Male pheromones modulate synaptic transmission at the C. elegans neuromuscular junction in a sexually dimorphic manner
Figures
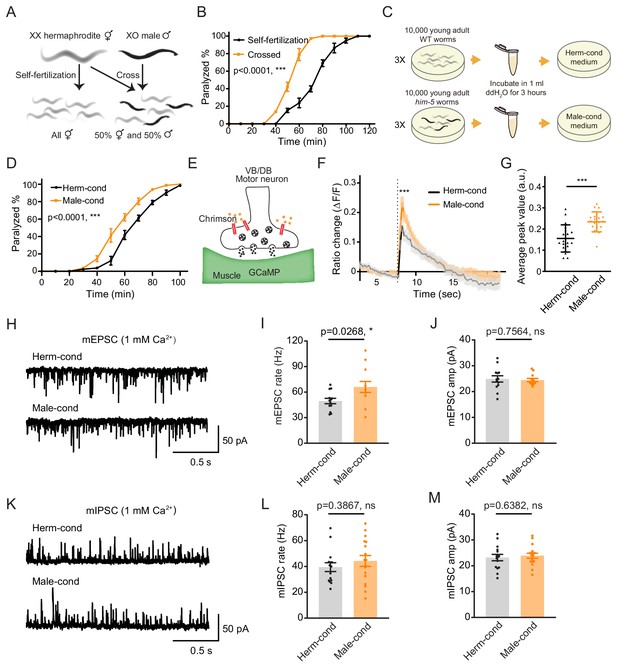
The male excretome increases cholinergic synaptic transmission at hermaphrodite NMJ.
(A) Schematic illustration of C. elegans reproduction. Hermaphrodites with two X chromosomes generate all hermaphrodite progeny via self-fertilization. While hermaphrodites are crossed with males that have a single X chromosome, an equal ratio of hermaphrodite and male offspring are generated. (B) Time course analysis of 1.4 mM aldicarb-induced paralysis in hermaphrodites generated from hermaphrodite self-fertilization (black, Self-fertilization) and hermaphrodite-male crossing (orange, Crossed). (C) Schematic illustration of conditioned medium preparation. 30,000 young-adult wild-type (WT) and him-5 mutant worms were collected and incubated in 1 ml ddH2O for 3 hr. Metabolites secreted by hermaphrodites and males were collected and used to make hermaphrodite-conditioned (Herm-cond) and male-conditioned (Male-cond) medium. (D) Time course analysis of Aldicarb-induced paralysis in hermaphrodites cultured in hermaphrodite-conditioned medium (black, Herm-cond) and male-conditioned medium (orange, Male-cond). (E) Schematic illustration showing calcium current recording at the C. elegans NMJ. Chrimson driven by the acr-5 promoter was expressed specifically in cholinergic motor neurons, and GCaMP3 under the myo-3 promoter was expressed in the body-wall muscle. (F–G) Chrimson-evoked calcium transients in body-wall muscle were analyzed using GCaMP3 as a calcium indicator. For adult hermaphrodites cultured in hermaphrodite-conditioned medium (black, Herm-cond) or male-conditioned medium (orange, Male-cond), the averaged responses (F) and the averaged and individual relative increase in GCaMP3 fluorescence intensity ΔF/F (G) are shown. The gray shadings in F indicate the SEM of GCaMP3 responses. The dashed line indicates when the illumination with nominal wavelength at 640 nm for Chrimson activation was applied. (H–J) Endogenous acetylcholine transmission was assessed by recording mEPSCs from body muscles of wild-type adult hermaphrodites cultured in hermaphrodite-conditioned or male-conditioned medium. Representative mEPSC traces (H), the mean mEPSC rates (I), and the mean mEPSC amplitudes (J) are shown. (K–M) Endogenous GABA transmission was assessed by recording mIPSCs from body muscles of wild-type adult hermaphrodites cultured in hermaphrodite-conditioned or male-conditioned medium. Representative mIPSC traces (K), the mean mIPSC rate (L), and the mean mIPSC amplitude (M) are shown. The data for individual animal analyzed are indicated. In (B), (D), (F–G), (I–J), (L–M), *p<0.05, ***p<0.001, ns not significant, two-way ANOVA comparing all of the time points for (B) and (D), unpaired Student’s t-test for (F–G), (I–J), and (L–M).
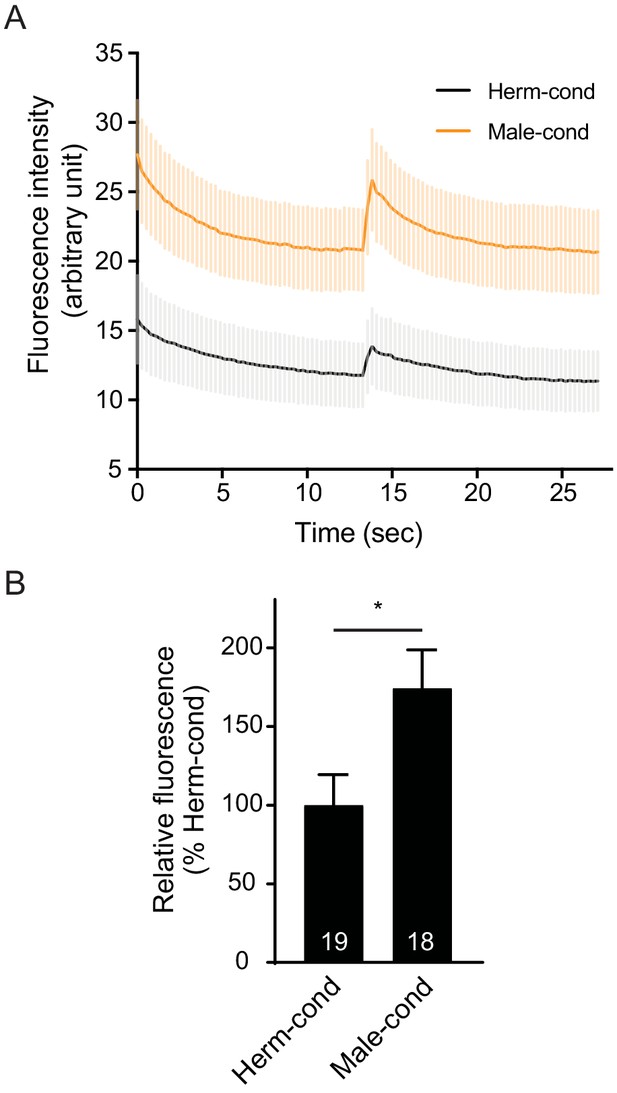
The physiological muscle excitability is potentiated in hermaphrodites from the male-conditioned medium.
