Osteogenic growth peptide is a potent anti-inflammatory and bone preserving hormone via cannabinoid receptor type 2
Figures
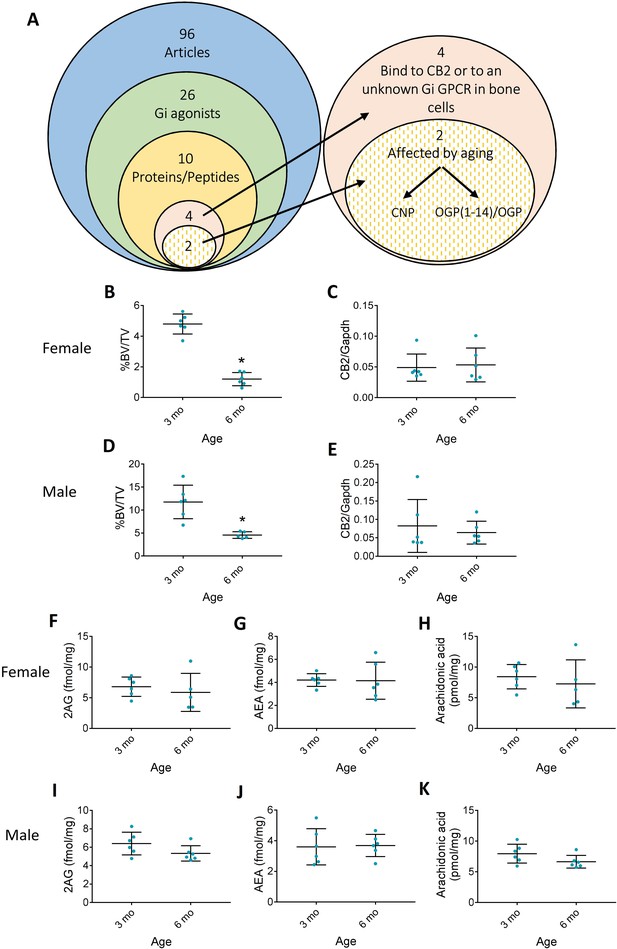
Aging differences in the endocannabinoid (EC) system.
(A) Literature scan isolated osteogenic growth peptide (OGP) as the only peptide that is mitogenic in osteoblasts and whose levels decline with age. (B–E) Age-related bone loss (decrease in trabecular bone volume density, %BV/TV) is not associated with changes in cannabinoid receptor type 2 (CB2) expression levels in 6 month (6 mo) vs. 3-month-old (3 mo) female (B and C, respectively) and male (D and E, respectively) mice. (F–K) Levels of EC ligands in the bone tissue of aging 6 mo vs. 3 mo female (F, G, and H) and male mice (I, J, and K). Data are the mean ± SD; n=6; *p<0.05 vs. 3 mo mice, t-test.
-
Figure 1—source data 1
BV/TV female and male mice 6 and 3 month old.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig1-data1-v2.xlsx
-
Figure 1—source data 2
Cnr2 expression male and female 3 and 6 month mice.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig1-data2-v2.xlsx
-
Figure 1—source data 3
Endocannabinoid levels in 3 and 6 month old mice.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig1-data3-v2.xlsx
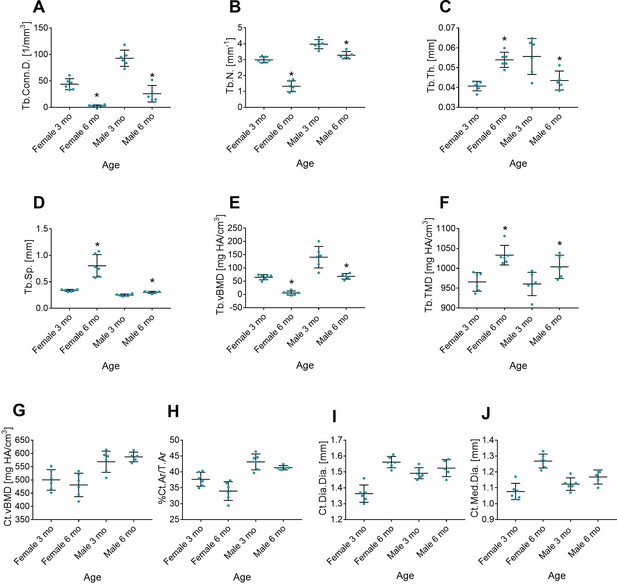
Age-related differences in femoral bone.
