Dwarf open reading frame (DWORF) is a direct activator of the sarcoplasmic reticulum calcium pump SERCA
Figures
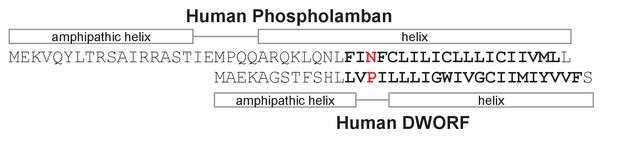
Sequence alignment, secondary structure prediction, and transmembrane domain prediction for phospholamban and dwarf open reading frame (DWORF).
The predicted transmembrane domains are in bold letters. The sequences are aligned between Asn34 of phospholamban and Pro15 of DWORF (Primeau et al., 2018). The helical regions of phospholamban are based on the NMR structure (PDB code 2KYV). The helical regions of DWORF are based on sequence predictions and molecular dynamics simulations in the present study.
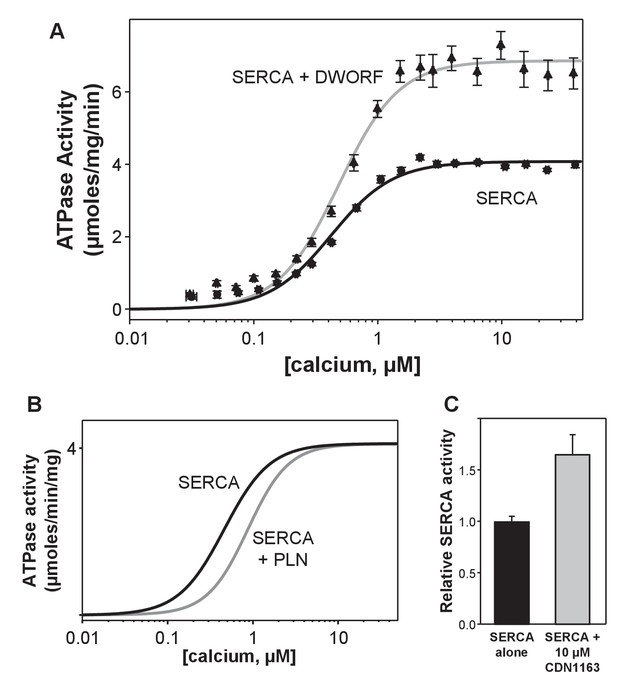
ATPase activity of sarco-endoplasmic reticulum calcium pump (SERCA) co-reconstituted into membrane vesicles in the absence and presence of dwarf open reading frame (DWORF).
(A) ATPase activity of SERCA membrane vesicles in the absence (black line) and presence of DWORF (gray line). (B) Comparison to previously published ATPase activity for SERCA in the absence (black line) and presence of phospholamban (gray line). Only the fitted curves from reference (Anderson et al., 2015) are shown. (C) ATPase activity of SERCA membrane vesicles in the absence (black bar) and presence (gray bar) of the small molecule activator CDN1163 at saturating calcium concentration (3 µM).
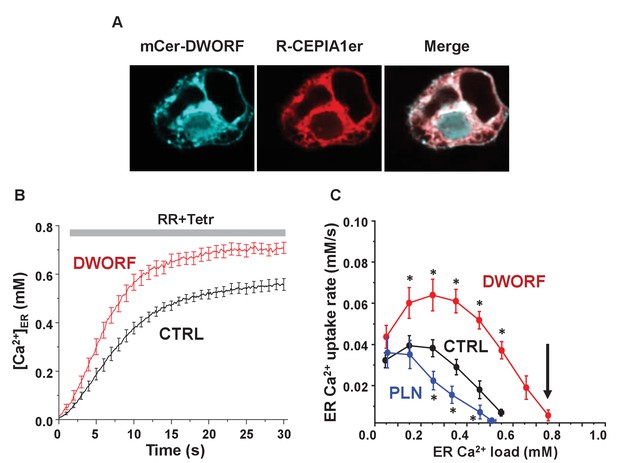
Dwarf open reading frame (DWORF) enhances sarco-endoplasmic reticulum calcium pump (SERCA)-dependent calcium dynamics in cells.