(A) Averaged Chrimson-evoked calcium transients in body-wall muscle were analyzed in hermaphrodites cultured in hermaphrodite-conditioned medium (black) or male-conditioned medium (orange). The averaged responses (A) and the endogenous GCaMP3 fluorescence intensity (B) were shown. The gray and orange shadings in (A) indicate SEM of GCaMP3 responses. The numbers of animals are indicated inside the bars. In (B), *p<0.05, unpaired Student’s t-test.
-
Figure 1—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig1-figsupp1-data1-v2.xlsx
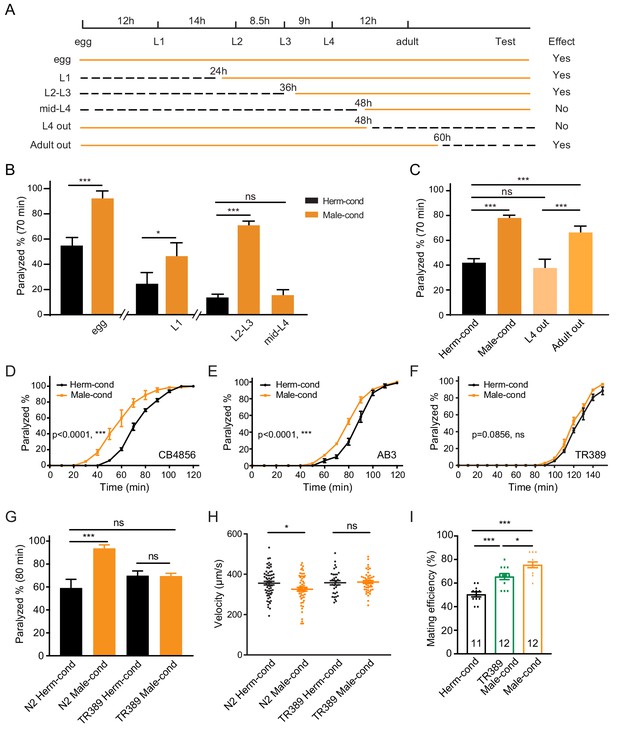
The male excretome modulates the hermaphrodite NMJ synaptic transmission in a developmental-stage-dependent manner.
(A) Schematic illustration of the life cycles of C. elegans and the time when the hermaphrodite-conditioned medium (dashed black lines) or male-conditioned medium (solid orange lines) was applied. (B) The percentage of animals paralyzed on 1.4 mM aldicarb at 70 min were plotted for hermaphrodites cultured in male-conditioned medium (orange) starting from the egg stage, L1 stage, L2–L3 stage, and mid-L4 stage. Hermaphrodites cultured in hermaphrodite-conditioned medium (black) served as controls. (C) The percentage of animals paralyzed on 1.4 mM aldicarb at 70 min were plotted for hermaphrodites cultured in male-conditioned medium from the egg stage to the mid-L4 stage (L4 out) and young adult stage (Adult out); hermaphrodites cultured in hermaphrodite-conditioned medium (black) or male-conditioned medium (orange) served as controls. (D–F) Time course analysis of aldicarb-induced paralysis in hermaphrodites cultured in hermaphrodite-conditioned medium (black) and male-conditioned medium (orange) in CB4856 (D), AB3 (E), and TR389 (F) strains. (G) The percentage of animals paralyzed on 1.4 mM aldicarb at 80 min were plotted for N2 hermaphrodites cultured in N2 hermaphrodite (N2 herm-cond)-, N2 male (N2 Male-cond)-, TR389 hermaphrodite (TR389 Herm-cond)- or TR389 male (TR389 Male-cond)-conditioned medium. (H) Locomotion behavior analysis of single adult hermaphrodite cultured in N2 hermaphrodite (Herm-cond)-, TR389 male (TR389 Male-cond)-, and N2 male (Male-cond)-conditioned medium. The averaged and individual crawling locomotion velocities were plotted. (I) Measurement of hermaphrodite mating efficiency cultured in N2 hermaphrodite-, TR389 male-, and N2 male-conditioned medium. In B-I, *p<0.05, ***p<0.001, ns not significant, two-way ANOVA with post hoc Sidak multiple comparisons for (B–C) and (G), two-way ANOVA comparing all of the time points for (D–F), one-way ANOVA with post hoc Dunnett multiple comparisons for (H and I).
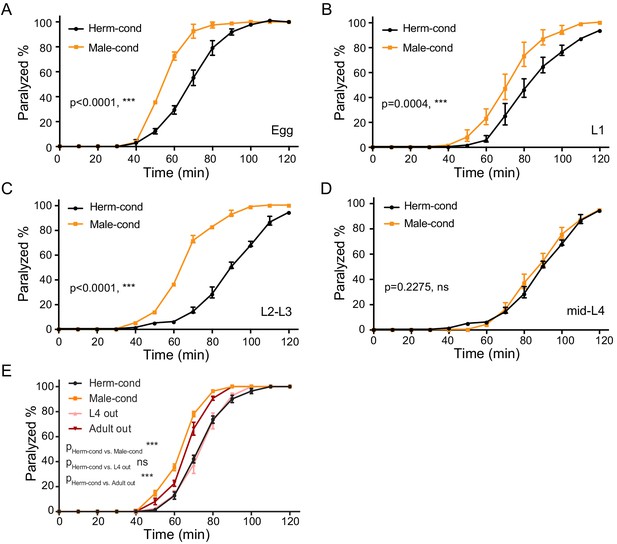
The male environment modulates the hermaphrodite NMJ synaptic transmission in a developmental-stage-dependent manner.
