The cooperative binding of TDP-43 to GU-rich RNA repeats antagonizes TDP-43 aggregation
Figures
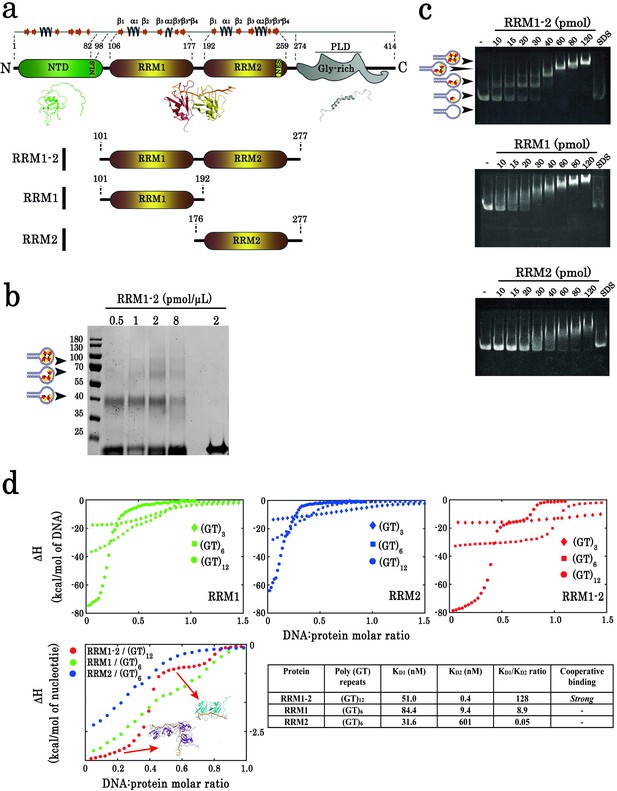
Biochemical characterization of TDP-43 fragments bound to oligonucleotide targets.
(a) Schematic representation of TDP-43 domains. Numbers indicate the boundaries according to the full-length protein sequence (NP_031401). The available 3D structure of N-terminal (PDB 2N4P), RRMs (PDB 4BS2), and C-terminal (PDB 2N3X) are also shown together with β-strands and α-helices. The boundaries of all three recombinant RRM fragments (RRM1–2, RRM1, and RRM2) used in this study are indicated. (b) Cross-linking experiments using increasing RRM1–2 protein concentrations, (GT)24-loop (10 pmol) and BS3 as cross-linking reagent. Cross-linked proteins are indicated with head-arrows. Last lane corresponds to the experiment in absence of BS3. (c) Electrophoretic Mobility-Shift Assay (EMSA) experiments were performed by using increasing protein concentrations and 10 pmol of a stem-loop DNA containing a (GT)24 repeats ((GT)24-loop). When indicated, the sample was treated with SDS in order to disassemble DNA-protein complexes. Free or protein-containing (GT)24-loop are indicated by head-arrows. (d) Binding of TDP-43 fragments to (GT)-rich oligonucleotides containing three (diamonds), six (squares) or twelve (circles) GT-repeats was monitored by ITC. At the bottom, plot of ITC titration curves for oligonucleotides that can bind two protein monomers (RRM1/(GT)6, RRM2/(GT)6 and RRM1–2/(GT)12). Plateaus corresponding to dimer and monomer states are indicated (red, green and blue curves correspond to RRM1–2, RRM1, and RRM2, respectively). Lower right panel: Kd1/Kd2 ratios obtained from (GT)12 or (GT)6 titration data. The binding of RRM1–2 to GT repeats is highly cooperative which is not observed for isolated RRM1 and RRM2. Thermodynamic parameters and ITC statistics are shown in Supplementary file 3. Raw thermograms are shown in Figure 1—figure supplement 2.
-
Figure 1—source data 1
SDS-polyacrylamide gel electrophoresis of samples from cross-linking experiments performed in absence of benzonase (see legend of Figure 1b).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-data1-v3.zip
-
Figure 1—source data 2
Electrophoretic Mobility-Shift Assay (EMSA) experiments on RRM1–2 (see legend of Figure 1c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-data2-v3.zip
-
Figure 1—source data 3
EMSA experiments on RRM1 (see legend of Figure 1c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-data3-v3.zip
-
Figure 1—source data 4
EMSA experiments on RRM2 (see legend of Figure 1c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-data4-v3.zip
-
Figure 1—source data 5
ITC data obtained from the binding of TDP-43 fragments to (GT)-rich oligonucleotides (See legend Figure 1d).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-data5-v3.xlsx
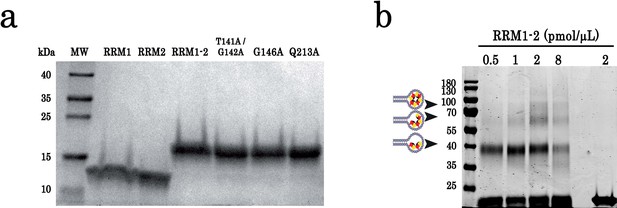
SDS-polyacrylamide gel electrophoresis of purified proteins and cross-linking experiments.