(A) Inducible human SERCA2a stable cell line (t-Rex-293 cells + Tetr) was transfected with mCer-DWORF together with the Ca2+ release channel ryanodine receptor (RyR)2 and the endoplasmic reticulum (ER)-targeted Ca2+ indicator R-CEPIA1er. MCer-DWORF expression in these cells showed a similar pattern as R-CEPIA1er. (B) The rate of [Ca2+]ER reuptake after full ER Ca2+ depletion by caffeine followed by RyR2 inhibition with ruthenium red (RR). (C) The ER Ca2+ uptake rate was plotted against the corresponding ER [Ca2+] load. DWORF expression almost doubled ER Ca2+ uptake rate through the entire range of physiological ER Ca2+ loads, which is the opposite trend seen for the SERCA inhibitor phospholamban. DWORF also increased the ER [Ca2+] load (arrow). *p<0.05 versus CTRL.
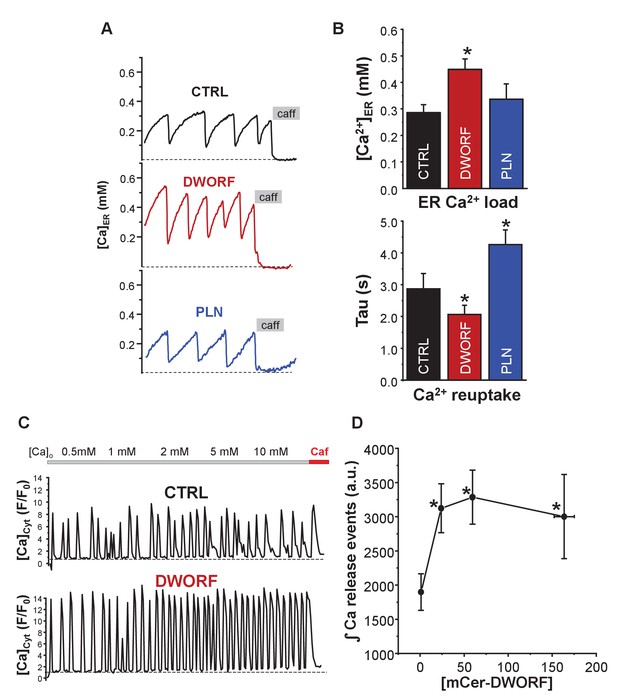
Effect of dwarf open reading frame (DWORF) on spontaneous calcium release from the endoplasmic reticulum (ER).
(A, B) Co-expression of sarco-endoplasmic reticulum calcium pump (SERCA)2a and ryanodine receptor (RyR2) produced Ca2+ waves due to spontaneous activation of RyR2 followed by SERCA Ca2+ reuptake. DWORF increased the magnitude ([Ca2+]ER) and frequency (rate of recovery of [Ca2+]ER, Tau(s)) of spontaneous Ca2+ waves, while phospholamban significantly decreased it. Similar effects of DWORF on spontaneous calcium release were observed when Ca2+ waves were measured as cytosolic Ca2+ fluctuations in intact cells. (C) Average amplitude and frequency of RyR-mediated Ca2+ release events were significantly increased in DWORF-expressing cells. (D) The integral of RyR-mediated Ca2+ release events was significantly increased in DWORF-expressing cells.
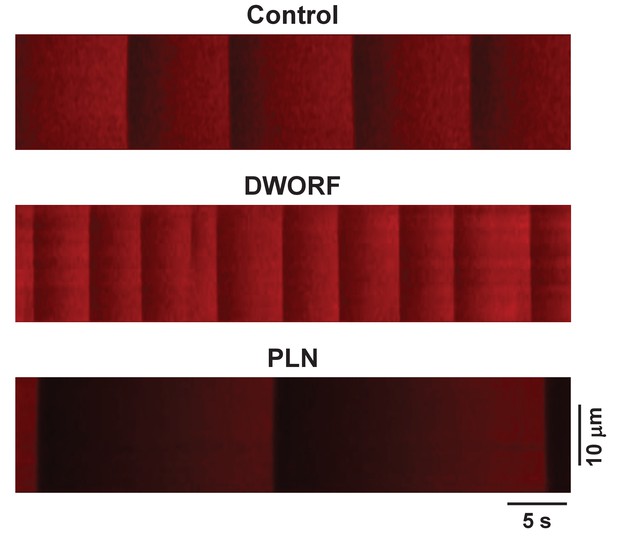
Examples of the experimental protocol used to measure sarco-endoplasmic reticulum calcium pump-mediated endoplasmic reticulum (ER) Ca2+ uptake.
