Information flow, cell types and stereotypy in a full olfactory connectome
Figures
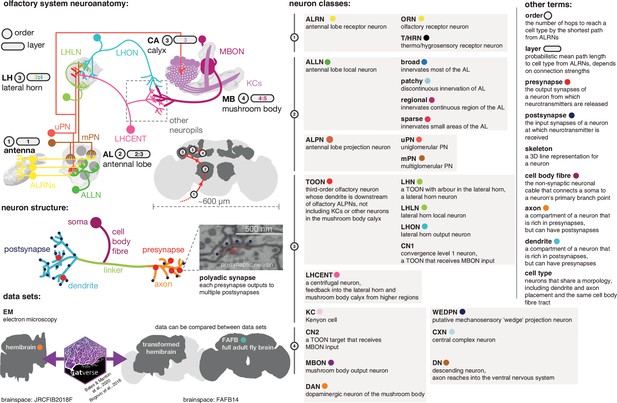
Graphical olfactory neuroanatomy glossary.
Top left: schematic of the D. melanogaster olfactory system showing all its major neuron classes. The ‘order’ of each neuropil is given in a grey circle, its average layers in a grey lozenge. Inset: the fly brain with a scale bar and early olfactory neuropils shown. Red path is the major feedforward course of olfactory information through the brain. Middle left: a neuron with its compartments is shown. Bottom left: the two EM data sets that feature in this work, the partial dense connectome, the hemibrain and a sparsely reconstructed data set, full adult fly brain (FAFB). Neuroanatomical data can be moved between the two spaces using a bridging registration (Bogovic et al., 2020; Bates et al., 2020a). Right: major neuron class acronyms are defined. Other neuroanatomical terms are also defined. Coloured dots indicate the colour used to signal these terms in the following figures.
Video of neurons typed in this study grouped by broad class.
Colours correspond to cell type (ALRNs), lineage (ALRNs) or are random (ALPNs, TOONs).
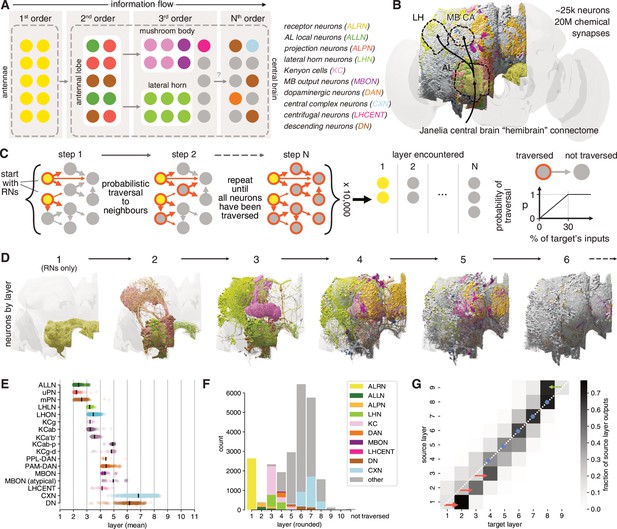
Identification of layers in the olfactory system.
(A) Schematic of the fly’s olfactory system. Colours reused in subsequent panels. (B) The Janelia Research Campus FlyEM hemibrain connectome. Principal olfactory neuropils as overlay; full brain plotted for reference. (C) Graph traversal model used to assign layers to individual neurons. (D) Neurons found in the first six layers. (E) Mean layer of individual neurons. Black line represents mean across a given neuron class. (F) Composition of each layer. (G) Connections between layers. AL: antennal lobe; CA: calyx; LH: lateral horn; MB: mushroom body; WEDPN: wedge; ALPN: antennal lobe projection neuron; uPN/mPN: uni-/multiglomerular ALPN.
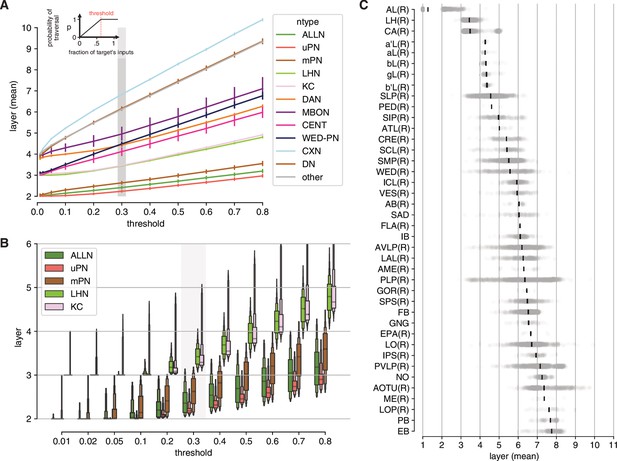
Graph traversal model extended data.
(A) Model parameterisation: relative positions are stable across parameter space (with the exception of WEDPNs). Grey bar indicates threshold used for final model (0.3). Error bars represent SEM. (B) Final threshold was chosen using known neuron classes as landmarks. (C) Mean layer by neuropil. Each neuron is assigned a ‘primary’ neuropil based on where it receives most of its inputs.
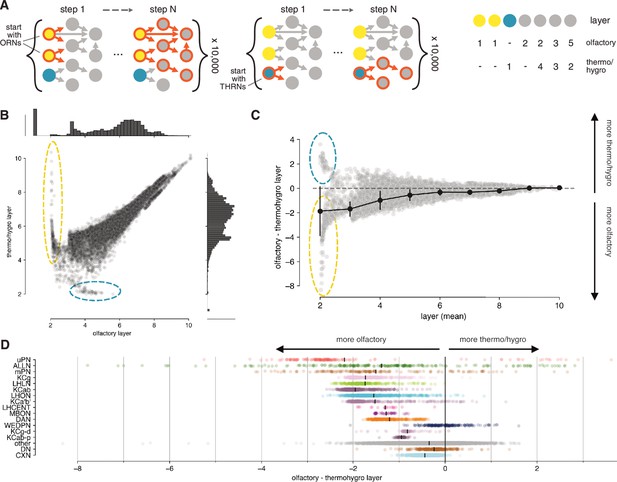
Olfactory vs. thermo/hygrosensory layers.
(A) Separate models with olfactory receptor neurons (ORNs) or thermo/hygro-receptor neurons (TRNs/HRNs) as seeds were run to assign layers with respect to the olfactory or thermo/hygrosensory system. (B, C) Comparison of olfactory vs. thermo/hygrosensory layer. Early on there are neurons that appear dedicated to either olfactory (yellow circle) or thermo/hygrosensory (blue circle) sensory information. This separation vanishes in higher layers. Error bars in (C) represent SEM. (D) Olfactory vs. thermo/hygrosensory layer by neuron class.
Video of neurons of the first 5 olfactory layers.
Colours correspond to neuron types (e.g. ALRNs, ALPNs, etc) also used elsewhere.
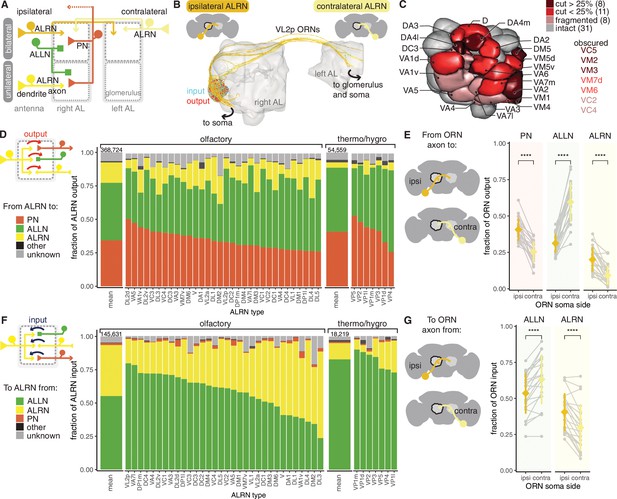
Antennal lobe (AL) receptor neurons (ALRNs) mostly target projection and local neurons.
(A) Summary schematic of antennal lobe ALRN classification and the major cell types present in the antennal lobe that interact with them. ALLN: antennal lobe local neurons; ALPN: antennal lobe projection neuron. (B) Ipsilateral and contralateral VL2p olfactory ALRNs (olfactory receptor neurons [ORNs]) in the right antennal lobe. The somas are not visible as they are cut off from the volume. Output synapses in red, input ones in blue. (C) Antennal lobe glomerular meshes (generated from ALRNs) showing which glomeruli are truncated and by how much (qualitative assessment). ALRN types in whole glomeruli but with fragmented ALRNs, which prevents assignment of soma side, are also shown. (D) Fraction of ALRN output per type. The leftmost bar is the mean for olfactory or thermo/hygrosensory ALRNs, with number of synapses on top. (E) Fraction of ipsilateral (ipsi) or contralateral (contra) ORN output to ALLNs, ALPNs and ALRNs. Means were compared using Wilcoxon two-sample tests. (F) Fraction of ALRN input per type. The leftmost bar is the mean for ORNs and thermo-receptor neurons (TRNs)/hygro-receptor neurons (HRNs), with number of synapses on top. (G) Fraction of ipsilateral (ipsi) or contralateral (contra) ORN input from ALLNs and ALRNs. Means were compared using Wilcoxon two-sample tests. Significance values: ns: p>0.05; *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
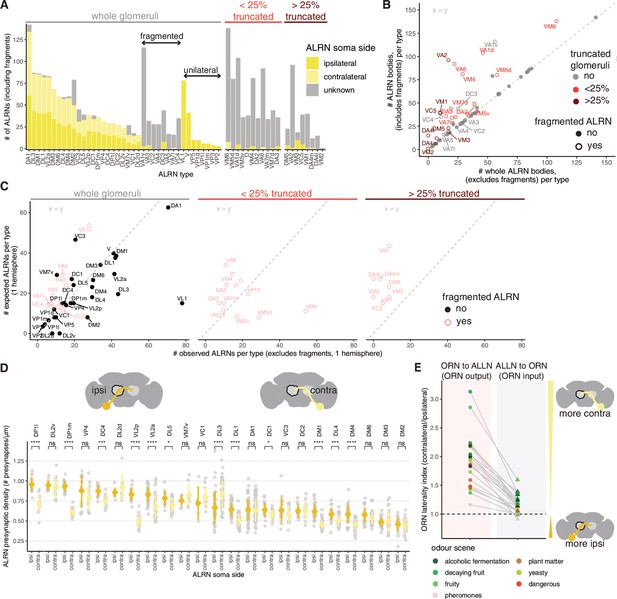
Annotation of antennal lobe receptor neuron (ALRN) bodies and connectivity features.