(A–D) Time course analysis of aldicarb-induced paralysis in hermaphrodites cultured in male-conditioned medium (orange) starting from egg stage (A), L1 (B), L2–L3 stage (C), and mid-L4 stage (D). Hermaphrodites cultured in hermaphrodite-conditioned medium (black) served as controls. (E) Time course analysis of aldicarb-induced paralysis in hermaphrodites cultured in male-conditioned medium from the egg stage to the L4 (pink) and young adult stage (dark red), hermaphrodites cultured in hermaphrodite-conditioned medium (black), and male-conditioned medium (orange). ***p<0.001, ns not significant, two-way ANOVA comparing all of the time points.
-
Figure 2—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig2-figsupp1-data1-v2.xlsx
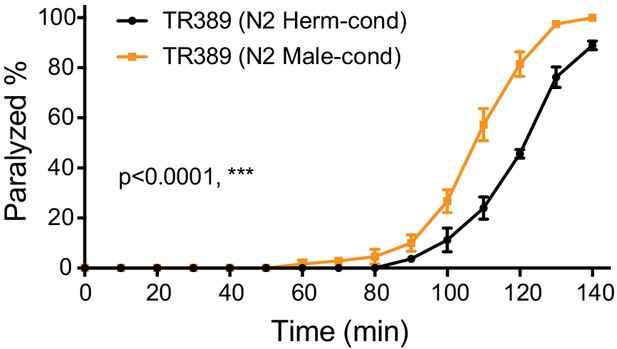
TR389 hermaphrodites can be modulated by the modulator ascarosides.
Time course analysis of aldicarb-induced paralysis in TR389 hermaphrodite grown under N2 hermaphrodite (black, N2 Herm-cond)- or N2 male-conditioned (orange, N2 Male-cond) medium. ***p<0.001, ns not significant, two-way ANOVA comparing all of the time points.
-
Figure 2—figure supplement 2—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig2-figsupp2-data1-v2.xlsx
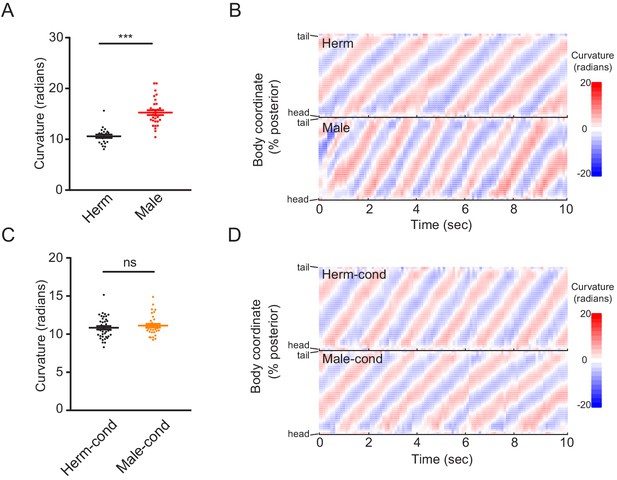
The male excretome does not change hermaphrodite body-bend curvature.
(A) Scatter plot showing the body-bend curvature in hermaphrodites (black) and males (red). (B) Color plot showing the waves of curvature propagating along the body of representative hermaphrodite (Herm, top) and male (Male, bottom). (C) Scatter plot showing the body-bend curvature in hermaphrodites from hermaphrodite- (black) and male-conditioned (orange) medium. (D) Color plot showing the waves of curvature propagating along the body of representative hermaphrodite from hermaphrodite- (top) and male-conditioned (bottom) medium. In A and C, ***p<0.001, ns not significant, unpaired Student’s t-test.
-
Figure 2—figure supplement 3—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig2-figsupp3-data1-v2.xlsx
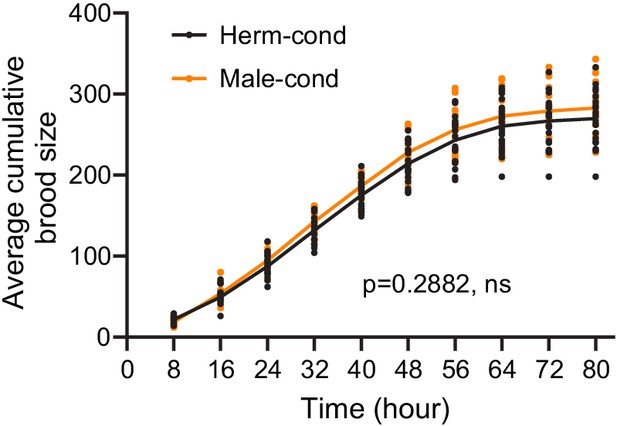
The male excretome does not modulate hermaphrodite brood size.
Time course analysis of the average number of eggs laid by hermaphrodites from hermaphrodite- and male-conditioned medium. ns, not significant, two-way ANOVA comparing all of the time points.
-
Figure 2—figure supplement 4—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig2-figsupp4-data1-v2.xlsx
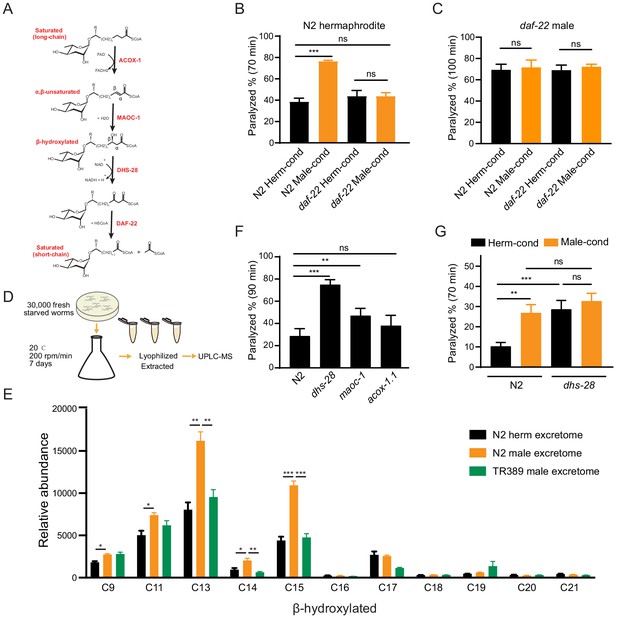
Ascarosides in the male environment modulate hermaphrodite NMJ synaptic transmission.