(a) SDS-polyacrylamide gel electrophoresis of purified TDP-43 protein fragments (RRM1, RRM2, and RRM1–2) and RRM1–2 mutants (T141/G142, G146A, and Q213A). Molecular mass standards are indicated on the left. (b) Cross-linking experiments using increasing protein concentrations, (GT)24-loop (10 pmol) and BS3 as cross-linking reagent. All samples were treated with benzonase as global control of crosslinking experiments showed in Figure 1. DNase treatment was performed at room temperature during 30 min. Cross-linked proteins are indicated with head-arrows. Different controls were also performed.
-
Figure 1—figure supplement 1—source data 1
SDS-polyacrylamide gel electrophoresis of purified proteins (See legend of Figure 1—figure supplement 1a).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-figsupp1-data1-v3.zip
-
Figure 1—figure supplement 1—source data 2
SDS-polyacrylamide gel electrophoresis of samples from cross-linking experiments performed in presence of benzonase (see legend of Figure 1—figure supplement 1b).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-figsupp1-data2-v3.zip
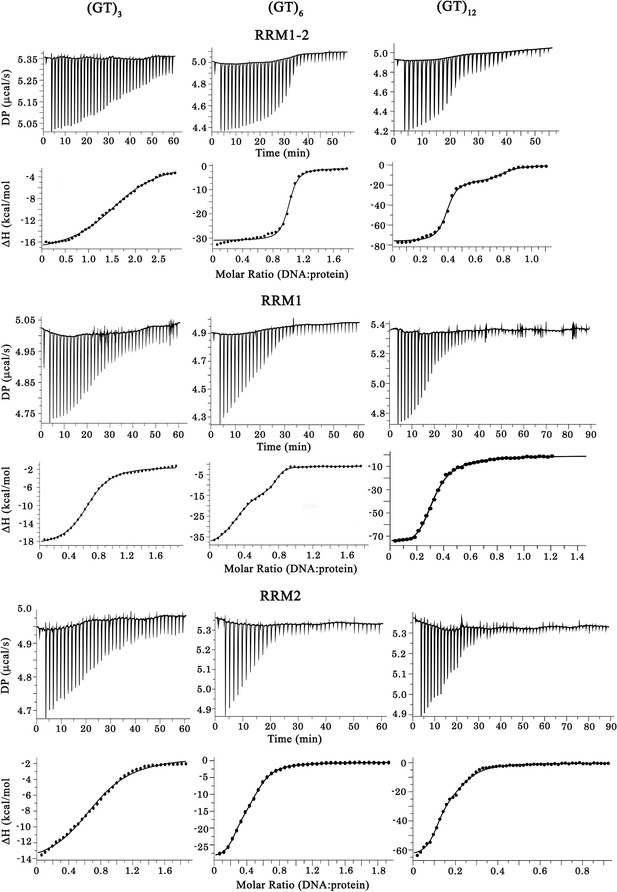
Raw calorigrams corresponding to the ITC curves displayed in Figure 1d.
-
Figure 1—figure supplement 2—source data 1
ITC data obtained from the binding of TDP-43 fragments to (GT)-rich oligonucleotides (See legend of Figure 1—figure supplement 2).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig1-figsupp2-data1-v3.xlsx
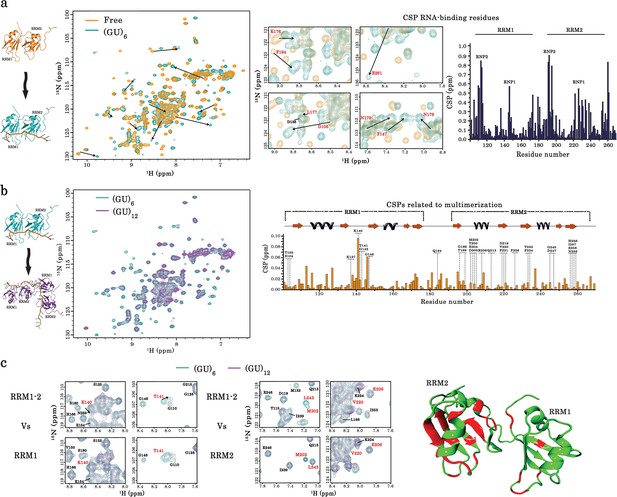
Identification of TDP-43 residues involved in its dimerization on GU-repeats.