Femurs were collected from female and male mice 6 month (6 mo) vs. 3-month-old (3 mo). Trabecular parameters were evaluated in the distal metaphysis; cortical parameters in the mid-diaphysis. (A) Trabecular connectivity density (Tb.Conn.D), (B) trabecular number (Tb.N), (C) trabecular thickness (Tb.Th), (D) trabecular separation (Tb.Sp), (E) trabecular volumetric bone mineral density (Tb.vBMD), (F) trabecular tissue mineral density (Tb.TMD), (G) cortical volumetric bone mineral density (Ct.vBMD), (H) cortical area over total bone area (Ct.Ar./T.Ar), (I) cortical mid-diaphyseal diameter (Ct.Dia.Dia), and (J) cortical medullary diameter (Ct.Med.Dia). Data are the mean ± SD; n=6; p<0.05 vs. 3 mo mice, t-test.
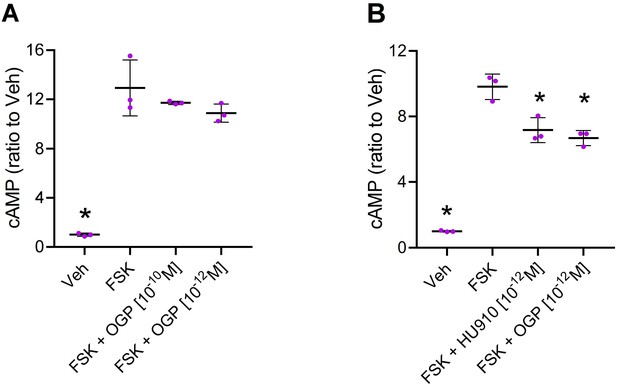
Osteogenic growth peptide (OGP) attenuates forskolin (FSK)-stimulated cAMP levels via cannabinoid receptor type 2 (CB2).
(A) CHO cells mock transfected with an empty vector (that does not express CB2) were treated with vehicle (Veh) or forskolin (FSK) to induce cAMP levels (with Veh only). FSK-treated cells were also pre-treated with two concentrations of OGP. (B) CHO cells transfected with human CNR2 received Veh only (Veh) or were treated with FSK and pre-treated with Veh (FSK), the CB2 agonist HU910 (FSK +HU910) or OGP (FSK +OGP). Data are the mean ± SD obtained in triplicate culture wells per condition and repeated three times. *p<0.05 vs. FSK alone, non-parametric one-way ANOVA.
-
Figure 2—source data 1
Mock and hCB2-CHO cAMP levels (binding assay).
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig2-data1-v2.xlsx
-
Figure 2—source data 2
cAMP levels in hCB2-CHO with CB2 antagonist (SR2).
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig2-data2-v2.xlsx
-
Figure 2—source data 3
hCB1-HEK293 cAMP levels.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig2-data3-v2.xlsx
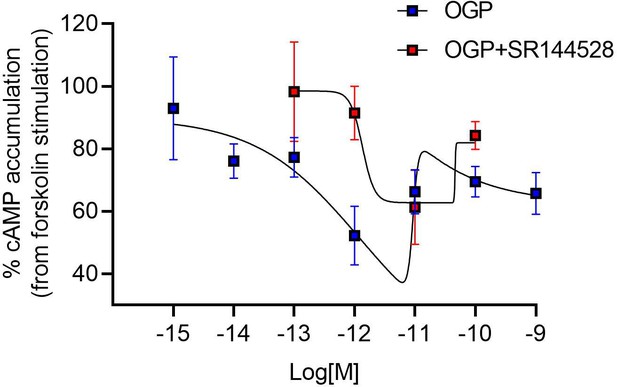
Osteogenic growth peptide (OGP) inhibits the forskolin-stimulated cAMP in CHO-hCB2 cells.
OGP and SR144528 (selective CB2 antagonist) were added at the indicated concentrations. The amount of [3H]cAMP in the absence of forskolin was subtracted. Data are the mean ± S.E.M. of two independent experiments (performed in triplicates).