(A) Number of unique bodies classified as ALRNs per type and per soma side. Truncated glomeruli (0, < 25%, >25%), fragmented ALRN types in whole glomeruli and unilateral ALRN types are indicated. (B) Relationship between the number of unique ALRN bodies (including fragments) and whole ALRN bodies (excluding fragments). (C) Comparison between the number of observed ALRNs (whole) and the expected number per type in one hemisphere. In (A–C) VM6 ALRNs are plotted as one population as not every body could be assigned to one of the three subpopulations because of the glomerulus truncation. (D) Presynaptic density for ipsilateral and contralateral ALRNs, per type. Types are ordered by mean ipsilateral density. (E) Laterality index for olfactory receptor neuron (ORN) and antennal lobe local neurons (ALLN) connectivity (ORN output and ORN input): fraction of contralateral ORN connectivity/fraction of ipsilateral ORN connectivity. Each ORN type is coloured by its functional relevance. Mean comparisons made by Wilcoxon two-sample tests. ns: p>0.05; *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
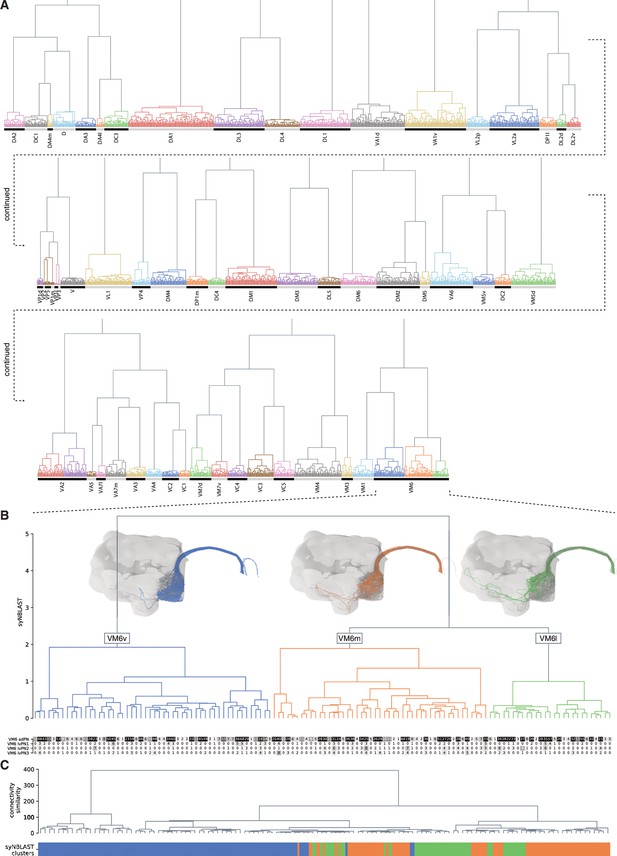
Antennal lobe receptor neuron (ALRN) clustering and subdivision of the VM6 glomerulus.
(A) Synapse-based hierarchical clustering (syNBLAST) of all ALRNs. (B) Zoom-in on VM6 ALRNs captures the partition into three subpopulations: VM6v, VM6m and VM6l. Heatmap shows connections of VM6 ALRNs onto uniglomerular VM6 ALPNs. (C) Clustering of VM6 ALRNs based on their downstream connectivity. Colour bar at the bottom corresponds to syNBLAST clusters in (B). The connectivity-based clustering does not align with the subpopulations which suggests that information from the different types of VM6 ALRNs is co-processed by the downstream networks.
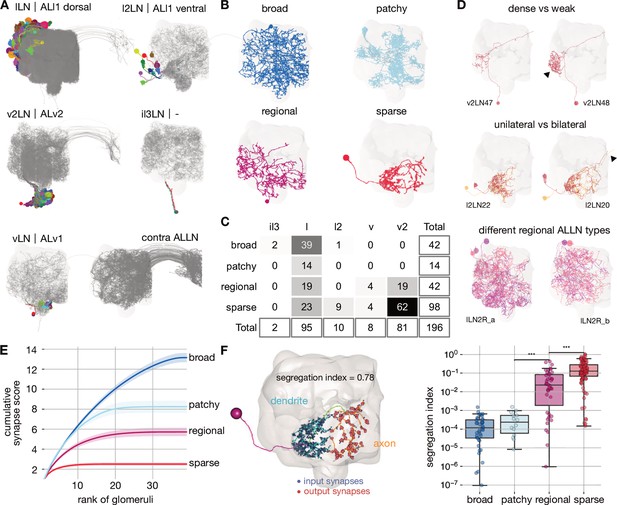
Cell typing, morphological classification and polarity of antennal lobe local neurons.
(A) Antennal lobe local neurons (ALLNs) classified by hemilineage and contralateral ALLNs (contra ALLN), along with the antennal lobe mesh in the background. Soma locations (circles) and primary neurite tracts are illustrated in multicolours. (B) Morphological classes of ALLNs. A representative example of each category is shown. (C) Number of ALLNs per hemilineage and morphological class. (D) Representative examples illustrating criteria used for typing: unilateral and bilateral neurites, lineage identity, area innervated by ALLN neurites and their density. Arrowheads point towards dense innervation and bilateral projection. (E) Synapse score per morphological class. Cumulative number of synapses is computed per ranked glomerulus (by number of synapses) and plotted against its rank. Envelopes represent standard error of the mean. (F) Polarisation of neurites per morphological class. Segregation index is a metric for how polarised a neuron is; the higher the score the more polarised the neuron (Schneider-Mizell et al., 2016). Left inset shows a sparse ALLN, l2LN21, as an example of a highly polarised ALLN. Significance values: *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001; pairwise Tukey-HSD post-hoc test.
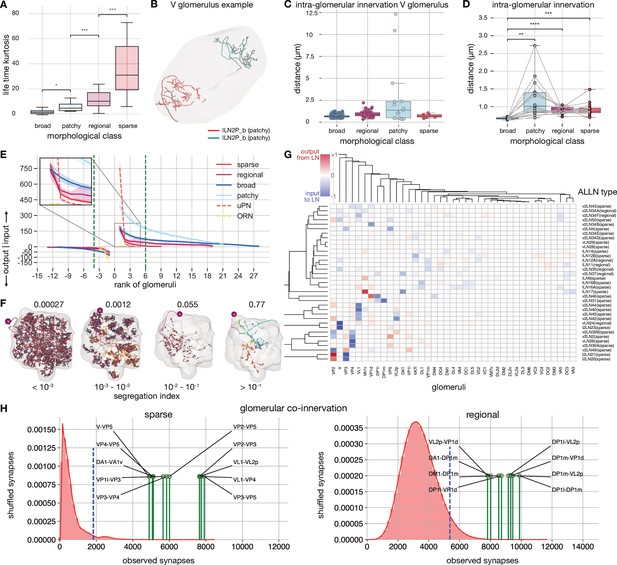
Antennal lobe local neuron (ALLN) glomerular innervation patterns.
(A) Sparseness of different ALLNs by morphological lifetime kurtosis class based on glomerular innervation (number of synapses) to calculate the lifetime kurtosis. (B) Example of two patchy ALLNs that are restricted to different areas of the V glomerulus. (C) Distances between ALLNs of the same morphology within the V glomerulus. (D) Distances between ALLNs of the same morphology in all glomeruli. (E) Input-output segregation by ALLN types. For each morphological class, input and output synapses per glomerulus are plotted in rank order. The inset shows that regional and sparse ALLNs asymptote faster to 0 compared with broad and patchy ALLNs consistent with the selective nature of their inputs. The green line indicates glomerular rank at which at least two of the ALLN types asymptote to 0. (F) Some ALLNs are polarised. The segregation index is a measure of how well they can be split into an axon and a dendrite; the higher the score, the more polarised the neuron. Images show splits for exemplary ALLNs across a range of segregation indices. (G) Heatmap showing, for all ALLN types with a segregation index above 0.1, their glomerular innervation. For each neuron, for each glomerulus, the proportion of dendritic input synapses is subtracted from the proportion of axonic output synapses in that glomerulus. Negative scores indicate dendritic input, positive ones axonic output. (H) Glomerular co-innervation per morphological class. Glomeruli that are frequently co-innervated are compared to the random distribution of synapses (in red). The blue dotted line represents the 95th percentile of the distribution of shuffled synapses. Co-innervation of significant pairs of glomeruli for sparse (left) and regional (right) ALLNs.
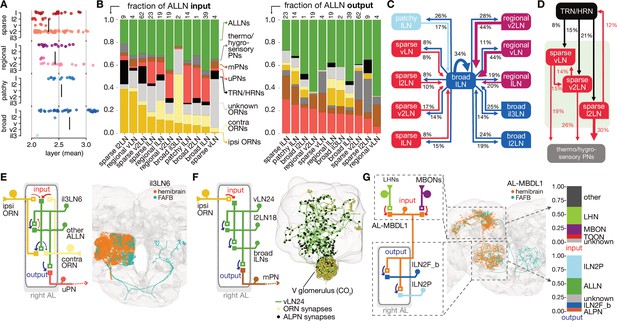
Antennal lobe (AL) local neuron (ALLN) connectivity and example circuit motifs.
(A) Layers of ALLNs. Vertical lines indicate group mean. (B) Fraction of ALLN input (left) and output (right) for different ALLN groups. Number of neurons per category is shown at the top of each bar. Where possible olfactory receptor neurons (ORNs) are split into ‘ipsi-’ and ‘contra’-lateral (‘unknown’ ORNs mostly correspond to those that are fragmented or belonging to truncated glomeruli). (C) Diagram illustrating ALLN-ALLN connectivity. ALLN groups are coloured by morphological class. (D) Diagram illustrating the most prominent ALLN connections to thermo/hygrosensory antennal lobe receptor neurons (ALRNs) and antennal lobe projection neurons (ALPNs). (E–G) Examples of ALLN connectivity. (E) A pair of broad ALLNs, il3LN6, cross-matched to the FAFB Keystone ALLNs. (F) An example of type regional ALLNs, vLN24, that receives specialised input in the V glomerulus. (G) A bilateral medial bundle neuron, AL-MBDL1, that integrates lateral horn neuron (LHN) and mushroom body output neuron (MBON) input and outputs to two specific types of ALLNs, broad lLN2F_b and patchy lLN2P.
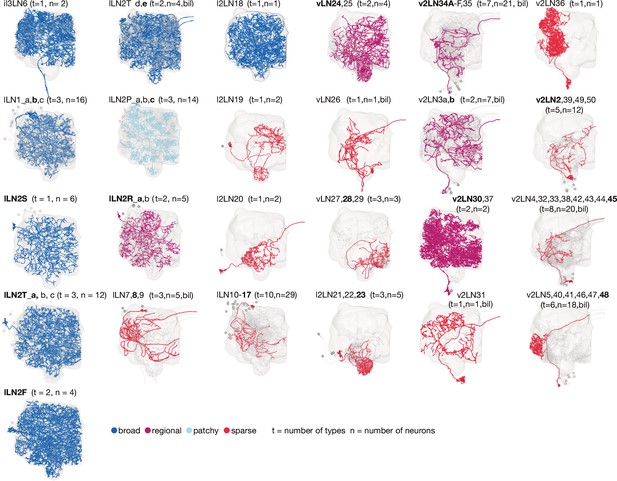
Antennal lobe local neuron (ALLN) groups.
ALLN types can be grouped into 25 anatomical groups that differ in their lineage, morphology, area of innervation and density of innervation. One neuron is plotted in colour as an example, the remaining are in grey. For groups with more than one type, the type of the coloured neuron is in bold. The group v2LN34A-F, 35 includes regional and sparse types. Note that each of the lLN2T_d extends several neurites towards the midline. bil: neurons project bilaterally.