Representative line-scan images of R-CEPIA1er during an initial 45 s recording are shown. Line-scan images of R-CEPIA1er were calibrated with ionomycin (see Materials and methods) to generate profiles of changes in ER Ca2+ concentration ([Ca2+]ER) as shown in Figure 4A. Experimental details can be found in Bovo et al., Am J Physiol Heart Circ Physiol (2019) 316:H1323-H1331.
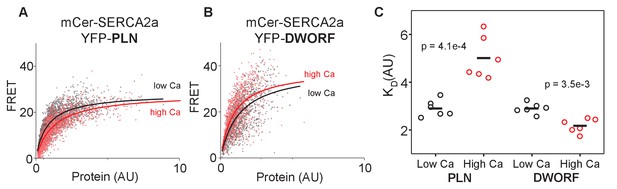
FRET analysis of sarco-endoplasmic reticulum calcium pump–dwarf open reading frame (SERCA-DWORF) interactions.
The average acceptor sensitization FRET efficiency of cells co-transfected with mCer-SERCA2a and either (A) YFP-PLN or (B) YFP-DWORF. FRET efficiency was measured at high and low calcium concentrations to assess the relative affinity of phospholamban (PLN) and DWORF for the calcium-free and calcium-bound conformations of SERCA. (C) Hyperbolic fits to data provide quantification of the apparent dissociation constant (KD) of the SERCA-PLN or SERCA-DWORF regulatory complexes. Ca decreases the apparent affinity of PLN for SERCA, yet it increases the affinity of DWORF for SERCA.
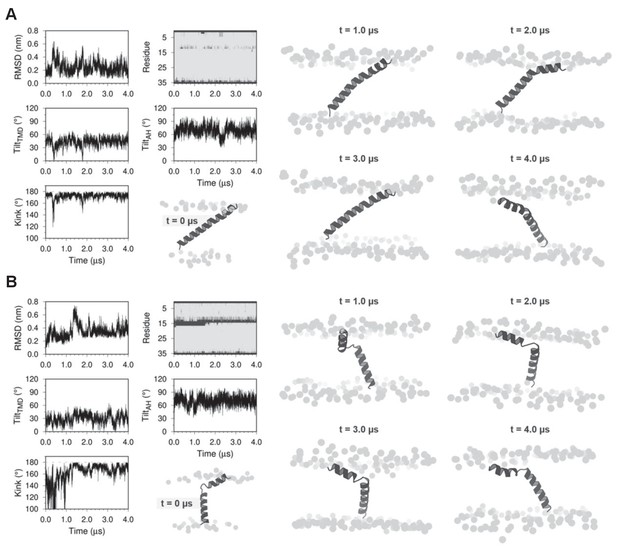
Molecular dynamics (MD) simulations of dwarf open reading frame (DWORF) modeled as a continuous helix (A) and as a helix-linker-helix (B).
Shown are snapshots during the simulations (0, 1, 2, 3, and 4 µs), as well as the RMSD (nm), kink angle, per-residue secondary structure (coil in dark gray and helix in light gray), and tilt angles of the transmembrane helix domain (TMD) and the amphipathic helix (AH). Notice that both simulations support a helix-linker-helix structure of DWORF. The MD simulation in (B) indicates that the helix-linker-helix structure is maintained, though it is dynamic during the time course of the simulation.