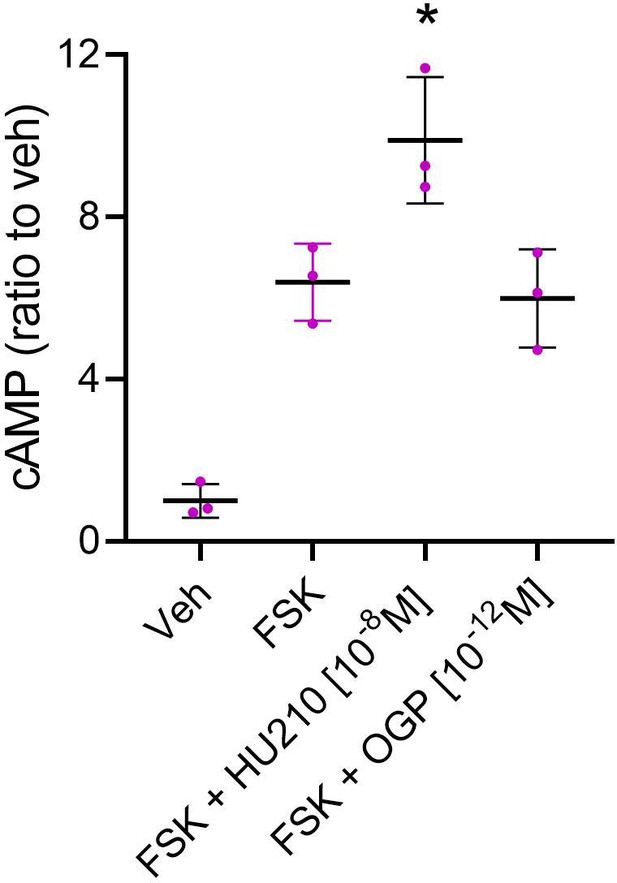
Osteogenic growth peptide (OGP) does not activate cannabinoid receptor type 1 (CB1) internal signaling in hCB1-HEK293 cells.
Forskolin (FSK) and the CB1 agonist HU210 increased cAMP levels in HEK293 cells transfected with human CNR1. Treatment with OGP had no effect on these CNR1-transfected cells. Data are mean ± SD obtained in triplicate. *p<0.05 vs. FSK, one-way ANOVA.
Interaction of the osteogenic growth peptide (OGP) with the allosteric site of human cannabinoid receptor type 2 (CB2).
(A) and (B) represent conformation of the peptide when OGP is bound to the extracellular surface in the absence (A) and presence (B) of CP55940. The conformations presented are taken from the last step of the molecular dynamics (MD) simulation. Residues forming direct hydrogen bonds with the peptide and the respective interactions are highlighted in purple. Residues forming hydrophobic interactions are labeled in gray color. The amino-π interaction is labeled in orange color. (C) Competitive binding assay between OGP and tritiated CP55940. CHO-CB2-derived membranes were used for a CB2 binding assay. No detection of CP55940 chase-out in the presence of increasing concentrations of OGP indicates that OGP and CP55940 do not compete over the same binding site at CB2. (D,E) Effect of OGP on the stimulation of binding of [35S]GTPγS induced by CP55940. CHO-CB2-derived membranes were used in a [35S]GTPγS binding assay. The white bar shows the relative activation of CB2 by 0.1 nM (D) or 10 nM (E) of CP55940 above basal levels (DMSO(Dimethyl sulfoxide) only) in the absence of OGP. Other graphs show relative activation of CB2 induced by CP55940 in the presence of increasing concentrations of OGP. *, p<0.05, vs. CP55940 only (no OGP).
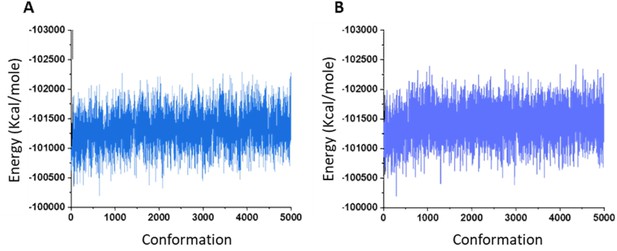
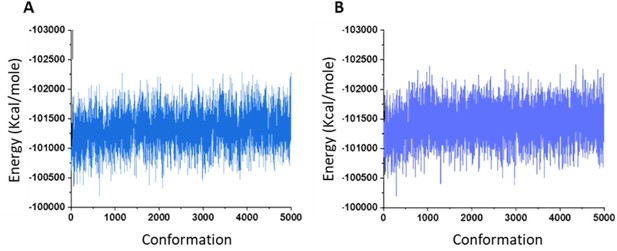
Molecular dynamics (MD) energy image.