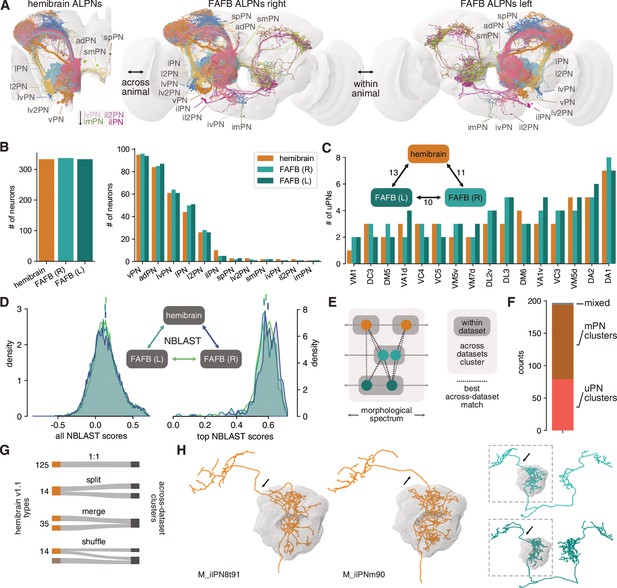
Numerical and morphological across – and within-animal stereotypy.
(A) Antennal lobe projection neurons (ALPNs) reconstructed in the hemibrain and from the left and right hemispheres of the full adult fly brain (FAFB) EM volume. (B) Overall ALPN counts are almost identical across hemispheres as well as across animals. (C) 17/56 uniglomerular projection neuron (uPN) types show variations in numbers. Numbers in triangle count instances of variation in numbers. (D) Across-data set NBLAST similarity scores are much the same. All scores on the left, only pairwise top scores on the right. Top lines represent means. (E) Clustering approach based on best across-data set matches. (F) Total number of across-data set clusters by composition. (G) Quantification of discrepancies between hemibrain v1.1 types and the across-data set clusters. See also Figure 6—figure supplement 1F. (H) Example where two hemibrain types merge into one across-data set cluster (2). One of the hemibrain neurons takes the ‘wrong’ antennal lobe tract (arrows) and has therefore been incorrectly given a separate type. See Figure 6—figure supplement 1G-J for more examples.
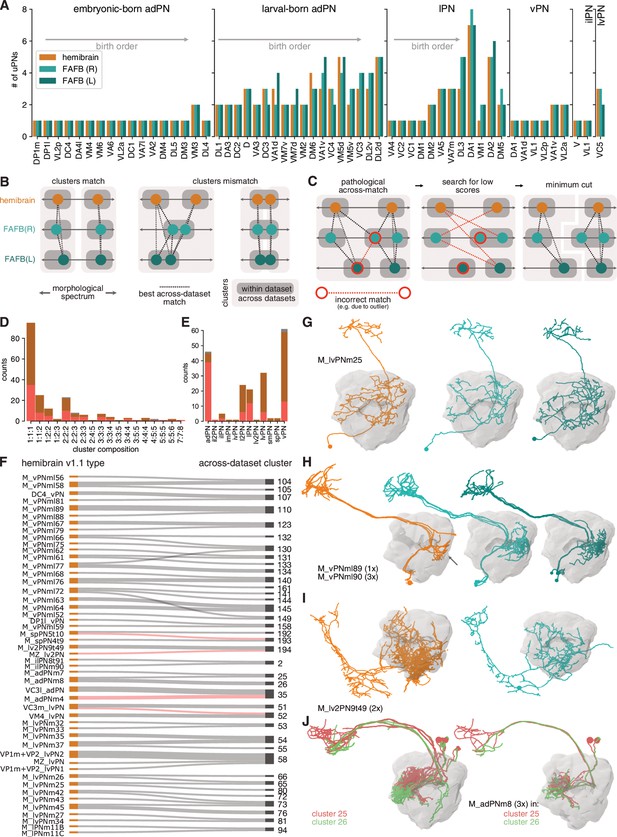
Comparison of antennal lobe projection neurons (ALPNs) across three hemispheres.
(A) Counts for 56 uniglomerular projection neuron (uPN) types across the three hemispheres. If known, order corresponds to order of birth. (B) Expanded explanation for across-data set clustering. (C) Illustration of refinement of initial clusters to deal with incorrect ‘pathological’ (red outline) across-data set matches. (D) Cluster composition as number of neurons from the three data sets. (E) Number of clusters per ALPN lineage. (F) Flow diagram for hemibrain v1.1 types that are merged or shuffled in the across-data set clusters. Across-data set merges identified as wrong are highlighted in red (see I for example). (G–J) Illustrative examples. (G) Single multiglomerular projection neuron (mPN) that can be tracked 1:1:1 across data sets. (H) Truncated (arrow) hemibrain mPNs matched to full adult fly brain (FAFB) ALPNs. (I) mPN without a match in FAFB (L) caused an incorrect merge into cluster 194. (J) M_adPNm8 mPNs are split into across-data set clusters 25 and 26.
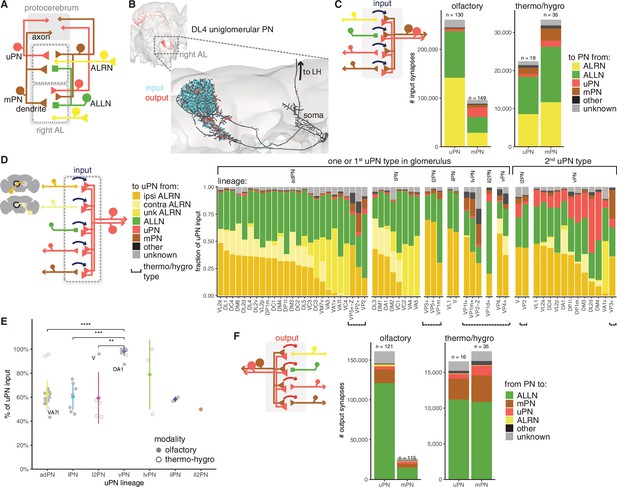
Antennal lobe (AL) projection neuron (ALPN) connectivity in the right antennal lobe.
(A) Summary schematic of ALPN classification and the major cell types present in the antennal lobe that interact with them. uPN: uniglomerular ALPN; mPN: multiglomerular ALPN. (B) DL4 uniglomerular PN showing inputs (cyan) and outputs (red). (C) Number of input synapses onto olfactory or thermo/hygrosensory uPNs and mPNs. Number of neurons in each category shown at the top of the bar. (D) Fraction of uPN input, grouped by type and lineage. The left group shows glomeruli that have only one uPN type, or one of the types for those with more than one. The right group shows the second uPN type for those glomeruli with more than one. Antennal lobe receptor neuron (ALRN) soma side indicated as ‘ipsi’ (ipsilateral), ‘contra’ (contralateral) or ‘unk’ (unknown, mostly corresponding to those glomeruli with fragmented ALRNs). Thermo/hygrosensory uPNs with suboesophageal zone (SEZ) innervation are indicated by ‘Z’ following the glomerulus. (E) Percentage of input onto uPN types relative to total connectivity (input + output), per lineage. Some of the outlier uPN types are labelled. Comparisons to categories with less than four data points were not done. Means per lineage were compared using Wilcoxon two-sample tests. Significance values: ns: p>0.05; *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001. (F) Number of output synapses from olfactory or thermo/hygrosensory uPNs and mPNs. Number of neurons in each category shown at the top of the bar.
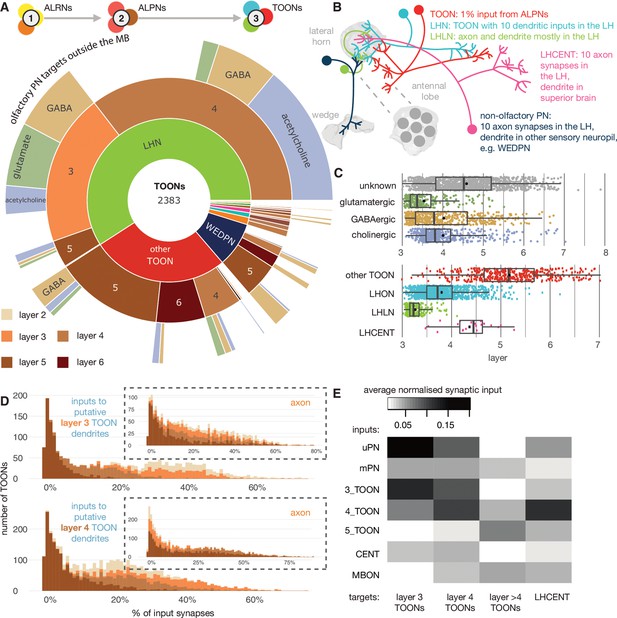
The targets of antennal lobe projection neuron (ALPN) axons.
(A) Starburst chart breakdown of the 2383 targets of ALPN axons, outside of the mushroom body, by various properties. We term these neurons ’third-order olfactory neurons’, or ’TOONs’ (see text for definition). From the inside out, neurons are grouped by broad neuron class, layer according to the traversal model and their putative neurotransmitter. Most TOONs receive the majority of this input at their dendrites: green, lateral horn neurons (LHNs); dark blue, wedge projection neurons (WEDPNs); orange, dopaminergic neurons of the mushroom body (DANs); brown, descending neurons to the ventral nervous system (DNs); pink, lateral horn centrifugal neurons (LHCENTs). The starburst plot also includes some neurons connected only or mainly at their axons, including a small number of: light blue, visual projection neurons; yellow, severed contralateral axons; dark green, putative gustatory projection neurons from the gnathal ganglia; yellow, putative axons ascending from the ventral nervous system. (B) Schematic illustrating the definitions used to group neurons into broad classes. For details see Materials and methods. (C) Jitter plot showing olfactory layers of TOONs broken down by predicted transmitter (if known) and broad class (LHONs, LH output neuron; LHLN, LH local neuron) (Frechter et al., 2019). (D) The percentage of input supplied onto third-order neurons by different classes of input neuron. Upper: inputs onto third-order neurons’ dendrite; lower: fourth-order neurons dendrites. Insets, input onto axons. (E) Normalised synaptic input to layer 3 and 4 neurons, as well as LH centrifugal neurons whose dendrites lie outside the LH but whose axons innervate it. Synaptic input is normalised by the total number of input synapses to the neuron’s predicted axon or dendrite.
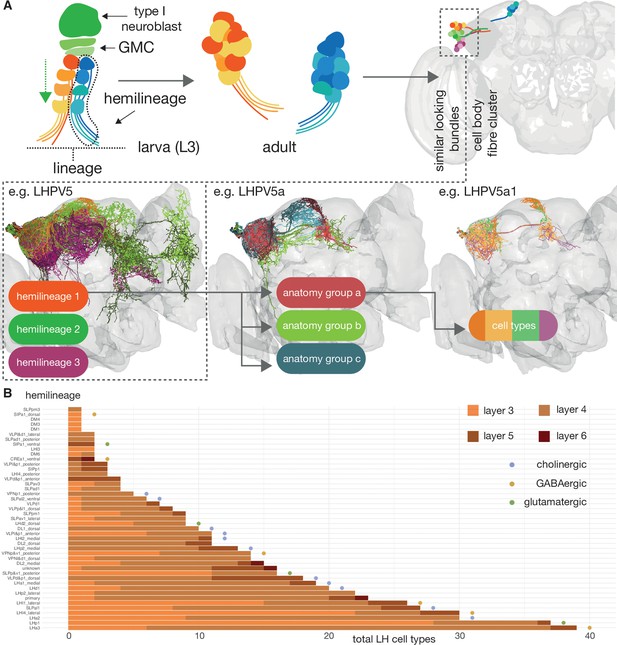
Defining cell types for third-order olfactory neurons.