(a) NMR spectra of free and bound RRM1–2. Left, superimposition of 1H-15N SOFAST-HMQC spectra of 15N-labeled RRM1–2 in the free (orange) and (GU)6 RNA-bound (turquoise) forms. Residues displaying the largest chemical shift perturbations (CSP) are indicated by arrows. Middle, zoom in on NMR spectra (left) showing the CSPs for some residues (highlighted in red) upon (GU)6 RNA binding. Right, plot of CSPs occurring in RRM1–2 upon (GU)6 RNA binding. The combined CSPs were calculated as reported (Williamson, 2013) and follow the same trajectories as previously published (Lukavsky et al., 2013) for RRM1–2 bound to AUG12 (PDB 4BS2). (b) NMR spectra of monomeric and dimeric forms of RRM1–2 bound to GU-repeats. Left, superimposition of 1H-15N SOFAST-HMQC spectra of 15N-labeled RRM1–2 bound to (GU)6 (turquoise) or (GU)12 (magenta). Right, combined CSPs, observed for monomeric and dimeric couples, plotted and linked to the secondary structures on top. (c) Left, zoom in on NMR spectra (b) showing RRM1–2 residues displaying particular CSPs, resonance disappearing, or peak broadening (in red) as compared to respective residues in RRM1 or RRM2 fragments. Right, all affected residues upon RRM1–2 dimerization are highlighted in red using molecular modelling approaches on RRM1–2 free fragment (see methods). Based on the above comparative NMR study, 28 residues were selected as candidates for mutagenesis approach combined to a detailed cellular and biochemical investigation.
-
Figure 2—source data 1
NMR data of RRM1-2 in free and bound forms (See legend of Figure 2a,b).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig2-data1-v3.xlsx
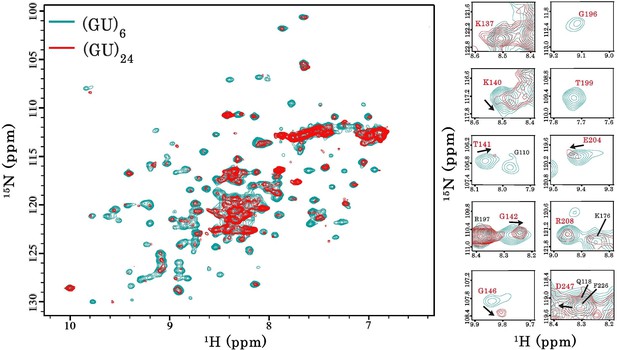
Interaction of RRM1–2 protein fragment with 24 GU-repeats.
Left, superimposition of 1H-15N SOFAST-HMQC spectra of 15N-labeled RRM1–2 bound to (GU)6 (turquoise) or bound to (GU)24 (red). Right, zoom in on NMR spectra showing RRM1–2 residues (highlighted in red) displaying particular CSPs, resonance disappearing or peak broadening upon protein multimerization.
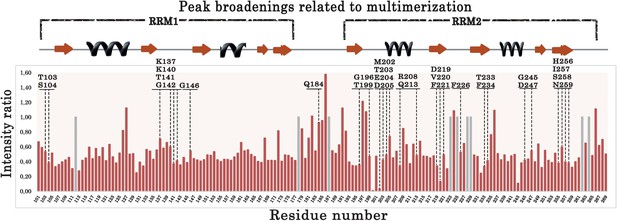
Plot showing the intensity ratios calculated between the 15N-labeled RRM1–2 bound to (GU)6 or (GU)12 RNA.
Gray bars correspond to non-assigned residues for which a value equal to 1.0 was arbitrary assigned. Residues located in the long RRM1 loop 3 (a.a.137–146) display low intensities ratios compared to residues located in unstructured domain such as the linker between RRM1 and RRM2 (177–191). In addition, residues around V220 and M202 also display a decreased intensity. Residues selected for the ‘microtubule bench’ assays are indicated by dashed lines.