Total energy of the system during MD simulation of osteogenic growth peptide at the ECL site of the inactive model of human cannabinoid receptor type 2 (CB2) in the absence (A) and presence (B) of CP55940.
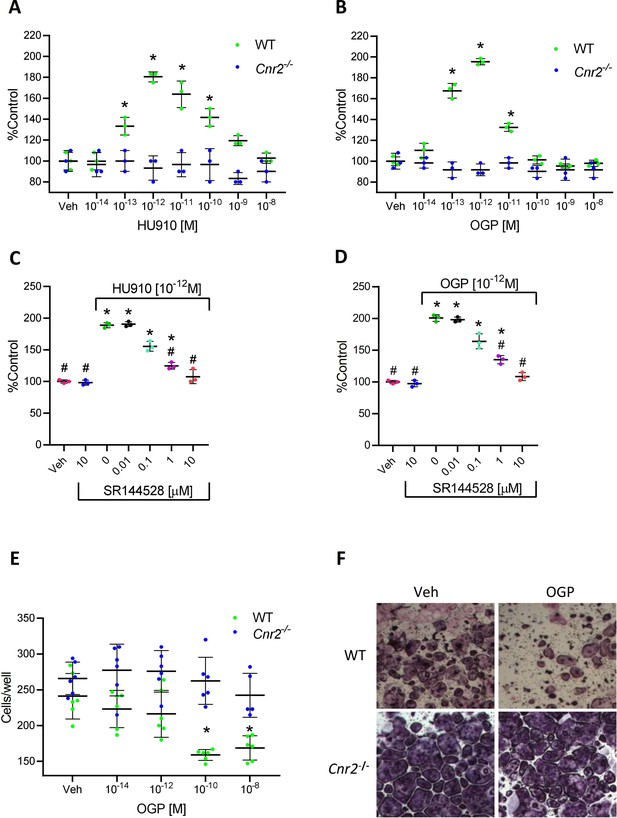
The effect of osteogenic growth peptide (OGP) in bone cells is dependent on cannabinoid receptor type 2 (CB2).
(A–B) Proliferative activity of HU910, a synthetic CB2 selective agonist (A), and of OGP (B) in wild type (WT) and Cnr2-/--derived murine osteoblasts. Data are the mean ± SD obtained in triplicate and were repeated at least two times. *p<0.05 vs. vehicle (Veh) in the same genotype, non-parametric oneway ANOVA. (C–D) The proliferative activity of HU910 (C) and OGP (D) in human osteoblasts is abrogated by the CB2-selective antagonist SR144528 (SR2). Data are mean ± SD obtained in triplicate. *p<0.05 vs. Veh, #p<0.05 vs. the CB2 agonist with no SR2, non-parametric oneway ANOVA. (E–F) OGP attenuates osteoclastogenesis (number of multinucleated tartrate-resistant acid phosphatase [TRAP] positive cells per well) in WT- but not in Cnr2-/--derived murine cultures. Data are the mean ± SD obtained in six wells per condition. *p<0.05 vs. Veh in the same genotype, non-parametric one-way ANOVA.
-
Figure 4—source data 1
Murine WT and Cnr2-/- osteoblasts proliferation.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig4-data1-v2.xlsx
-
Figure 4—source data 2
Human osteoblasts proliferation with CB2 antagonist.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig4-data2-v2.xlsx
-
Figure 4—source data 3
Murine WT and Cnr2-/- osteoclasts differentiation.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig4-data3-v2.xlsx
-
Figure 4—source data 4
BrdU on WT osteoblasts with OGP.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig4-data4-v2.xls
-
Figure 4—source data 5
BrdU on Cnr2-/- osteoblasts with OGP.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig4-data5-v2.xls
-
Figure 4—source data 6
Murine osteoblasts with OGP, HU910 and CB2 antagonist.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig4-data6-v2.xlsx
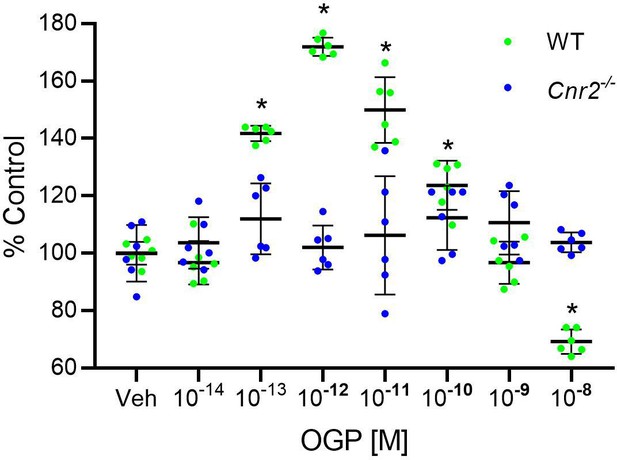
The effect of osteogenic growth peptide (OGP) in murine bone cells is dependent on CB2.