(A) Proposed model of peroxisomal β-oxidation enzymes ACOX-1, MAOC-1, DHS-28, and DAF-22 in ascaroside side-chain biosynthesis. (B) The percentage of animals paralyzed on 1.4 mM aldicarb at 70 min were plotted for N2 hermaphrodites cultured in hermaphrodite (N2 Herm-cond)-, male (N2 Male-cond)-, daf-22 mutants herm (daf-22 Herm-cond)-, or daf-22 mutant male (daf-22 Male-cond)-conditioned medium. (C) The percentage of animals paralyzed on 0.5 mM aldicarb at 100 min were plotted for daf-22 mutant males cultured in hermaphrodite (N2 Herm-cond)-, male (N2 Male-cond)-, daf-22 mutants herm (daf-22 Herm-cond)-, or daf-22 mutant male (daf-22 Male-cond)-conditioned medium. (D) Schematic illustration of excretome preparation for UPLC-MS. Around 30,000 freshly starved worms were cultured in S medium supplemented with concentrated OP50 for 7 days. The excretomes were collected by centrifugation, filtration, and lyophilized extraction, followed by UPLC-MS analysis. (E) β-hydroxylated ascaroside profiles in excretomes obtained from N2 hermaphrodites (N2 herm excretome), N2 mixed-gender animals of him-5 mutants (N2 male excretome), and TR389 mixed-gender animals (TR389 male excretome). (F) The percentage of animals paralyzed on 1.4 mM aldicarb at 90 min were plotted for β-oxidation mutants (acox-1.1, maoc-1, and dhs-28). (G) The percentage of animals paralyzed on 1.4 mM aldicarb at 70 min were plotted for N2 and dhs-28 mutant hermaphrodites cultured in hermaphrodite-conditioned medium (Herm-cond), male-conditioned medium (Male-cond). In (B–C), (E–G), *p<0.05, **p<0.01, ***p<0.001, ns not significant, two-way ANOVA with post hoc Sidak multiple comparisons for (B–C) and (F–G). one-way ANOVA with post hoc Dunnett multiple comparisons for (E).
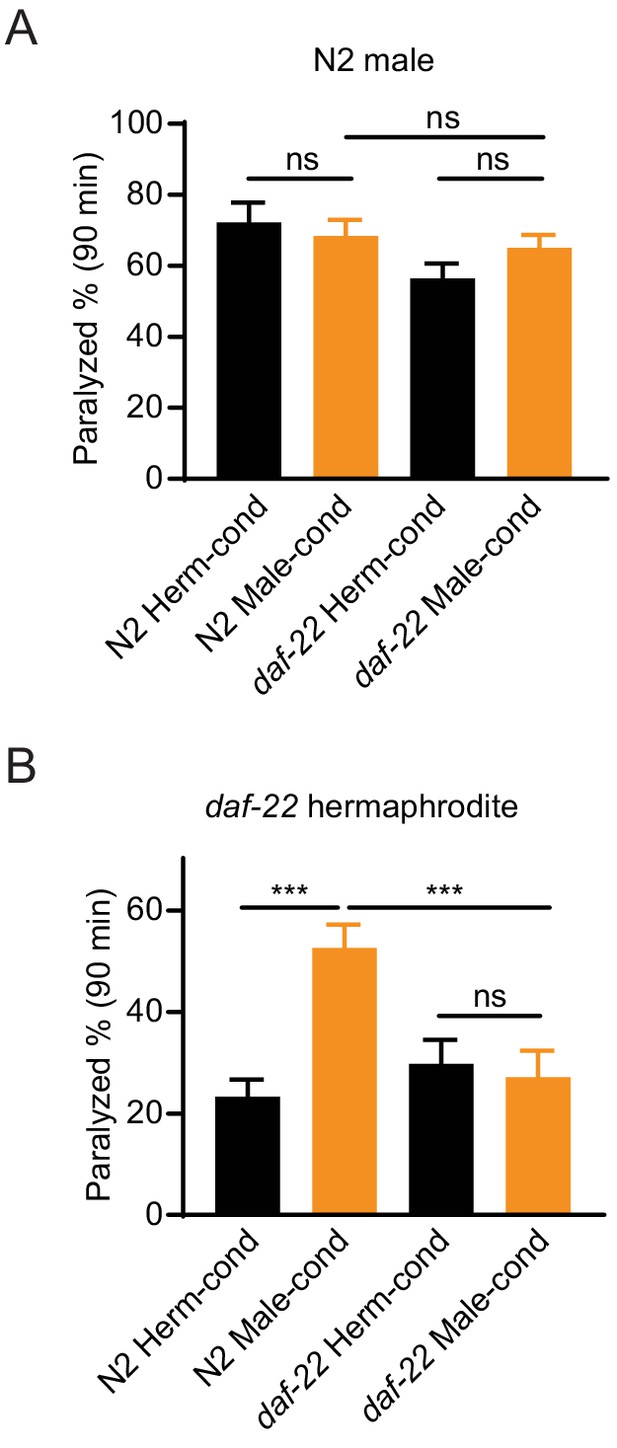
The male environment cannot modulate NMJ synaptic transmission in males.
The percentage of animals paralyzed on 0.5 mM (A) or 1.4 mM (B) aldicarb at 90 min were plotted for wild-type males (A) and daf-22 mutant hermaphrodites (B) cultured in N2- and daf-22 mutant-conditioned medium. ***p<0.001, ns not significant, two-way ANOVA with post hoc Sidak multiple comparisons.
