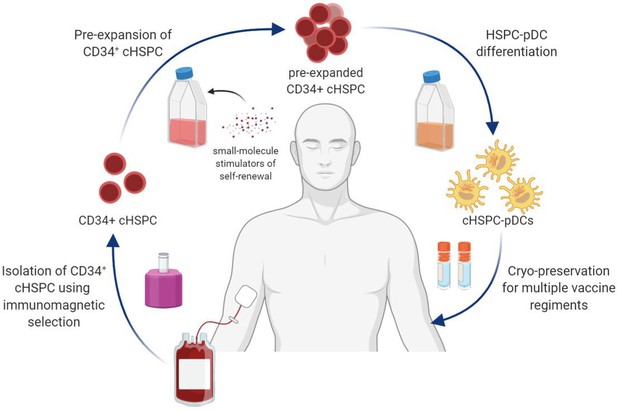
Schematic illustration showing the collective procedure of generating circulating CD34+ hematopoietic stem and progenitor cell-derived plasmacytoid dendritic cells (cHSPC-pDCs) for therapeutic purposes starting from a patient blood sample.
CD34+ cHSPCs are initially isolated using immunomagnetic selection. cHSPCs are then pre-expanded at low density using small molecules that promote self-renewal. Subsequently, pre-expanded cHSPCs are differentiated into cHSPC-pDCs, which can either be readily used for immunotherapeutic purposes or cryopreserved to allow for multiple vaccine regiments.