Bromodeoxyuridin assay determination of the proliferative activity of OGP in wild type (WT) and Cnr2-/--derived new-born mouse calvarial osteoblasts. Data are the mean ± SD obtained in six replicates. *p<0.05 vs. vehicle (Veh) in two-way ANOVA.
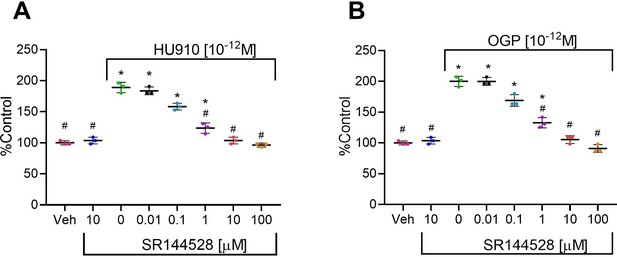
The effect of osteogenic growth peptide (OGP) in murine bone cells is dependent on cannabinoid receptor type 2 (CB2).
(A,B) The proliferative activity of HU910 (A) and OGP (B) in murine osteoblasts is abrogated by the CB2 selective antagonist SR144528. Data are mean ± SD obtained in triplicates. *p<0.05 vs. vehicle (Veh); #p<0.05 vs. the CB2 agonist with no SR144528, one-way ANOVA.
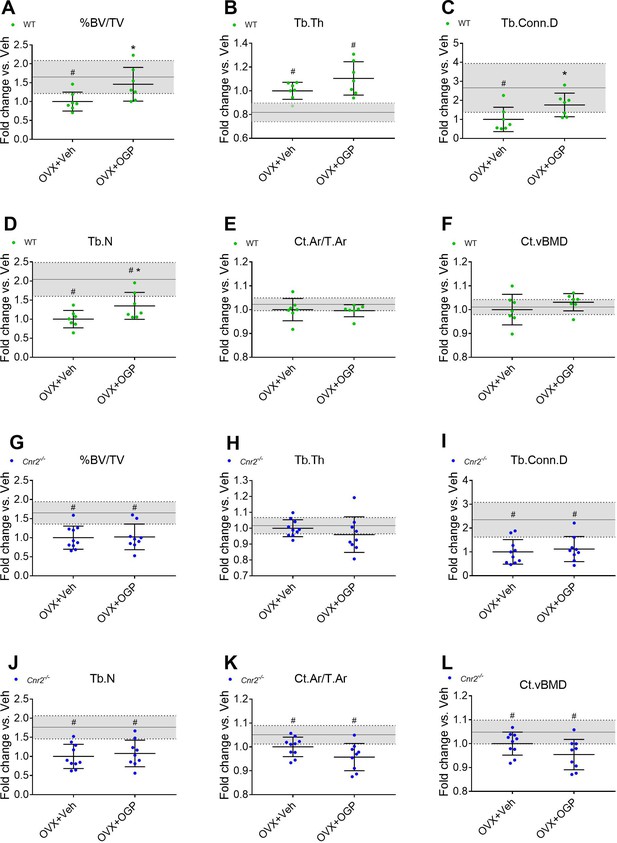
Osteogenic growth peptide (OGP) effect on bone recovery in an ovariectomized (OVX) model is cannabinoid receptor type 2 dependent.