-
Figure 3—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig3-figsupp1-data1-v2.xlsx
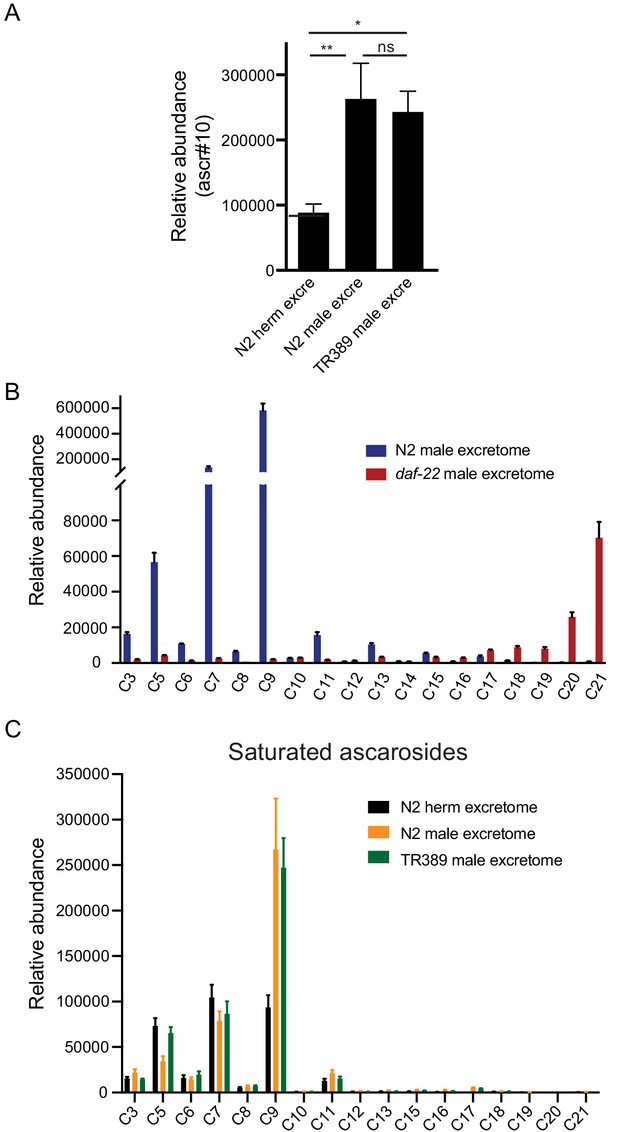
UPLC-MS analysis of excretome from animal cultures.
(A) The relative abundance of ascr#10 in excretomes obtained from N2 hermaphrodites (N2 herm excre), N2 mixed-gender animals of him-5 mutants (N2 male excre), and TR389 mixed-gender animals (TR389 male excre). (B) Ascaroside profiles in excretomes obtained from N2 mixed-gender animals of him-5 mutants (N2 male excretome) and daf-22 mixed-gender animals (daf-22 male excretome) showed that long-chain ascarosides were accumulated in daf-22 mutant cultures. (C) Saturated ascaroside profiles in excretomes obtained from N2 hermaphrodites (N2 excretome), N2 mixed-gender animals of him-5 mutants (N2 male excretome), and TR389 mixed-gender animals (TR389 male excretome). *p<0.05, **p<0.01, one-way ANOVA with post hoc Dunnett multiple comparisons.
-
Figure 3—figure supplement 2—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig3-figsupp2-data1-v2.xlsx
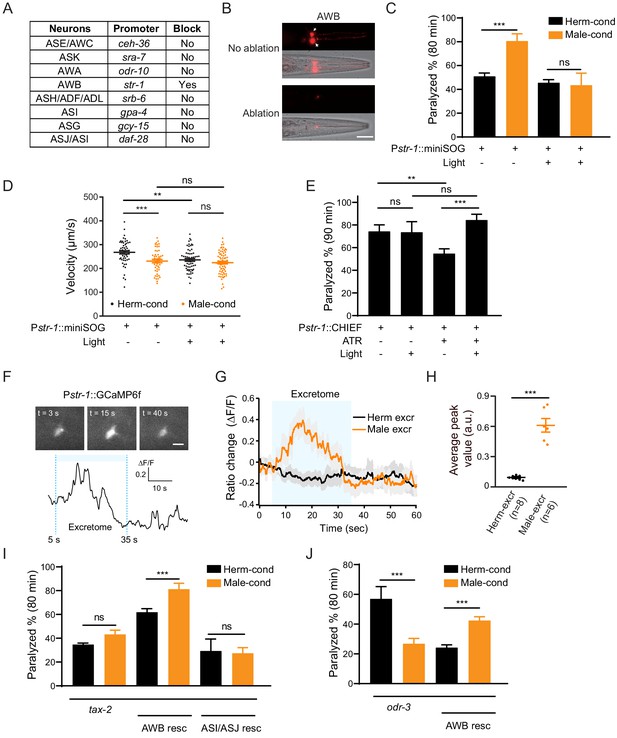
AWB neurons sense the modulator ascarosides.
(A) The table lists all of the chemosensory neurons examined in the screen, the neuron-specific promoters used to drive miniSOG expression, and the impact of neuron ablation on sensing of modulator ascarosides. (B) Representative images showing that mCherry-labeled AWB neurons were specifically ablated by blue light-induced miniSOG activation. Scale bar, 40 μm. (C) The percentage of animals paralyzed on 1.4 mM aldicarb at 80 min were plotted for hermaphrodites expressing miniSOG in AWB neurons (str-1 promoter) with and without blue-light-induced ablation. Black: cultured in hermaphrodite-conditioned medium; Orange: cultured in male-conditioned medium. (D) Locomotion behavior analysis of single adult worm from AWB-ablated hermaphrodites in hermaphrodite- and male-conditioned medium. The averaged crawling locomotion velocities were plotted. (E) The percentage of animals paralyzed on 1.4 mM aldicarb at 90 min were plotted for hermaphrodites with AWB neurons optogenetically activated during the L4 stage. The channelrhodopsin variant CHIEF was expressed in AWB chemosensory neurons driven by the str-1 promoter. The blue light was turned on to excite AWBs in transgenetic animals fed with or without ATR. (F) Top: snapshots of GCaMP6f fluorescent signals of an AWB neuron before, during, and after addition of male excretome. Scale bar, 10 μm. Bottom: the calcium trace showing the AWB neuron activated by male excretome. (G) Curves and average intensities of Ca2+ signals evoked by the hermaphrodite or male excretome in the soma of AWB with GCaMP6f expression. The shaded box represents the addition of the hermaphrodite or male excretome. (H) Scatter diagram and quantification of the Ca2+ change. Each point represents Ca2+ peak value from one animal. (I) The percentages of animals paralyzed on 1.4 mM aldicarb at 80 min were plotted for tax-2(p691) mutant hermaphrodites and TAX-2 expression restored in AWB or ASJ/ASI neurons cultured in hermaphrodite- (black) and male-conditioned medium (orange). (J) The percentages of animals paralyzed on 1.4 mM aldicarb at 80 min were plotted for odr-3(n1605) mutant hermaphrodites and ODR-3 expression restored in AWB neurons cultured in hermaphrodite- (black) and male-conditioned medium (orange). In (C–E), (H–J), ***p<0.001, **p<0.01, ns not significant, two-way ANOVA with post hoc Sidak multiple comparisons for (C), (E), and (I–J), one-way ANOVA with post hoc Dunnett multiple comparisons for (D), unpaired Student’s t-test for (H).