-
Figure 2—figure supplement 2—source data 1
NMR data displaying Peak Intensity of RRM1-2 residues in presence of (GU)6 or (GU)12 (See legend of Figure 2—figure supplement 2).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig2-figsupp2-data1-v3.xlsx
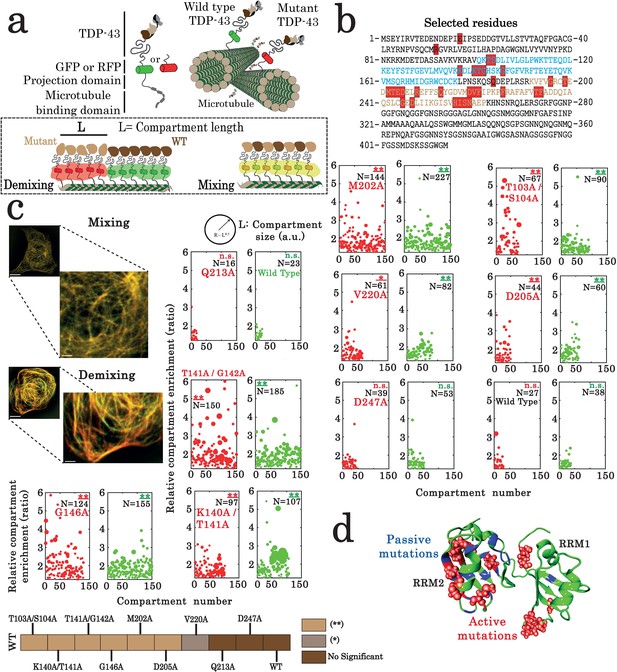
Assessment of interactions between wild-type and mutant TDP-43 by using the microtubule bench assay (Maucuer et al., 2018).
(a) Scheme representing the method used to probe homotypic interactions between wild-type and mutant forms of full-length TDP-43. To track their subcellular protein localization, wild-type and mutant TDP-43 were labeled with the Green Fluorescent Protein (GFP) and the Red Fluorescent Protein (RFP), respectively (b) Amino acid sequence of full-length TDP-43 showing RRM1 (blue) and RRM2 (brown) motifs. Amino acids subjected to mutagenesis are highlighted by red boxes. (c) HeLa cells co-expressing GFP- and RFP-labeled TDP-43 in order to assess mixing/demixing on microtubules. Relative enrichment of both wild-type and mutated TDP-43 compartments is expressed as a function of compartment number according to the procedure described in the Materials and methods section. Several mutations such as G146A display an elevated enrichment and many more compartments than when two wild-type TDP-43 are interacting. Scale bar: 10 µm. p<0.05*; p<0.01** (paired two-sample Kolmogorov–Smirnov test compared to control). (n.s.) non-significant. (N), number of compartments. (d) TDP-43 mutants displaying mixing or demixing are referred as ‘passive mutations’ or ‘active mutations’, respectively. Demixing denotes a perturbation in interactions between wild-type and mutant of TDP-43. The amino acid residues corresponding to the ‘passive mutations’ or ‘active mutations’ are shown in blue or red, respectively.