Rescue of wild type (WT) (A–F) but not Cnr2-/- (G–L) OVX-induced bone loss by OGP administration. 8-week old mice were OVX (or Sham-OVX), and treatment with OGP or vehicle started after 6 weeks for 6 weeks. (A, G) trabecular bone volume fraction (BV/TV); (B, H) trabecular thickness (Tb.Th); (C, I) trabecular connectivity density (Tb.Conn.D); (D, J) trabecular number (Tb.N); (E, K) cortical area over total bone area (Ct.Ar/T.Ar); (F, L) cortical volumetric bone mineral density (CT.vBMD). Results obtained from WT Sham-OVX, WT OVX+Veh and WT OVX+OGP (n=7); Cnr2-/- Sham-OVX, Cnr2-/- OVX+Veh and Cnr2-/- OVX+OGP (n=9–10). Data are mean ± SD normalized to the OVX+Veh group. Sham-OVX mean and SD are represented by continuous line and shaded area, respectively. *p<0.05 vs. OVX+Veh in the same genotype. #p<0.05 vs. Sham-OVX.
-
Figure 5—source data 1
MicroCT morphometric data of OVX and Sham-OVX mice.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig5-data1-v2.xlsx
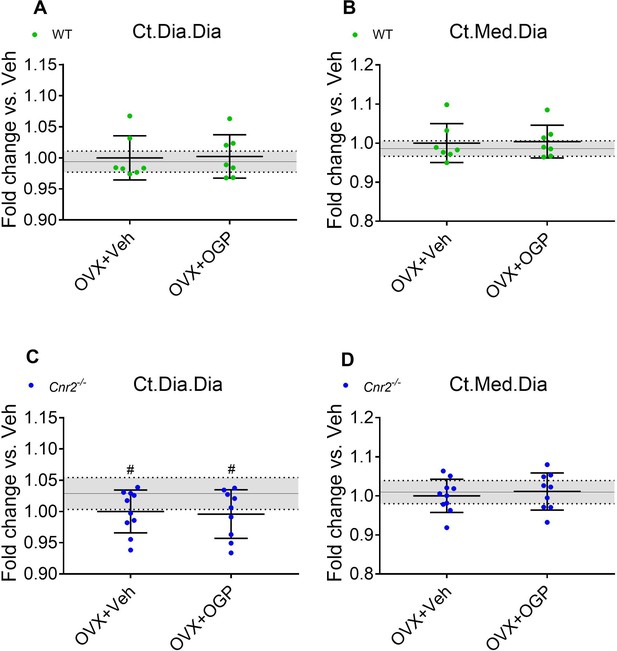
Osteogenic growth peptide (OGP) effect on bone recovery in ovariectomized (OVX) mice is cannabinoid receptor type 2 dependent.
Data from the same mice as in Figure 5. 8-week old mice were OVX (or Sham-OVX), and treatment with OGP or vehicle (Veh) started after 6 weeks for 6 weeks. (A, C) Cortical mid-diaphyseal diameter (Ct.Dia.Dia); (B, D) cortical medullary diameter (Ct.Med.Dia). Results obtained from wild type (WT) Sham-OVX, WT OVX+Veh and WT OVX+OGP (n=7); Cnr2-/- Sham (n=10), Cnr2-/- OVX+Veh and Cnr2-/- OVX+OGP (n=9–10). Data are mean ± SD normalized to the OVX+Veh group. Sham-OVX mean and SD are represented by continuous line and shaded area, respectively. *p<0.05 vs. OVX+Veh in the same genotype. #p<0.05 vs. Sham-OVX.
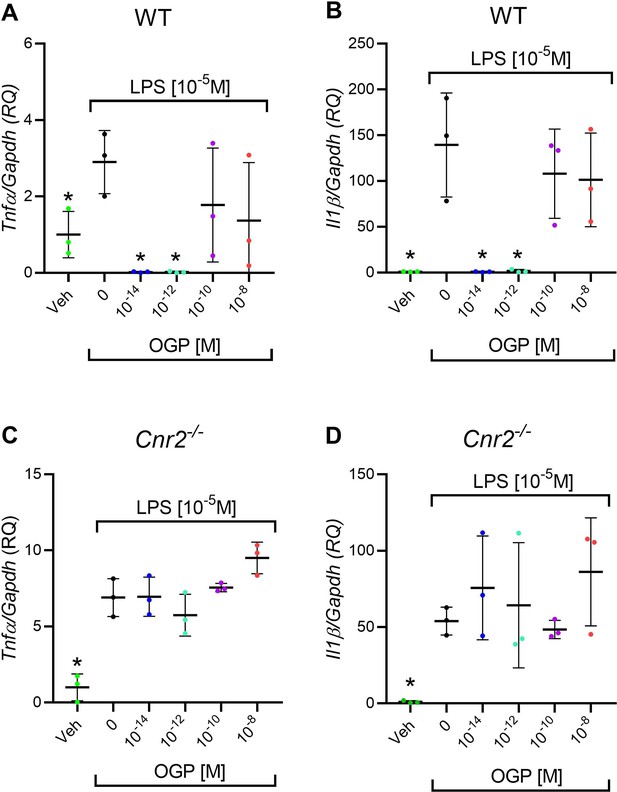
In vitro anti-inflammatory activity of osteogenic growth peptide (OGP) in wild type (WT) but not in Cnr2-/- macrophage cultures.