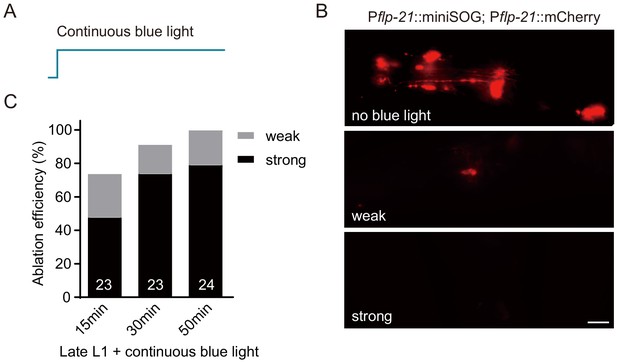
Genetic ablation efficiency by miniSOG.
(A) Schematic illustration of blue light stimulation pattern. (B) Representative images of multiple chemosensory neurons expressing miniSOG and mCherry (flp-21 promoter) before (no blue light) and after (weak and strong) blue light stimulation (scale bar, 5 μm). (C) Ablation efficiency was calculated after 15, 30, and 50 min of blue light stimulation. Weak and strong labels indicated ablation efficiencies by examining mCherry fluorescent signals in chemosensory neurons. The numbers of animals analyzed were indicated for each condition.
-
Figure 4—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig4-figsupp1-data1-v2.xlsx
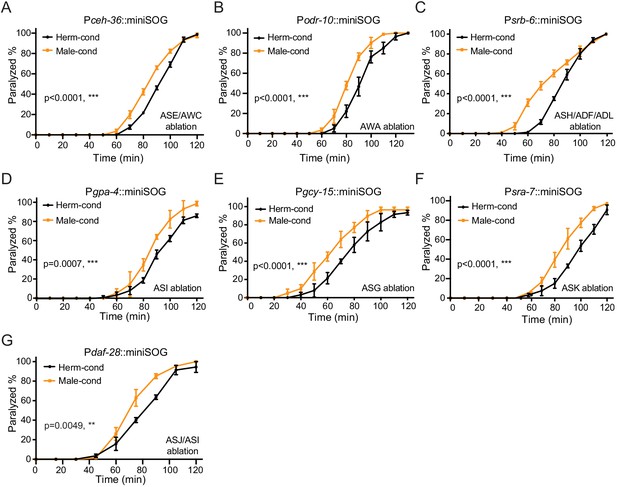
ASE, AWC, AWA, ASH, ASI, ADF, ASG, ASK, ADL, and ASJ chemosensory neurons are dispensable for sensing modulator ascarosides.
Time course analysis of aldicarb-induced paralysis in hermaphrodites with ablated ASE/AWC (ceh-36 promoter, A), AWA (odr-10 promoter, B), ASH/ADF/ADL (srb-6 promoter, C), ASI (gpa-4 promoter, D), ASG (gcy-15 promoter, E), ASK (sra-7 promoter, F), and ASJ/ASI (daf-28 promoter, G) grown in hermaphrodite-conditioned medium (black) or male-conditioned medium (orange). **p<0.01, ***p<0.001, two-way ANOVA comparing all of the time points.
-
Figure 4—figure supplement 2—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig4-figsupp2-data1-v2.xlsx
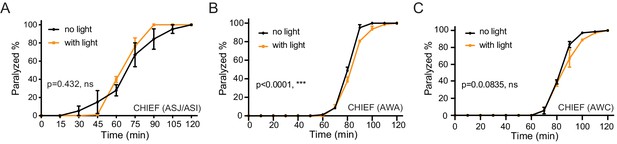
Activation of other sensory neurons does not change aldicarb sensitivity in hermaphrodites.
(A–C) Time course analysis of aldicarb-induced paralysis in hermaphrodites with ASJ/ASI (A), AWA (B), or AWC (C) optogenetically activated during the L3–L4 stage. The channelrhodopsin variant CHIEF was expressed in ASJ/ASI, AWA or AWC chemosensory neurons driven by the neuron-specific daf-28, odr-10, and str-2 promoters. The blue light was turned on (orange) or off (black) in the presence of ATR. ***p<0.001, ns not significant, two-way ANOVA comparing all of the time points.