The scheme by which we have named lateral horn neurons (LHNs) derives from the system we implemented in Frechter et al., 2019. (A) Similar-looking hemilineages are grouped together, neurons of similar coarse morphology are grouped together into ‘anatomy groups’ and each anatomy group is broken down into approximately isomorphic cell type (Bates et al., 2019). (B) The number of LHN cell types contributed by different hemilineages, which approximate cell body fibre tracts (Wong et al., 2013; Lovick et al., 2013). Names from the scheme by the K. Ito and T. Lee groups (Yu et al., 2013; Ito et al., 2013). Colours give a breakdown by their layer. Putative transmitter indicated by coloured circles.
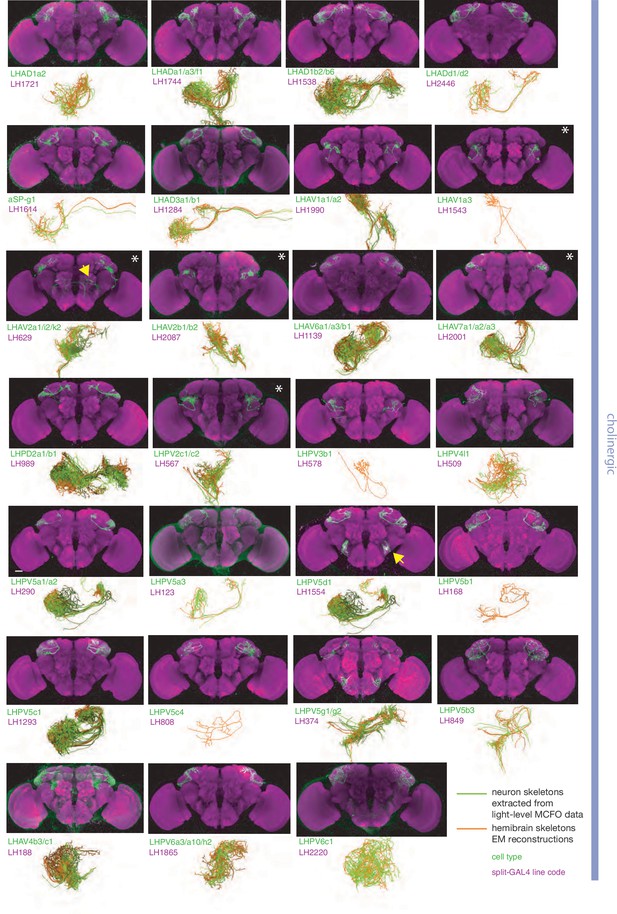
Split-GAL4 lines for excitatory lateral horn output neurons.
Putative excitatory output neurons of the lateral horn for which there are targeted genetic reagents as well as EM reconstructions (Dolan et al., 2019; Bates et al., 2020b; Scheffer et al., 2020). Expression of split-GAL4 lines are visualised using UAS-csChrimson::mVenus in attP18 (green), with nc82 as a neuropil stain (magenta) (Dolan et al., 2019). Off-target expression in the brain for non-ideal lines labelled with a yellow arrow. See https://www.janelia.org/split-gal4 for image data.
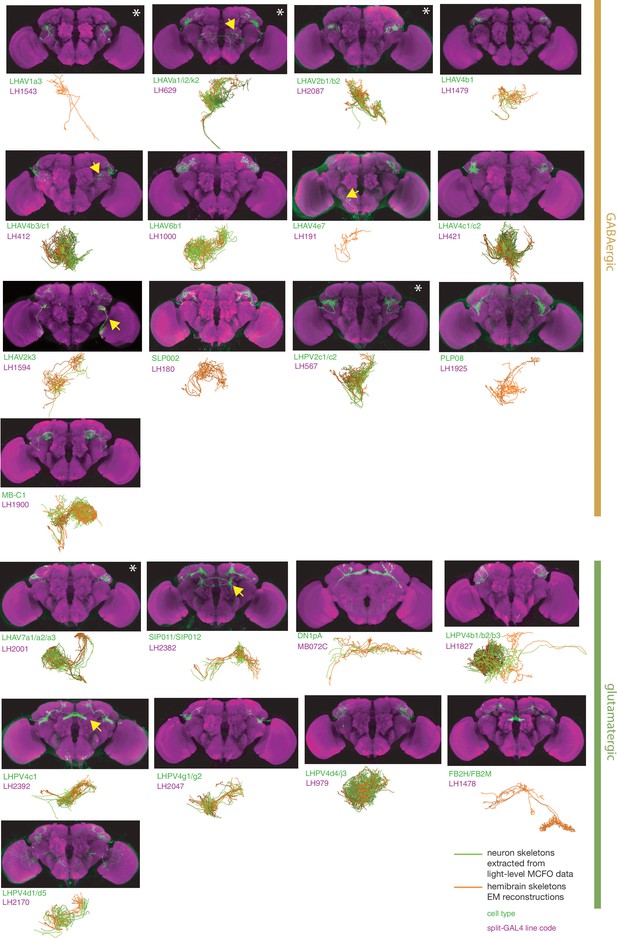
Split-GAL4 lines for inhibitory lateral horn output neurons.
Putative inhibitory output neurons of the lateral horn for which there are targeted genetic reagents as well as EM reconstructions (Dolan et al., 2019; Scheffer et al., 2020; Bates et al., 2020b). See http://www.janelia.org/split-gal4 for image data.
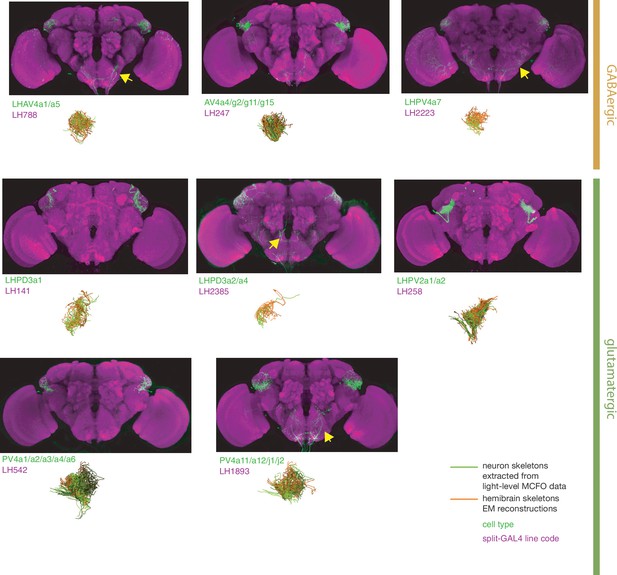
Split-GAL4 lines for lateral horn local neurons.
Putative local neurons of the lateral horn for which there are targeted genetic reagents as well as EM reconstructions (Dolan et al., 2019; Bates et al., 2020b; Scheffer et al., 2020). See http://www.janelia.org/split-gal4 for image data.
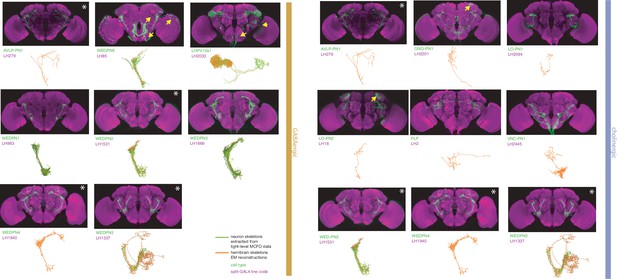
Split-GAL4 lines for lateral horn input neurons.
Putative non-olfactory input neurons to the lateral horn for which there are targeted genetic reagents as well as EM reconstructions (Dolan et al., 2019; Scheffer et al., 2020; Bates et al., 2020b). See http://www.janelia.org/split-gal4 for image data.
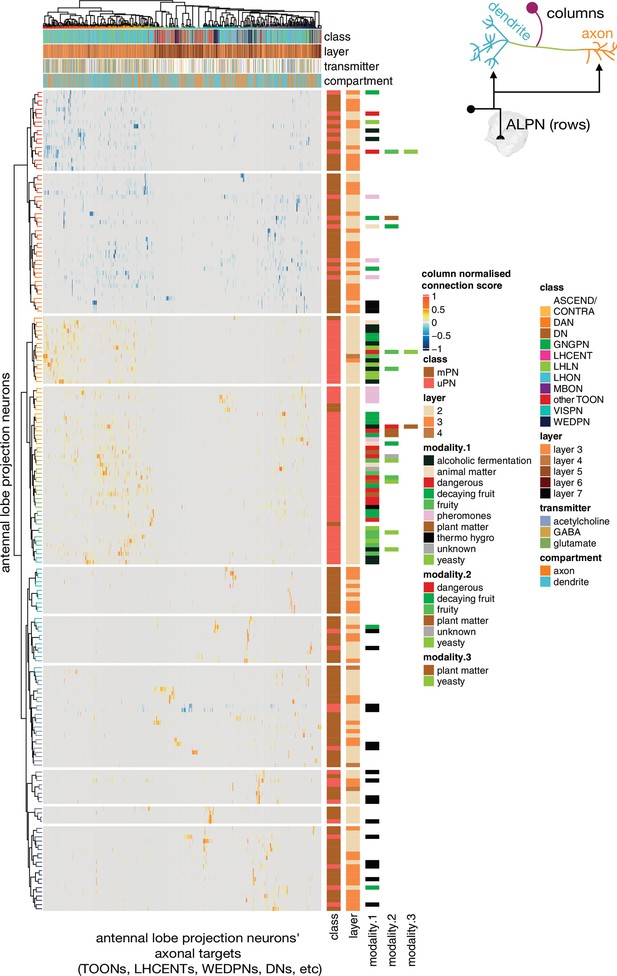
Antennal lobe projection neuron (ALPN) connectivity onto downstream targets.
Annotated heatmap showing the ALPN cell types (188, rows) → target (column) connection strengths. These connection strengths have been max normalised per column (target). ALPNs known to be glutamatergic or GABAergic have been given negative connection strengths, those that are unknown or cholinergic, positive. Each target column represents an entire connectivity types’ dendrites or axons (964 connectivity types’ dendrites, 534 connectivity types’ axons), in which each neuron has to have at least a 10 synapse or 1% postsynapse-normalised connection from an ALPN. Annotation bars indicate axons versus dendrites, as well as other metadata. Row and column clusters based on cosine similarity between connection strengths; see Figure 9—figure supplement 1. Where ‘modality’ is left white, the cell type in question combines information from multiple antennal lobe glomeruli. Clustering based on Ward’s distance, ALPNs grouped into 10 blocks for visualisation.