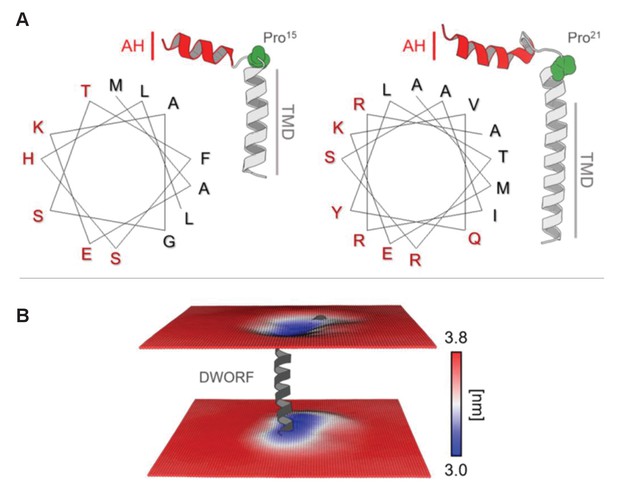
Helix-linker-helix model of dwarf open reading frame (DWORF).
(A) Hydrophobic moment analysis revealed amphipathic helices at the N-terminus of DWORF (left panel) and phospholamban (PLN) (right panel). Notice the similar distribution of polar (red) and apolar (black) residues in the helix-kink structure of DWORF and PLN N-terminal to a proline residue (Pro15 in DWORF and Pro21 in PLN). (B) Time-averaged local membrane thickness in the molecular dynamics simulation of the DWORF helix-linker-helix model. Notice how the bilayer becomes thinner around the short transmembrane helix and amphipathic helix of DWORF. The maximum and minimum widths of the lipid bilayer are indicated by the blue-white-red color-coded bar.
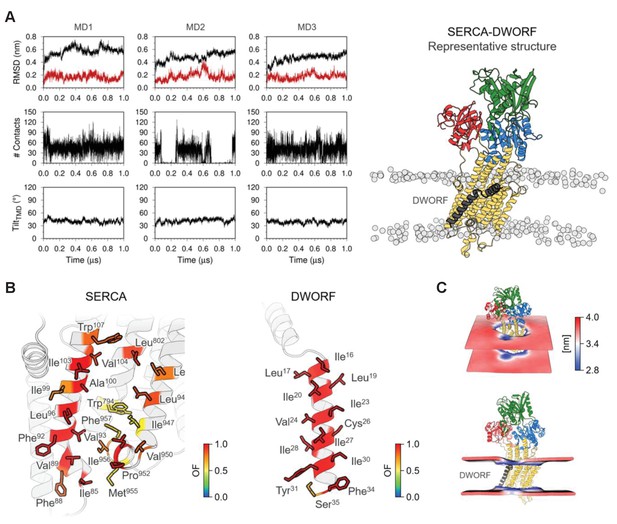
Molecular model for the interaction of sarco-endoplasmic reticulum calcium pump (SERCA) with dwarf open reading frame (DWORF).
(A) Backbone RMSD, number of contacts between SERCA and DWORF, and tilt angle of DWORF transmembrane helix domain in the three 1 µs replicates of SERCA-DWORF molecular dynamics (MD) simulations. The figure to the right shows the representative structure of the SERCA-DWORF complex from the RMSD clustering analysis of the three simulations. SERCA is colored yellow, with the nucleotide-binding domain in green, the phosphorylation domain in blue, and the actuator domain in red. DWORF is shown in gray. (B) SERCA residues located within 3.0 Å of DWORF (left panel) and DWORF residues located within 3.0 Å of SERCA (right panel) colored by the computed occupancy fraction (OF) of the three independent simulations. Only the residues that presented an OF ≥ 0.6 are shown in the figure. (C) Representation of the averaged local membrane thickness of SERCA-DWORF complex calculated with the first replicate MD1.
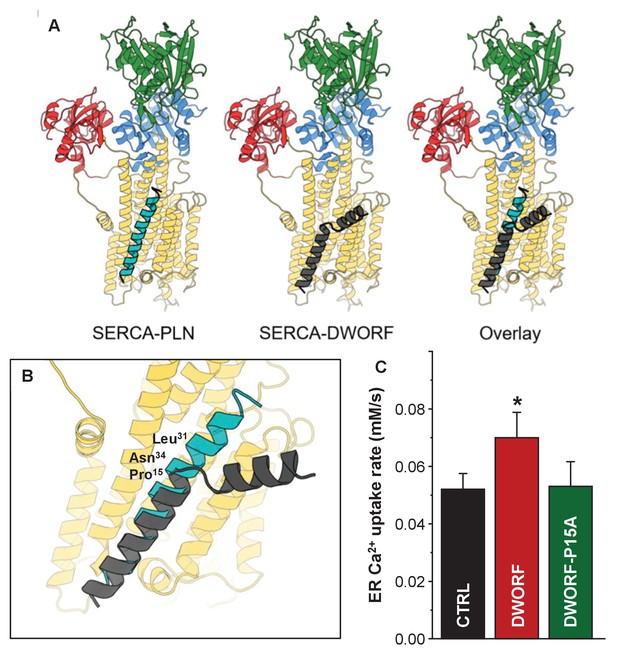
Molecular model for the interaction of sarco-endoplasmic reticulum calcium pump (SERCA) with dwarf open reading frame (DWORF).