-
Figure 4—figure supplement 3—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig4-figsupp3-data1-v2.xlsx
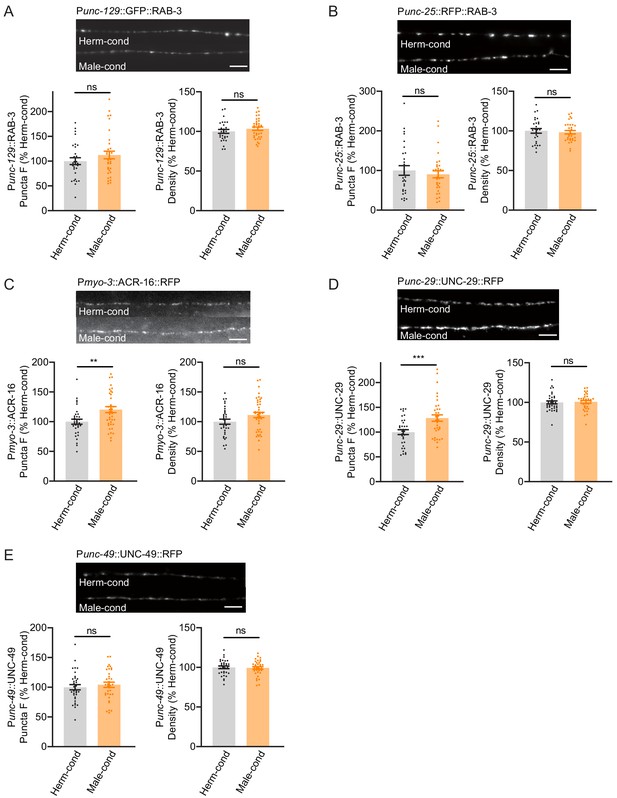
Modulator ascarosides promote postsynaptic AchR synaptic localization.
(A) The puncta fluorescence intensities and densities marked by the excitatory synaptic GFP::RAB-3 (under unc-129 promoter) in dorsal nerve cord axons were unaltered by modulator ascarosides. Representative images (top panel), mean puncta intensities and puncta density (bottom panel) are shown for hermaphrodites grown in hermaphrodite- or male-conditioned medium. (B) The puncta fluorescence intensities and densities marked by the inhibitory synaptic RFP::RAB-3 (under unc-25 promoter) were unaltered in hermaphrodites cultured in male-conditioned medium. Representative images (top), mean puncta intensities and puncta density (bottom) are shown. (C–E) The muscle-specific ACR-16::RFP, UNC-29::RFP, and UNC-49::RFP fluorescence intensities and densities in hermaphrodites cultured in hermaphrodite- and male-conditioned medium. Representative images, mean puncta intensities and puncta density are shown separately. Scale bars, 5 μm. **p<0.005, ***p<0.001, ns not significant, unpaired Student’s t-test.
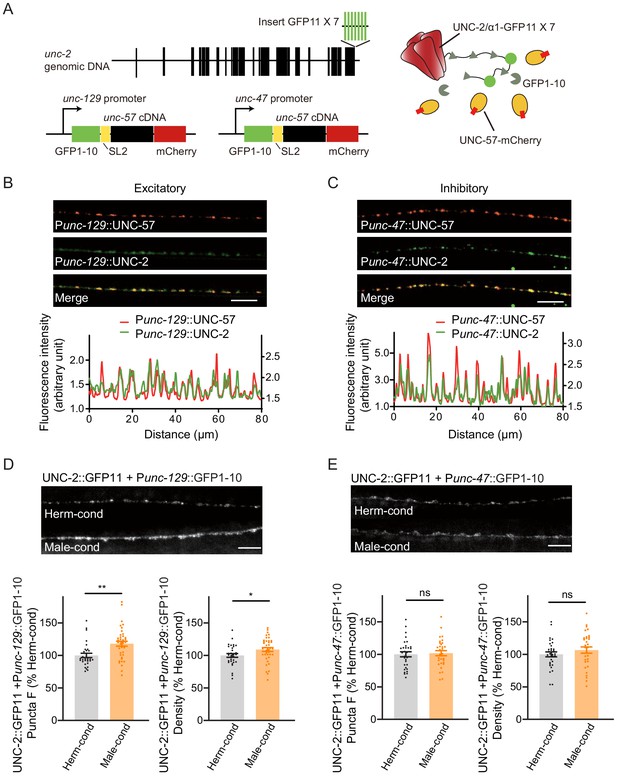
Modulator ascarosides increase the abundance of excitatory presynaptic CaV2 calcium channels at the NMJ.
(A) Schematic illustration of split GFP experimental design. Seven copies of the splitGFP 11 were inserted into the C-terminal of unc-2 genomic loci by CRISPR-Cas9 system. The splitGFP1-10 was expressed in B-type cholinergic and GABAergic motor neurons by unc-129 and unc-47 promoters. The unc-57-mCherry under the same promoter was separated with splitGFP1-10 by SL2 and was also used as a coexpressed presynaptic marker. (B, C) Presynaptic UNC-2::splitGFP (green) and UNC-57::mCherry (red) were co-localized in the dorsal nerve cord at both excitatory (B) and inhibitory (C) synapses. Representative images (top, scale bar, 10 μm) and linescan curves (bottom) are shown. For linescan curves, the mCherry signals were plotted on the left Y-axis, while the splitGFP signals were plotted on the right. one arbitrary fluorescence intensity unit equals 100 gray value. (D, E) The puncta fluorescence intensities and densities of UNC-2::splitGFP in B-type motor neurons (D) and GABAergic motor neurons (E) of hermaphrodites cultured in hermaphrodite- or male-conditioned medium. Representative images (scale bar, 5 μm), mean puncta intensities, and puncta densities are shown. In D and E, *p<0.05, **p<0.01, ns not significant, unpaired Student’s t-test.
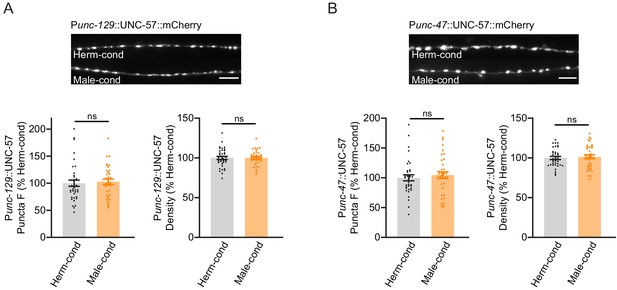
Excitatory and inhibitory synapse structures are not affected by the modulator ascarosides.