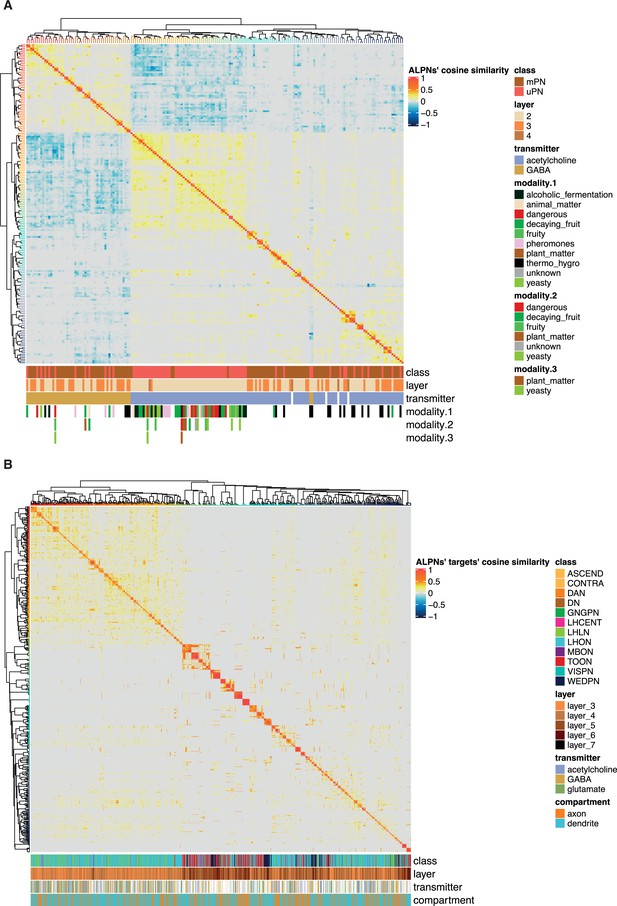
Neurons at the antennal lobe projection neuron (ALPN) axon → target connection, clustered by connection similarity.
(A) Cosine similarity calculated between ALPN cell types, based on ALPN→targets connection strengths, see Figure 9. (B) Cosine similarity calculated between ALPN’s target connectivity types, broken into axon and dendrite and based on ALPN→targets connection strengths. Clustering by Ward’s method, method ’ward.D2’ with the base R function hclust.
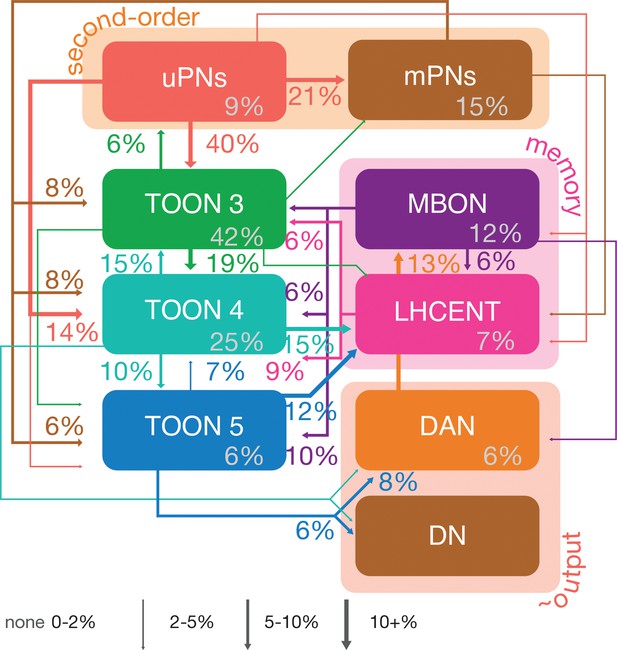
Neuron class-level network diagram of higher olfactory layers.
A circuit schematic of third-order olfactory neurons, showing the average connection strength between different classes of neurons (mean percentage of input synapses), broken into their layers, as well as the antennal lobe projection neuron (ALPN), lateral horn centrifugal neuron (LHCENT) and mushroom body output neuron (MBON) inputs to this system and dopaminergic neuron (DAN) and descending neuron (DN) outputs. The percentage in grey, within coloured lozenges, indicates the mean input that class provides to its own members. The threshold for a connection to be reported here is 5%, and >2% for a line to be shown.
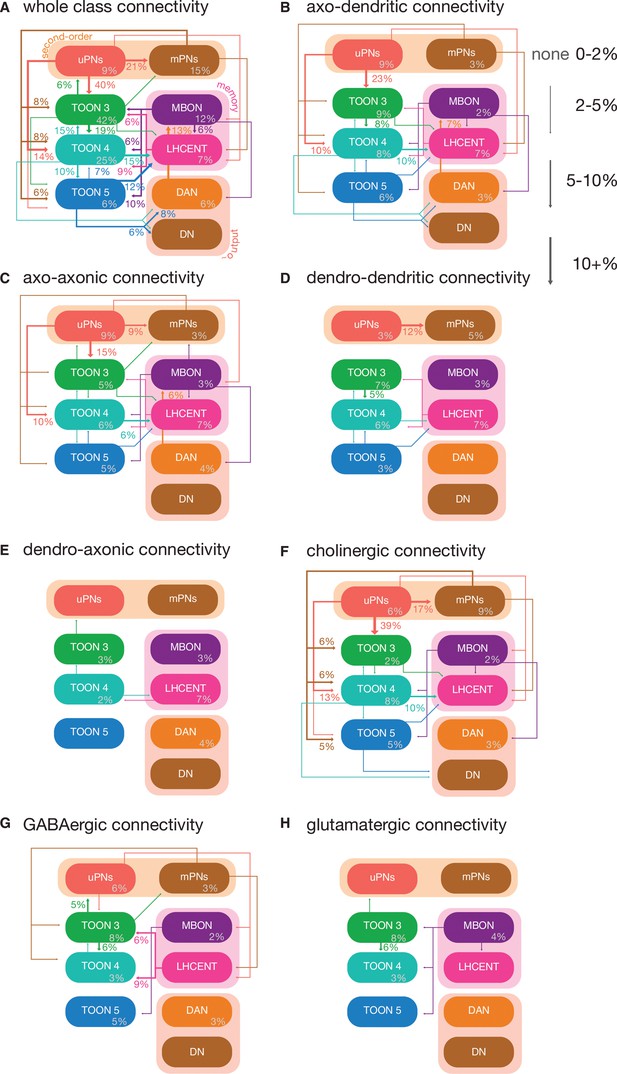
Neuron class-level network diagrams of higher olfactory layers, broken down by neuron compartments and putative transmitters.
(A) A circuit schematic of third-order olfactory neurons, showing the average connection strength between different classes of neurons (mean percentage of input synapses), broken into their layers, as well as the antennal lobe projection neuron (ALPN), lateral horn centrifugal neuron (LHCENT) and mushroom body output neuron (MBON) inputs to this system and dopaminergic neuron (DAN) and descending neuron (DN) outputs. The percentage in grey, within coloured lozenges, indicates the mean input that class provides to its own members. The threshold for a connection to be reported here is 5%, and >2% for a line to be shown. Subsequent plots just show a subset of this connectivity, that is, (B) axo-dendritic connections, (C) axo-axonic connections, (D) dendro-dendritic connections, (E) dendro-axonic connections, (F) putative cholinergic connections, (G) putative GABAergic connections and (H) putative glutamatergic connections.
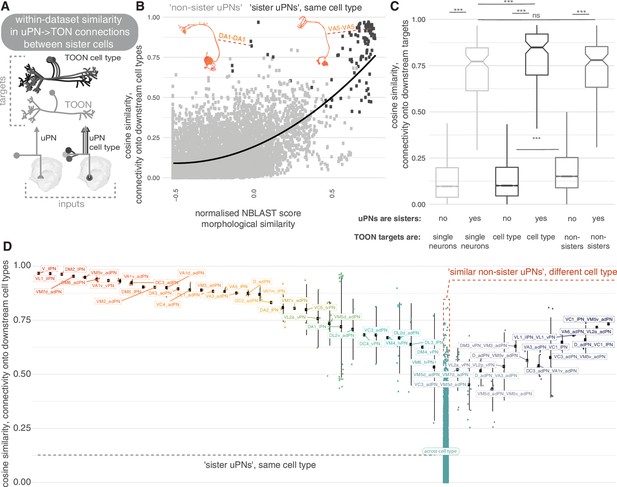
Within-data set connectivity similarity for key olfactory cell types.
(A) The synaptic targets of uniglomerular projection neurons (uPNs) (left) and uPN cell types (right) can be thought of as both individual downstream cells (lower) as well as cell types (upper). (B) For each pair of uPNs, the cosine similarity for their outputs onto downstream cell types is compared against their morphological similarity. The uPN-uPN pairs where both neurons are from the same cell type, ‘sisters’, shown in dark grey, otherwise in light grey. (C) The cosine similarity in the downstream target pool for sister and non-sister uPN pairs is compared. Targets can either be considered as separate cells (light grey, leftmost boxplots) or pooled by cell type (dark grey, middle boxplots). Shuffled data, for which cell type labels were shuffled for neurons downstream of each uPN to produce random small out-of-cell-type groupings of cells, shown in mid grey (rightmost box plots). Non-sister third-order olfactory neurons (TOONs) are shuffled pairs of TOONs from different cell types. There are 113 different sister PN-PN comparisons and 9157 non-sister PN-PN comparisons from our pool of 136 uniglomerular PNs. (D) The cosine similarity between connections to downstream cell. Left, all reconstructed lateral horn neurons (LHNs) types, for uPN-uPN pairs. Pairs shown are from the same cell type (left) or different cell types, where at least one comparison has a similarity of above >0.6. Significance values, Wilcoxon test: ***p≤0.001.
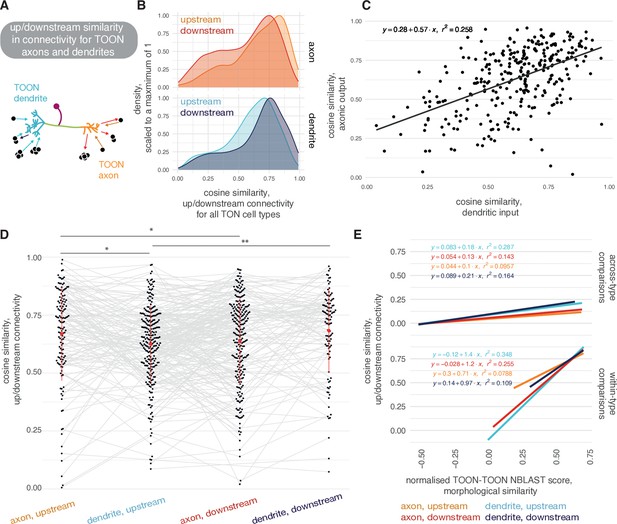
Similarity in connectivity up- and downstream of olfactory neurons.