(A–B) Lipopolysaccharide (LPS)-induced expression of Tnfa (A) and Il1b (B) in WT macrophages pre-treated with OGP. (C–D) LPS-induced expression of Tnfa (C) and Il1b (D) in Cnr2-/- macrophages pre-treated with OGP. Data are normalized to the vehicle (Veh) value (RQ) and are presented as the mean ± SD obtained in triplicate. *p<0.05 vs. LPS alone, non-parametric one-way ANOVA.
-
Figure 6—source data 1
Murine WT and Cnr2 macrophages cytokine levels.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig6-data1-v2.xlsx
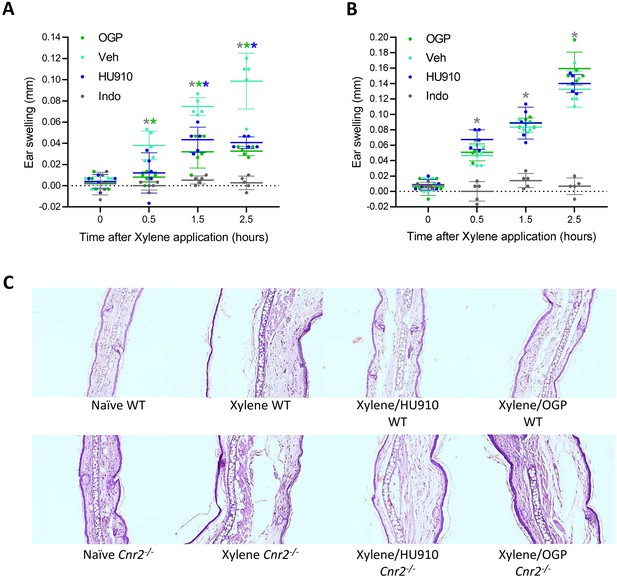
Osteogenic growth peptide (OGP) attenuates xylene-induced ear swelling.
(A) Wild type (WT) mice and (B) Cnr2-/- mice treated with PBS (vehicle [Veh]), indomethacin (positive control), HU910 (a selective CB2 agonist and positive control), or OGP prior to xylene application. Ear swelling is presented as the difference between the xylene-treated and the untreated ear. Data are the mean ± SD, n=6. Color-coded * for p<0.05, Veh (turquoise) vs. indomethacin (gray), HU910 (blue), or OGP (green) treated mice at the same time point, two-way ANOVA. In either genotype, p<0.001 for the effect of xylene in the Veh group. (C) Photomicrographs of the mid-ear region from median representative mice.
-
Figure 7—source data 1
Ear edema in WT and Cnr2-/- mice.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig7-data1-v2.xlsx
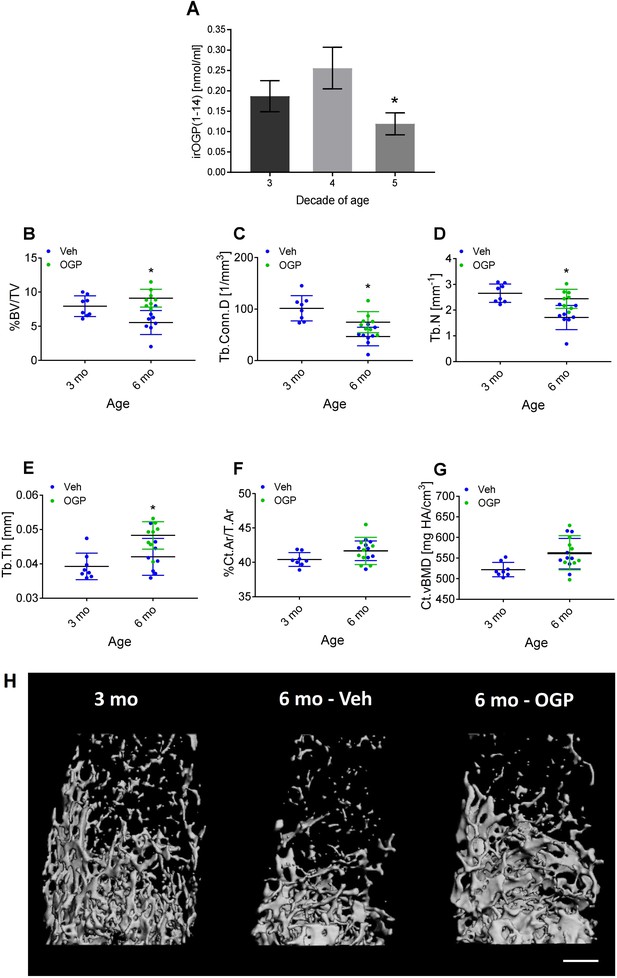
Osteogenic growth peptide (OGP[1-14]) levels in women and the effect of OGP on age-related bone loss in male mice.