(A) The puncta fluorescence intensities and densities marked by the excitatory synaptic UNC-57::mCherry (under unc-129 promoter) in dorsal nerve cord axons were unaltered by modulator ascarosides. Representative images (top), mean puncta intensities and puncta density (bottom) are shown for hermaphrodites grown in hermaphrodite- or male-conditioned medium. (B) The puncta fluorescence intensities and densities marked by the inhibitory synaptic UNC-57::mCherry (under unc-47 promoter) were unaltered in hermaphrodites cultured in male-conditioned medium. Representative images (top), mean puncta intensities and puncta density (bottom) are shown. ns, not significant, unpaired Student’s t-test.
-
Figure 6—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/67170/elife-67170-fig6-figsupp1-data1-v2.xlsx
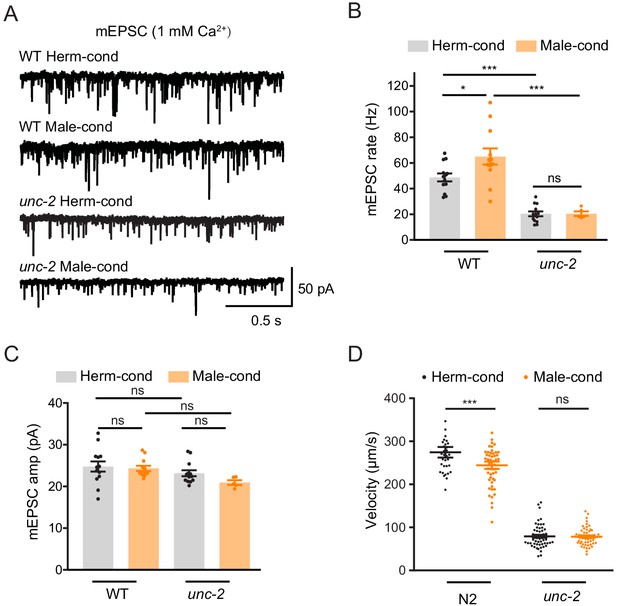
CaV2 calcium channel is the synaptic target of the modulator ascarosides.
(A–C) Endogenous acetylcholine transmission was assessed by recording mEPSCs from body muscles of wild-type N2 and unc-2 mutant adult hermaphrodites cultured in hermaphrodite- or male-conditioned medium. Representative mEPSC traces (A), the mean mEPSC rates (B), and the mean mEPSC amplitudes (C) are shown. The data for wild type (N2) is the same as in Figure 1H–J. (D) Locomotion behavior analysis of the single wild-type and unc-2 mutant hermaphrodite in hermaphrodite- and male-conditioned medium. The averaged and individual locomotion velocities were plotted. In (B)–(D), *p<0.05, ***p<0.001, ns not significant, one-way ANOVA with post hoc Dunnett multiple comparisons.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (C. elegans) | C. elegans strains used in this study are listed in Supplementary file 1 | |||
| Strain (Escherichia coli) | OP50 | CGC | RRID:WB-STRAIN:OP50 | |
| Sequence-based reagent | Sequence information is listed in Supplementary file 2 | |||
| Recombinant DNA reagent | pPD49.26 | Addgene (Andrew Fire) | https://www.addgene.org/1686/ | |
| Recombinant DNA reagent | pPD95.75 | Addgene (Andrew Fire) | https://www.addgene.org/1494/ | |
| Recombinant DNA reagent | pCFJ910 | Addgene (Erik Jorgensen) | https://www.addgene.org/44481/ | |
| Recombinant DNA reagent | CZ14527 | Yingchuan B. Qi Qi et al., 2012 | Plasmid: Punc-17β::tomm-20N::miniSOG | |
| Recombinant DNA reagent | quan0071 | Quan Wen | Xu, T et al., 2018 | Plasmid: Pacr-5::chrimson |
| Recombinant DNA reagent | pSG368 | Shangbang Gao | Gao, S et al., 2018 | Plasmid: GCaMP6f |
| Commercial assay or kit | PureLink HiPure Plasmid Miniprep Kit | Invitrogen | Cat#: K210002 | |
| Commercial assay or kit | QIAprep Spin Miniprep Kit | Qiagen | Cat#: 27106 | |
| Commercial assay or kit | PrimeSTAR Max DNA Polymerase | Takara | Cat#: R045A | |
| Commercial assay or kit | hyPerFUsion high-fidelity DNA polymerase | ApexBio | Cat#: K1032 | |
| Commercial assay or kit | Hieff CLoneTM Plus One Step Cloing Kit | Yeasen | Cat#: 10911ES62 | |
| Chemical compound, drug | Aldicarb | ApexBio | Cat#: B4778 | |
| Chemical compound, drug | All-trans-Retinal | Sigma | Cat#: R2500 | |
| Chemical compound, drug | Geneticin, G418 Sulfate | GOLDBIO | Cat#: G-418–1 | |
| Chemical compound, drug | 2,3-Butanedione monoxime | Sigma | Cat#: B0753 | |
| Chemical compound, drug | Polybead Microspheres0.10 μm | Polysciences | Cat#: 00876–15 | |
| Chemical compound, drug | Fluospheres carboxylat | Life Science | Cat#: F8813 | |
| Software, algorithm | ImageJ | NIH | https://imagej.nih.gov/ij/download.html | |
| Software, algorithm | Igor pro 6.3 | WaveMetrics | https://www.wavemetrics.com/products/igorpro/igorpro.htm | |
| Software, algorithm | GraphPad Prism 8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ | |
| Software, algorithm | MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html?s_tid=hp_products_matlab | |
| Software, algorithm | MetaMorph | Molecular Devices | https://www.moleculardevices.com/systems/metamorph-research-imaging/metamorph-microscopy-automation-and-image-analysis-software | |
| Software, algorithm | WormLab | MBF Bioscience | https://www.mbfbioscience.com/wormlab |
Additional files
-
Supplementary file 1
C. elegans strains used in this study.
- https://cdn.elifesciences.org/articles/67170/elife-67170-supp1-v2.docx
-
Supplementary file 2
Sequence information.
- https://cdn.elifesciences.org/articles/67170/elife-67170-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/67170/elife-67170-transrepform-v2.docx