(A) Neuron can give and receive output from both their axons and their dendrites. (B) Density plots showing cosine similarity scores for the cell types downstream of third-order olfactory neurons (TOON)-TOON pairs, where both members of the pair are from the same cell type. Upper: cosine similarity between the two populations upstream and downstream of the TOONs’ axons. Lower: cosine similarity between the two populations upstream and downstream of the TOONs’ dendrites. (C) Correlation between the mean cosine similarity between members of a TOON cell type’s dendritic input populations (x-axis) and axonic target populations (y-axis). (D) Cosine similarity between connections from/onto TOON axons/dendrites, for TOON-TOON pairs of the same cell type. (E) Correlations between morphological similarity and connectivity similarity shown, for both out-of-cell-type comparisons (top) and within-cell-type comparisons (bottom). Significance values: ns: p>0.05; *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
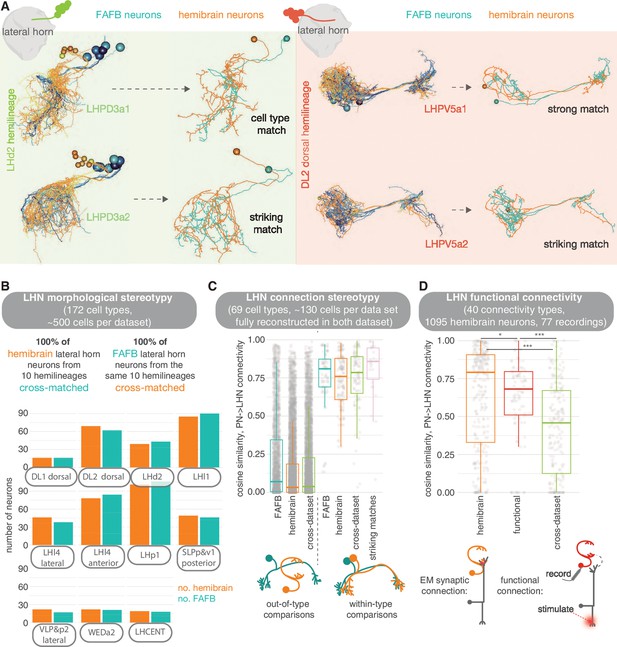
Stereotypy in morphology and connectivity between lateral horn neurons (LHNs) in the hemibrain, full adult fly brain (FAFB) and functional data sets.
(A) Cell types and individual neurons that have been cross-matched between data sets. Examples from the hemilineages LHd2 (i.e. the dorsal most cell body group in the LHd2 lineage clone, otherwise known as DPLm2 dorsal) and DL2 dorsal (otherwise known as CP3 dorsal). (B) We were able to cross-match >600 neurons across 10 hemilineages between the hemibrain and FAFB. (C) For neurons that had been fully synaptically reconstructed in FAFB, we calculate the cosine similarity for their antennal lobe projection neuron (ALPN) → LHN connectivity vectors to hemibrain neurons, both out-of-cell-type (left) and within-cell-type (right), as well as between the two data sets. In pink, same-cell-type between data set comparisons are made for only our ‘best’ morphological matches; matches for which the two neurons look so similar they could be the ‘same cell’. (D) Within-cell-type cosine similarity for ALPN→LHN connectivity for within the hemibrain data set, within the Jeanne and Wilson, 2018 functional connectivity data set and between members of the same cell type across data sets. Significance values, Student’s t-test: ns: p>0.05; *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
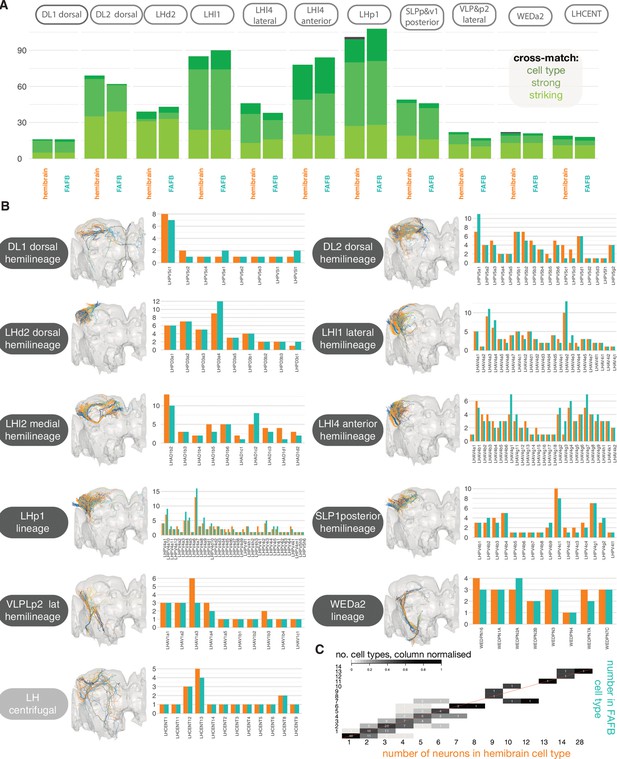
Stereotypy in morphology between lateral horn neurons (LHNs) in the hemibrain and full adult fly brain (FAFB) data sets.
(A) Tallies for the number of matches made from hemibrain → FAFB neurons (right) and hemibrain → FAFB neurons, and hemibrain → FAFB neurons (left), in both sets of ‘secondary’ hemilineages, plus LH centrifugal neurons, most of which are ‘primary’. ’Striking’ indicates that the two neurons look so similar they could be the ‘same cell’, ‘strong’ means that these cells look to belong to the same cell type, ‘cell type’ means that the two cells most likely belong to at least the same cell type. (B) Hemibrain image shows all reconstructed LHNs from both hemilineages are plotted together in the same brain space (hemibrain, grey) after a bridging registration had been applied (Bates et al., 2020a). Right: counts for neurons per identified LHN cell type, in each hemilineage in each data set. (C) Comparing the number of neurons in matched hemibrain-FAFB cell types. Red unity line.
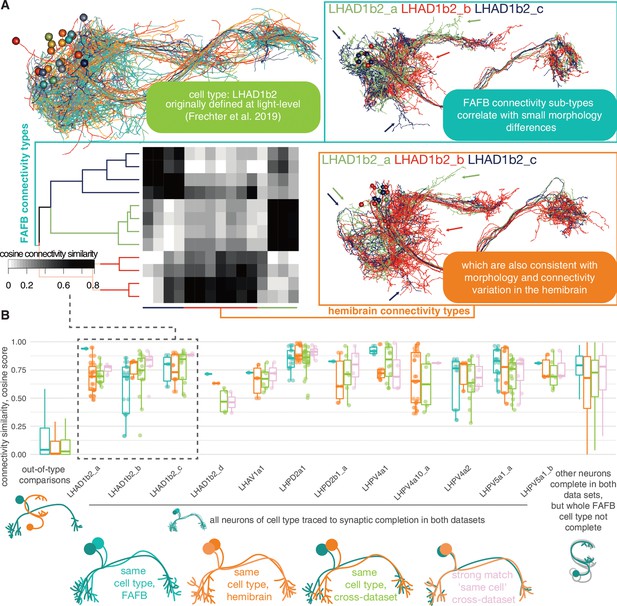
Stereotypy in connectivity between lateral horn neurons (LHNs) in the hemibrain and full adult fly brain (FAFB).
(A) An example of a cell type that looked cohesive at light-level resolution (Frechter et al., 2019), which actually breaks down into several connectivity subtypes on examination of the hemibrain data (Scheffer et al., 2020). Only uniglomerular antennal lobe projection neuron (ALPN) (uPN) inputs are considered for the cross-correlation plot. (B) Cosine similarity scores for uPN -> LHN inputs. The cell types shown have been ‘completely’ synaptically reconstructed in both data sets (total of 34 FAFB reconstructions), and the cosine similarity score calculated for every pairing within each data set (FAFB, blue; hemibrain, orange), between the two data sets (green) and between all ‘strongly’ cross-data set matched pairs (pink). Each completed FAFB cell type comprises a mean of 3.4 Â ± 1.1 s.d. neurons. Out-of-cell-type comparisons also made (leftmost), as well as for other neurons completed in FAFB, where not all members of the cell type have been completed (rightmost, 48 FAFB reconstructions) (Bates et al., 2020b).
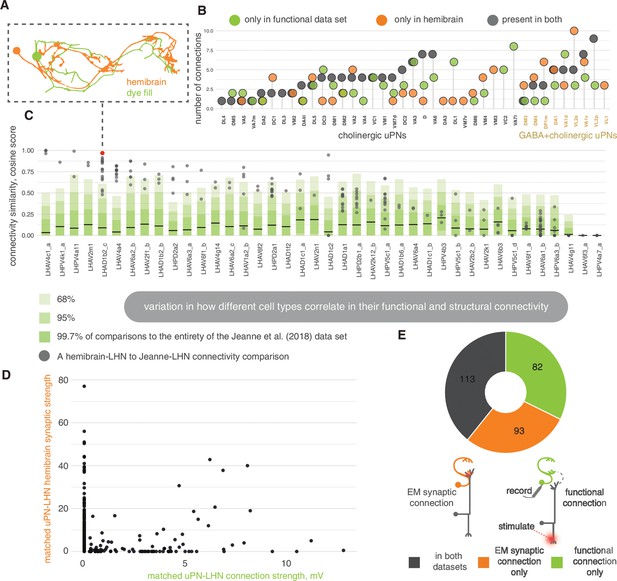
Stereotypy in connectivity between lateral horn neurons (LHNs) in the hemibrain and a functional data set.
(A) We matched light-level neuron skeletons from Jeanne and Wilson, 2018 to hemibrain reconstructions; these light-level skeletons are associated with functional glomeruli → LHN connections ascertained by electrophysiology (Jeanne and Wilson, 2018). (B) We calculate the number of equivalent connections, present by any degree, between both data sets. (C) The cosine similarity score for antennal lobe projection neuron (ALPN) → LHN connections. Horizontal bars, mean of the cosine comparison of each Jeanne and Wilson, 2018 cell type against all other cells in the Jeanne and Wilson, 2018 data set; dark green is one standard deviation from the mean, mid-green is two standard deviations, light green is three. Grey, comparison to matched hemibrain cell type, each point is one neuron-neuron comparison. (D) Scatter plot showing the strength of the recorded functional connections, in mV, and the number of connecting synapses in their cross-matched hemibrain neurons, for the corresponding uniglomerular projection neuron (uPN)→LHN contact. (E) The number of putative ALPN→LHN connections from a study on functional connectivity (Jeanne and Wilson, 2018), which can be found in the hemibrain data set, for cross-matched neurons. A threshold of four synapses has been applied for the hemibrain data.

Matching synaptically complete neurons between two EM data sets.
(A) Each full hemibrain lateral horn neuron (LHN) cell type is compared with as many of its cognates in full adult fly brain (FAFB) as possible, that is, from those neurons reconstructed in Bates et al., 2020b. Each point represents the normalised connection strength of a single uniglomerular projection neuron (uPN) type onto the target cell type in question (total connecting synapses / number of postsynapses in the target cell type). (B) Scatter plot showing the cosine similarity in uPN→LHN connectivity for LHN-LHN pairs, and LHN-LHN NBLAST scores. Every hemibrain neuron in (A) is compared with every FAFB neuron in (A). Neurons of the same cell type are shown in red. (C) For each uPN cell type, the mean normalised connection strength to each hemibrain cell type is taken as in (A), and the normalised connection strength to its cognate FAFB cell type is subtracted. Each point represents a different cell-type comparison. (D) Inset: insect synapses are polyadic meaning that one presynaptic site connects with multiple postsynaptic sites. We previously manually marked up presynapse-postsynapse connections for dozens of presynapses over a limited number of cell types in FAFB (green) (Bates et al., 2020b). The number of automatically detected postsynapses for each presynapse is also given for those same cell types in the hemibrain data set.