(A) Immunoreactive (ir) OGP(1-14) levels were measured in serum from human female subjects aged 18–49 years. irOGP(1-14) levels were significantly lower in the fifth decade compared with the third and fourth decades of life. Data are the mean ± SD. n=28 (third decade), n=6 (fourth decade), n=6 (fifth decade). *p<0.05 vs. fifth decade aged women, Kruskal-Willis oneway ANOVA and Mann Whitney-Wilcoxon rank sum tests. (B–E) Exogenous administration of OGP prevents age-related decline in (B) trabecular bone volume fraction (BV/TV), (C) trabecular connectivity density (Conn.D), (D) trabecular number (Tb.N) and (E) trabecular thickness (Tb.Th) in the distal femur of 3 month (3 mo) and 6 month old (6 mo) male mice. Data are the mean ± SD, n=8. *p<0.05 vs. vehicle (Veh)-treated mice, one-way ANOVA. (H) Representative µCT images of the distal femur from each group. Bar = 0.5 mm.
-
Figure 8—source data 1
OGP serum levels in women.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig8-data1-v2.xlsx
-
Figure 8—source data 2
MicroCT data for 3- and 6-mo mice treated with OGP.
- https://cdn.elifesciences.org/articles/65834/elife-65834-fig8-data2-v2.xlsx
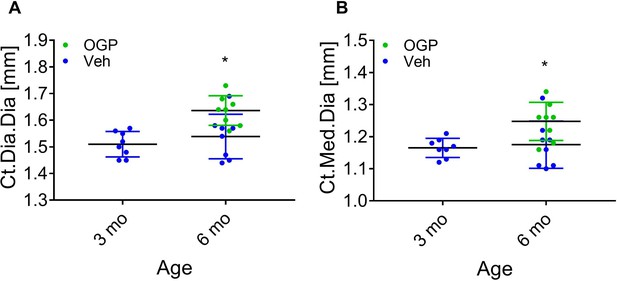
Effect of osteogenic growth peptide (OGP) on age-related cortical bone loss in male mice.
(A, B) Data are the mean ± SD for mid-diaphyseal diameter (A) and medullary diameter from the same mice as in Figure 8 showing a significant cortical expansion, n=8. *p<0.05 vs. vehicle (Veh)-treated mice, one-way ANOVA.
Additional files
-
Supplementary file 1
Literature search results summary, indicating the results of a Pubmed search (keywords [‘peptide’ or ‘protein’] and [‘Gi protein’ or ‘G(i)’ or ‘GPCR’ or ‘G*coupled receptor’] and [osteoblast* or osteoclast* OR osteocyte*]) where the activator interacts with a Gi-GPCR (26 agonists in total).
The 10 agonists that are also endogenous proteins/peptides are highlighted in bold fonts. PTX, pertussis toxin.
- https://cdn.elifesciences.org/articles/65834/elife-65834-supp1-v2.docx
-
Supplementary file 2
Glide docking score (in kcal/mol) obtained for binding OGP in active and inactive models of CB2.
- https://cdn.elifesciences.org/articles/65834/elife-65834-supp2-v2.docx
-
Supplementary file 3
Percent identity and similarity between CB1 and CB2 calculated using the online resource by Munk et al., 2019.
- https://cdn.elifesciences.org/articles/65834/elife-65834-supp3-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/65834/elife-65834-transrepform1-v2.docx