(A) SERCA-PLN, SERCA-DWORF, and the overlay are shown in cartoon format. The molecular model of DWORF (Figure 6A) was superimposed on the structure of the SERCA-PLN complex (PDB ID: 4KYT) according to the sequence alignment in Figure 1. SERCA is colored yellow, with the nucleotide-binding domain in green, the phosphorylation domain in blue, and the actuator domain in red. Phospholamban (PLN) is shown in cyan and DWORF in gray. (B) Close-up view of the SERCA-PLN and SERCA-DWORF complexes. The relative positions of Leu31 and Asn34 are indicated, as well as Pro15 of DWORF, which aligns with Asn34 of PLN. (C) Endoplasmic reticulum calcium uptake rate for the Pro15-Ala (P15A) mutant of DWORF compared to SERCA alone (CTRL) and SERCA in the presence of wild-type DWORF (DWORF). *p<0.01.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Escherichia coli) | BL21(DE3) | Sigma-Aldrich | CMC0016 | Chemically competent cells |
| Recombinant DNA reagent | pMAL c2x DWORF plasmid | This study | MBP-DWORF fusion; H.S. Young lab | |
| Peptide, recombinant protein | Human DWORF | This study | NCBI: NP_001339058.1 | Purified human DWORF peptide; H.S. Young lab |
| Chemical compound, drug | CDN1163; 4-(1-methylethoxy)-N-(2-methyl-8-quinolinyl)-benzamide | Sigma-Aldrich | SML1682 | Previously published SERCA activator (PMID:26702054) |
| Chemical compound, drug | C12E8; octaethylene glycol monododecyl ether | Nikkol | BL-8SY | Detergent for solubilizing SERCA |
| Chemical compound, drug | Egg PC; L-α-phosphatidylcholine (Egg, Chicken) | Avanti | 840051 | Natural lipid |
| Chemical compound, drug | Egg PE; L-α-phosphatidylethanolamine (Egg, Chicken) | Avanti | 840021 | Natural lipid |
| Chemical compound, drug | Egg PA; L-α-phosphatidic acid (Egg, Chicken) | Avanti | 840101 | Natural lipid |
| Chemical compound, drug | Ionomycin, calcium salt | Sigma-Aldrich | I3909 | Facilitates the transport of calcium across the plasma membrane |
| Chemical compound, drug | Ruthenium red | Sigma-Aldrich | R2751 | RyR inhibitor |
| Chemical compound, drug | Caffeine | Sigma-Aldrich | C0750 | RyR2 agonist |
| Recombinant DNA reagent | pEGFP_RyR2 | Donated by Dr. C. George (PMID:15047862) | Mammalian expression construct containing GFP fused RyR2 | |
| Cell line (HEK 293) | T-Rex-293 Cell Line | Thermo Fisher Scientific | R71007 | Mammalian cell line for the stable expression of proteins of interest |
| Recombinant DNA reagent | EYFP-PLN | Singh et al., 2019 | Constitutively fluorescent green/yellow fluorescent protein fusion construct | |
| Recombinant DNA reagent | EYFP-DWORF | Singh et al., 2019 | Constitutively fluorescent green/yellow fluorescent protein fusion construct | |
| Recombinant DNA reagent | mCerulean-SERCA | Bovo et al., 2019 | Fluorescent protein fusion construct | |
| Cell line (HEK 293) | AAV-293 cells | Agilent Technologies | 240073 | HEK293 cell line optimized for AAV transfection |