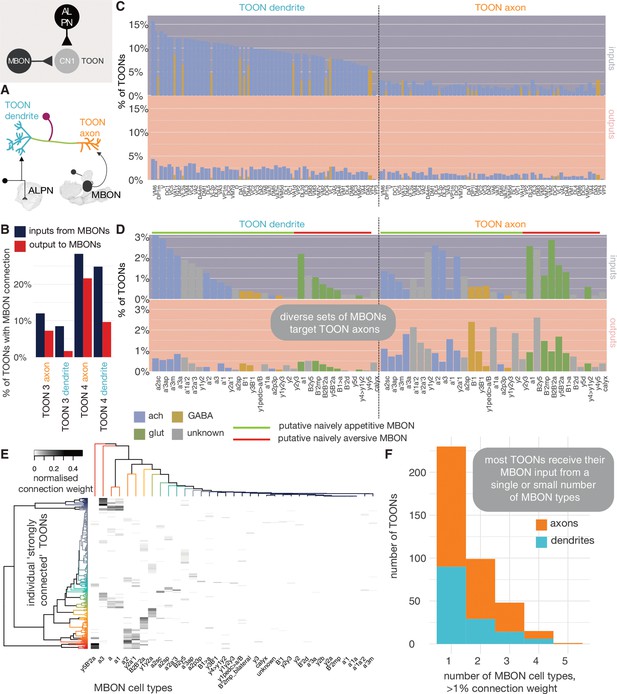
Mushroom body output neuron (MBON) innervation of lateral horn neurons.
(A) Olfactory projection neurons and MBONs seem to target different ends of lateral horn output neurons. (B) The percentage of third-order olfactory neurons (TOONs) (2383 neurons in total) that receive a ‘strong’ connection from an MBON type (71 neurons in total) (>1% of their dendrite’s/axon’s input synapses). (C) Percentages are broken down by MBON cell type. (D) The percentage of TOONs that receive a ‘strong’ connection from a uniglomerular projection neuron (uPN) type (136 neurons in total), broken down by type (>1% of their dendrite’s/axon’s input synapses). (E) A heatmap showing the normalised input of different MBONs onto TOONs’ axons. (F) A histogram showing the number of downstream TOONs that receive input from different numbers of MBONs. A threshold of >1% the input synapse count is used, axons and dendrites treated separately.

Propagating known odour information to third-order olfactory neurons (TOONs) and mushroom body output neurons (MBONs).
(A) Calcium responses recorded from antennal lobe projection neuron (ALPN) dendrites in the antennal lobe to odour presentations in Badel et al., 2016. (B) ’Co-connectivity’ and ‘odour influence’ scores calculated by matrix multiplication of uniglomerular projection neuron (uPN)→TOON or uPN→lateral horn centrifugal neuron (LHCENT) connectivity, MBON connectivity and previously published odour response data (Badel et al., 2016). TOONs that have PN innervation at their dendrites and MBON innervation at their axons were used. All matrices are minmax normalised across their columns. (C, D) Scores calculated using both MBON→TOON axon connectivity and MBON→LHCENT dendrite connectivity. Groupings referred to in text labelled in red dashed boxes.
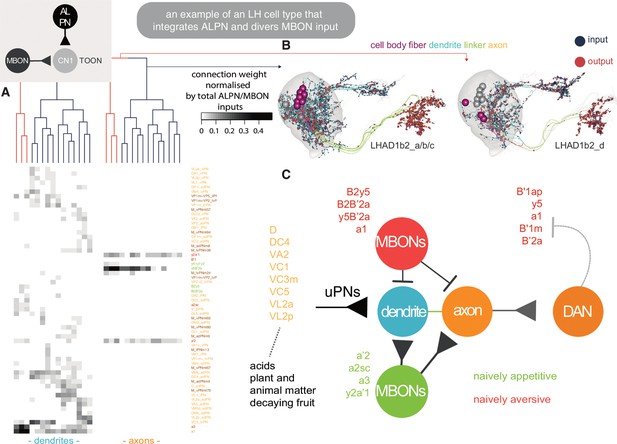
An exemplar convergence cell type of the lateral horn (LH) and mushroom body (MB).
(A) Heatmap showing the normalised connectivity (weight/total number of lateral horn neuron [LHN] inputs) of antennal lobe projection neuron (ALPN) and mushroom body output neuron (MBON) input (rows) onto 15 LHAD1b2 neurons, axons (right) and dendrites (left). Clustering by Ward’s method on dendrite data, cut at Euclidean linkage distance 0.2. MBON-dendrite connects can happen on distinct sub-branches, see Dolan et al., 2019. (B) Visualisation of the two connectivity clusters split into their dendrite-axon compartments (Schneider-Mizell et al., 2016; Bates et al., 2020b), which also correspond to small deviations in morphology. The other cluster is shown in grey in each panel. (C) An LHAD1b2 specific schematic for an emerging circuit motif integrating LH and MB output, based on the available labelled LHN data. MBONs coloured by naive valence, ALPNs by class.
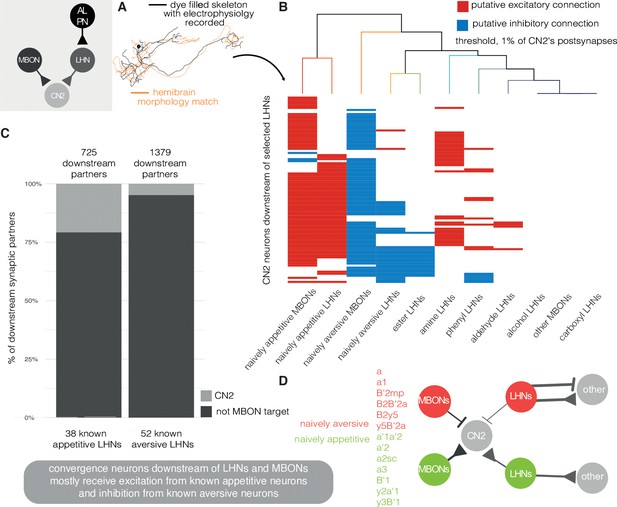
Convergence neurons of the lateral horn (LH) and mushroom body (MB).
(A) Matches were made between hemibrain reconstructions and lateral horn neuron (LHN) morphologies of electrophysiologically recorded cells (Frechter et al., 2019) and MultiColor FlpOut (Nern et al., 2015) data from LHN split-GAL4 lines used in behavioural studies (Dolan et al., 2019). A neuron is ‘appetitive’ if its optogenetic activation causes attraction to the stimulating light, and aversive if the opposite behaviour is significant (Dolan et al., 2019; Aso et al., 2014b). (B) Connections onto downstream targets (rows) by mushroom body output neurons (MBONs) and LHNs, grouped by putative valence or odour coding. Note that LHN valence and odour coding categories are not mutually exclusive. Connections have been binarised: if the upstream neuron class accounts for greater than 1% of inputs onto a given target, the connection is shown. Putative excitatory connections in red (i.e. cholinergic) and inhibitory in blue (i.e. GABAergic or glutamatergic). (C) The proportion of downstream targets from putatively aversive and appetitive LHNs, which also receive direct MBON input. (D) A general schematic for an emerging circuit motif integrating LH and MB output, based on the available labelled LHN data.
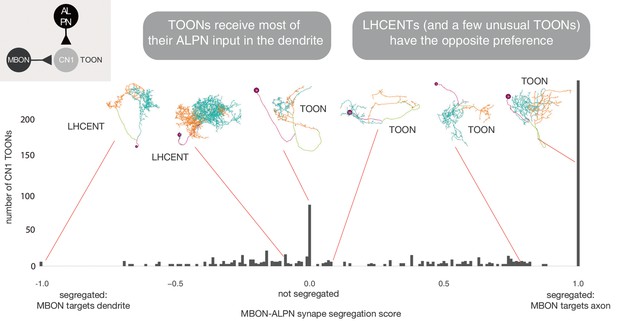
A class-compartment separation score.
The more positive the score, the more polarised the neuron such that antennal lobe projection neuron (ALPN) innervation is seen at the dendrite and mushroom body output neuron (MBON) innervation at the axon. Negative scores show the opposite segregation. See Materials and methods.
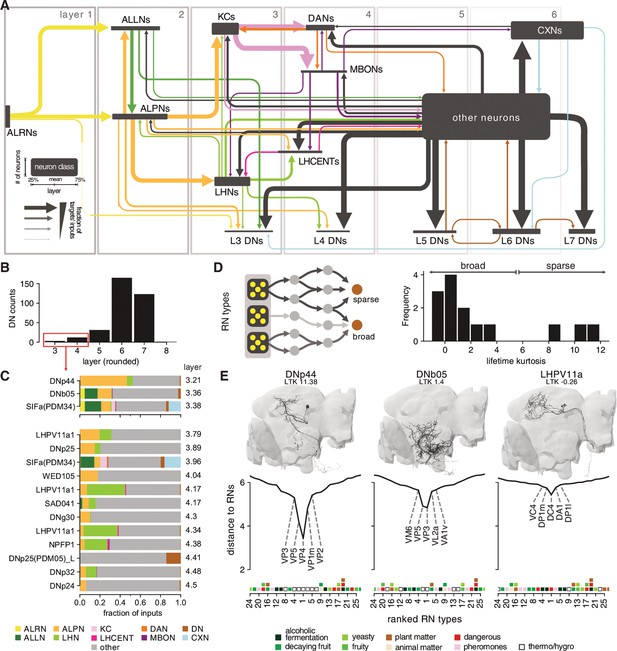
Connections between the olfactory system and descending neurons (DNs).
(A) Summary of olfactory circuits organised by layers. Box heights and widths correspond to the number and layer of neurons represented, respectively; arrow widths correspond to fraction of the targets’ inputs. See also legend in lower left. (B) The number of ‘early’ (layers 3 and 4) DNs is low. (C) Inputs to early DNs are diverse. Labels represent names in neuPrint. (D) Sparseness (lifetime kurtosis, LTK) of early DNs with respect to individual receptor neuron (antennal lobe receptor neuron [ALRN]) types. Most early DNs receive indirect inputs from a broad range of ALRNs. (E) Exemplary DNs and their connectivity to individual ALRN types. A low distance indicates a more direct connection between an olfactory receptor neurons (ORNs) or thermo-receptor neurons (TRN)/hygro-receptor neurons (HRN) type and the DN. Only the top 25 ALRN types shown. Hemibrain DNs are shown in black, and their homologs in the FlyWire data set as reference in grey. Heatmap shows glomeruli odour scenes.
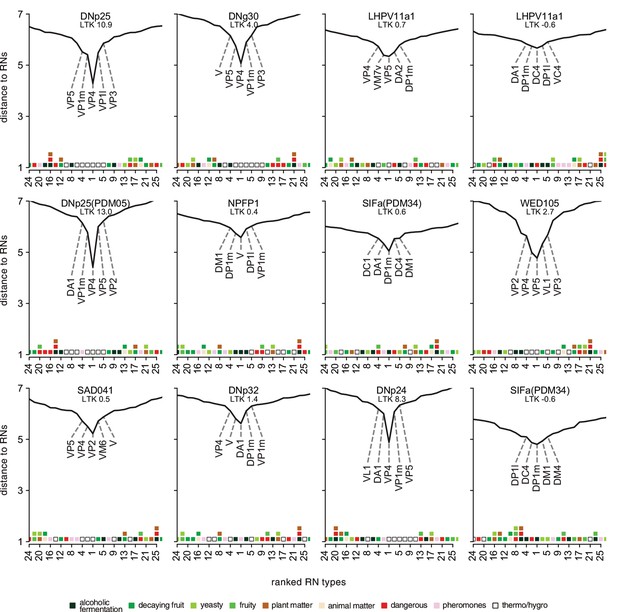
Extended data for Figure 14.
Antennal lobe receptor neuron (ALRN)→descending neuron (DN) distances for DNs not shown in main figure. A low distance indicates a more direct connection between an ALRN type and given DN. Only the top 25 ALRN types shown. Heatmap shows glomeruli odour scenes.
Tables
R and Python packages used and developed in this study.
| Language | Name | Github repository | Versioned DOI | Description | |
|---|---|---|---|---|---|
| By the authors | R | neuprintr | natverse/neuprintr | 10.5281/zenodo.3843545 | Query data from neuPrint |
| R | hemibrainr | natverse/hemibrainr | 10.5281/zenodo.4969908 | Analyse hemibrain data and metadata | |
| R | catmaid | natverse/rcatmaid | 10.5281/zenodo.3357143 | Query CATMAID data (e.g. for FAFB) | |
| R | nat.jrcbrains | natverse/nat.jrcbrains | 10.5281/zenodo.4966564 | Map between brain templates (inc hemibrain and FAFB) | |
| R | nat.nblast | natverse/nat.nblast | 10.5281/zenodo.4966689 | Morphological comparison | |
| Python | navis | schlegelp/navis | 10.5281/zenodo.4751181 | Query and process neuron data | |
| Python | navis-flybrains | schlegelp/navis-flybrains | 10.5281/zenodo.4966641 | Map between brain templates (inc hemibrain and FAFB) | |
| Python | pymaid | schlegelp/pymaid | 10.5281/zenodo.4724559 | Query CATMAID data (e.g. for FAFB) | |
| Python | fafbseg | flyconnectome/fafbseg-py | 10.5281/zenodo.4966610 | Work with autosegmented FAFB data (e.g. FlyWire) | |
| Third party | Python | neuprint-python | connectome-neuprint/ neuprint-python | 10.5281/zenodo.4968061 | Query data from neuPrint, developed by Stuart Berg (Janelia Research Campus) |
Names of posterior glomeruli across data sets and publications, and supporting reference for names used in this study.
| Glomerulus | Hemibrain v1.1 + 1.2 | Bates et al., 2020b | Tanaka et al., 2012b | Yu et al., 2010 | Receptor | Supporting references |
|---|---|---|---|---|---|---|
| VC5 | VC3m | VC3m | VC3m | – | Ir41a | Silbering et al., 2011; Task et al., 2020; Hussain et al., 2016; Min et al., 2013; Chai et al., 2019 |
| VC3 | VC3l | VC3l | VC3l | VC3 | Or35a | Couto et al., 2005; Grabe et al., 2016; Silbering et al., 2011; Task et al., 2020; Min et al., 2013 |
| VM6 (v + m + l) | VC5 | VC5 | VC5 | VM6+ VP1 | Rh50/Amt | Endo et al., 2007; Li et al., 2016; Chai et al., 2019; Vulpe et al., 2021; Task et al., 2020 |
Description of neuron metadata listed in supplementary files.
| Column name | Description |
|---|---|
| bodyid | A unique identifier for a single hemibrain neuron |
| pre | The number of presynapses (outputs) a neuron contains, each of these is polyadic |
| post | The number of postsynapses (inputs) to the neuron |
| upstream | The number of incoming connections to a neuron |
| downstream | The number of outgoing connections from a neuron |
| voxels | Neuron size in voxels |
| soma | Whether the neuron has a soma in the hemibrain volume |
| name | The name of this neuron, as read from neuPrint |
| side | Which brain hemisphere contains the neuron’s soma |
| connectivity.type | A subset of neurons within a cell type that share similar connectivity, a connectivity type is distinguished from a cell type by an ending _letter unless there is only one connectivity type for the cell type, defined using CBLAST (Scheffer et al., 2020) |
| cell.type | Neurons of a shared morphology that take the same cell body fibre tract and come from the same hemilineage (Bates et al., 2019) |
| class | The greater anatomical group to which a neuron belongs, see Figure 1 |
| cellBodyFiber | The cell body fibre for a neuron, as read from neuPrint (Scheffer et al., 2020) |
| ItoLee_Hemilineage | The hemilineage that we reckon this cell type belongs to, based on expert review of light-level data from the K. Ito and T. Lee groups (Yu et al., 2013; Ito et al., 2013) |
| Hartenstein_Hemilineage | The hemilineage that we reckon this cell type belongs to, based on expert review of light-level data from the V. Hartenstein group (Wong et al., 2013; Lovick et al., 2013) |
| putative.classic.transmitter | Putative neurotransmitter based on what neurons in the hemilineage in question have been shown to express, out of acetylcholine, GABA and/or glutamate |
| putative.other.transmitter | Potential second neurotransmitter |
| FAFB.match | The ID of the manual match from the FAFB data set, ID indicates a neuron reconstructed in FAFBv14 CATMAID, many of these neurons will be available through Virtual Fly Brain, https://v2.virtualflybrain.org/ |
| FAFB.match.quality | The matcher makers’ qualitative assessment of how good this match is: a poor match could be a neuron from a very similar cell type or a highly untraced neuron that may be the correct cell type; an okay match should be a neuron that looks to be from the same morphological cell type but there may be some discrepancies in its arbour; a good match is a neuron that corresponds well between FAFB and the hemibrain data |
| layer | Probabilistic mean path length to neuron from ALRNs, depends on connection strengths |
| layer.ct | The mean layer for cell type, rounded to the nearest whole number |
| axon.outputs | Number of outgoing connections from the neuron’s predicted axon |
| dend.outputs | Number of outgoing connections from the neuron’s predicted dendrite |
| axon.inputs | Number of incoming connections from the neuron’s predicted axon |
| dend.inputs | Number of incoming connections from the neuron’s predicted dendrite |
| total.length | Total cable length of the neuron in micrometres |
| axon.length | Total axon cable length of the neuron in micrometres |
| dend.length | Total dendrite cable length of the neuron in micrometres |
| pd.length | Total cable length of the primary dendrite ’linker’ between axon and dendrite |
| segregation_index | A quantification of how polarised a neuron is, in terms of its segregation of inputs onto its predicted dendrite and outputs onto its axon, where 0 is no-polarisation and 1 is totally polarised (Schneider-Mizell et al., 2016) |
| notes | Other notes from annotators |
Additional files
-
Supplementary file 1
Layers assigned by the probabilistic graph traversal model.
bodyId refers to neurons’ unique ID in neuPrint. layer_mean contains the mean layer after 10,000 iterations of the main model (Figure 2). layer_olf_mean and layer_th_mean contain the mean layers from running the traversal model with ORNs and THN/HRNs, respectively (Figure 2—figure supplement 2).
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp1-v2.csv
-
Supplementary file 2
Sensory meta-information related to each glomerulus.
Columns: glomerulus (canonical name for one of the 51 olfactory + 7 thermo/hygrosensory antennal lobe glomeruli), laterality (whether the glomerulus receives bilateral or only unilateral innervation from ALRNs), expected_cit (a citation that describes the expected number of RNs in this glomerulus), expected_RN_female_1 h (number of expected RNs in one hemisphere), expected_RN_female_SD (standard deviation in the expected number of RNs), missing (qualitative assessment of glomeruli truncation), RN_frag (if the RNs in that glomerulus are fragmented), receptor (the OR or IR expressed by cognate ALRNs, Bates et al., 2020b; Task et al., 2020), odour_scenes the general ‘odour scene(s)’ which this glomerulus may help signal, (Mansourian and Stensmyr, 2015; Bates et al., 2020b), key_ligand (the ligand that excites the cognate ALRN or receptor the most, based on pooled data from multiple studies, Münch and Münch and Galizia, 2016), valence (the presumed valence of this odour channel, Badel et al., 2016). Exists as hemibrain_glomeruli_summary in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp2-v2.csv
-
Supplementary file 3
File listing all identified antennal lobe receptor neurons (ALRNs) in the hemibrain, including information shown in neuPrint.
See above for column explanations. Exists as rn.info in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp3-v2.csv
-
Supplementary file 4
All the hemibrain neurons we have classed as antennal lobe local neurons (ALLNs).
See above for column explanations. Exists as alln.info in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp4-v2.csv
-
Supplementary file 5
All the hemibrain neurons we have classed as antennal lobe projection neurons (ALPNs).
See above for column explanations. In addition, across_dataset_cluster refers to the clustering with left and right FAFB PNs; is_canonical indicates whether that ALPN is one of the well studied ‘‘canonical’’ uPNs. Exists as pn.info in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp5-v2.csv
-
Supplementary file 6
All the hemibrain neurons we have classed as third-order olfactory neurons (TOONs) including lateral horn neurons (LHNs), as well as wedge projection neurons (WEDPNs), lateral horn centrifugal neurons (LHCENT) and other projection neuron classes (Figure 1).
See above for column explanations. Exists as ton.info in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp6-v2.csv
-
Supplementary file 7
All the hemibrain neurons we have classed as neurons that descend to the ventral nervous system (DNs).
See above for column explanations. Exists as dn.info in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp7-v2.csv
-
Supplementary file 8
The root point in hemibrain voxel space, for each hemibrain neuron.
This is either the location of the soma, or the tip of a severed cell body fibre tract, where possible. Exists as hemibrain_somas in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp8-v2.csv
-
Supplementary file 9
The start points for different neuron compartments.
Nodes downstream of this position in the 3D structure of the neuron indicated with bodyid, belong to the compartment type designated by Label. A product of running flow_centrality on hemibrain neurons, exists as hemibrain_splitpoints in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp9-v2.csv
-
Supplementary file 10
3D triangle mesh for the hemibrain surface as a.obj file.
This mesh was generated by first merging individual ROI meshes from neuPrint and then filling the gaps in between in a semi-manual process. It also exists as hemibrain.surf in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp10-v2.obj
-
Supplementary file 11
3D meshes of 51 olfactory + 7 thermo/hygrosensory antennal lobe glomeruli for the hemibrain volume, generated from ALRN presynapses.
These meshes follow the subdivision of VM6 and hence contain 60 meshes in total. Note that hemibrain coordinate system has the anterior-posterior axis aligned with the Y axis (rather than the Z axis, which is more commonly observed).
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp11-v2.zip
-
Supplementary file 12
3D meshes of 51 olfactory + 7 thermo/hygrosensory antennal lobe glomeruli for the hemibrain volume, generated from ALPN postsynapses.
Note that hemibrain coordinate system has the anterior-posterior axis aligned with the Y axis (rather than the Z axis, which is more commonly observed). These meshes are also available as hemibrain_al.surf in our R package hemibrainr.
- https://cdn.elifesciences.org/articles/66018/elife-66018-supp12-v2.zip
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/66018/elife-66018-transrepform1-v2.docx