-
Figure 3—source data 1
Assessment of interactions between wild-type and mutant full-length TDP-43 by using the microtubule bench assay (See of legend Figure 3c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig3-data1-v3.xlsx
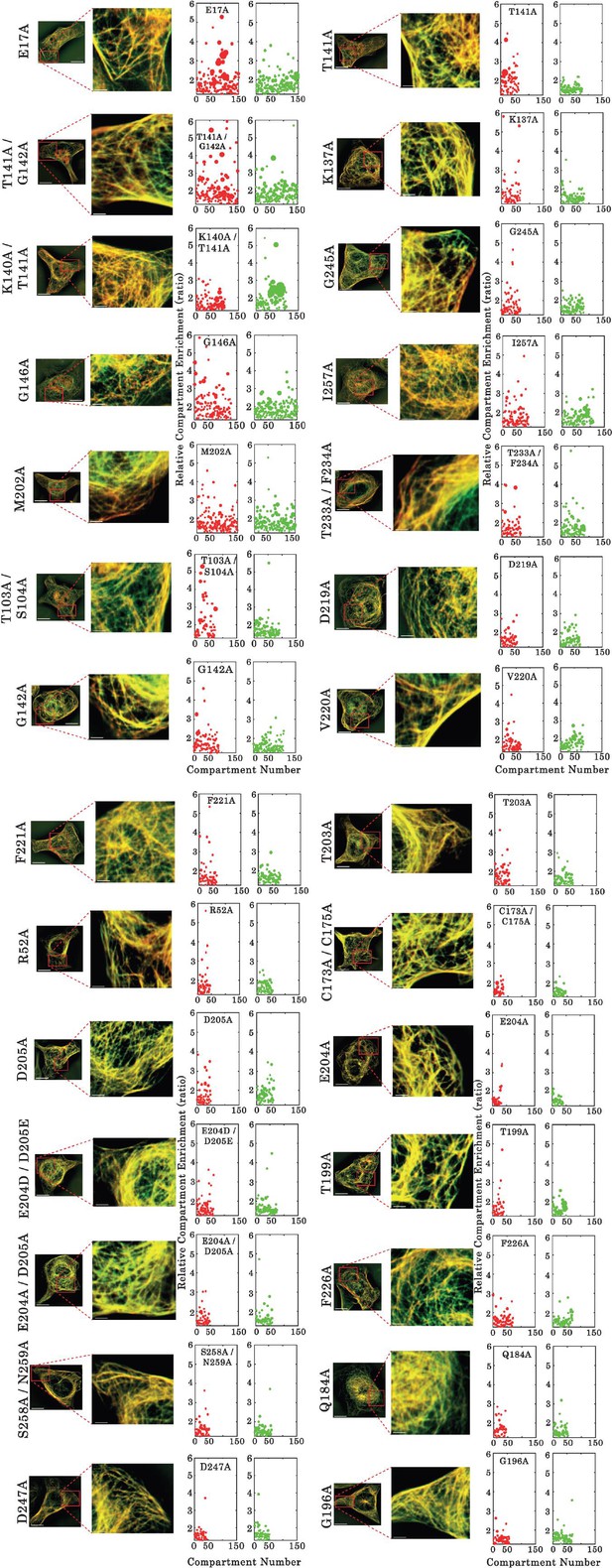
Assessment of interactions between wild type and mutants of full-length TDP-43 by using the microtubule bench assay (Maucuer et al., 2018).
ImageJ-treated HeLa cells co-expressing GFP- and RFP-fused TDP-43 in order to assess mixing/demixing on microtubules. Relative enrichment of both wild -type and mutated TDP-43 (fused to GFP and RFP, respectively) compartments is expressed as a function of the compartment number according to the described procedure in Materials and methods section. Scale bar: 10 µm.
-
Figure 3—figure supplement 1—source data 1
Assessment of interactions between wild type and mutants of full-length TDP-43 by using the microtubule bench assay (See legend of Figure 3—figure supplement 1).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig3-figsupp1-data1-v3.xlsx
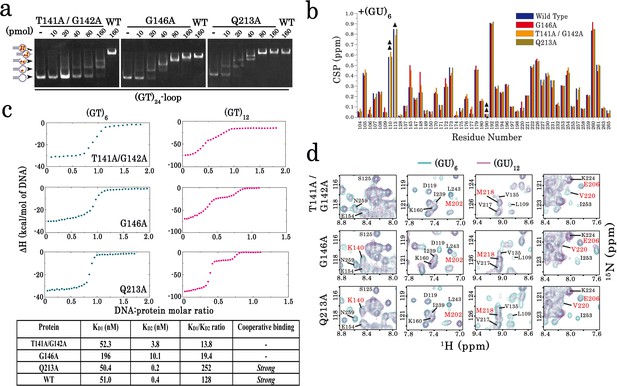
Characterization of TDP-43 mutants with an impaired cooperative binding to RNA.
(a) EMSA experiments were performed by using increasing protein concentrations of T141A/G142A, G146A, and Q213A mutants and 10 pmol of a stem-loop DNA to assess multimerization changes on RRM1–2 (as in Figure 1c). Saturated amounts of wild-type RRM1–2 were used as a control (last lane). DNA-protein complexes are pointed out with head-arrows. (b) Plot showing CSPs for RNA-binding residues along RRM1–2 mutants bound to (GU)6 compared to their free forms. In most cases, the binding to (GU)6 RNA provokes CSPs comparable to the wild-type RRM1–2. ΔΔ, ambiguous assignment; Δ, signal vanishing. (c) The binding of RRM1–2 mutants to (GT)6 or (GT)12 oligonucleotides, was monitored by ITC. Whereas the ITC curves of wild type and Q213A are similar (see Figure 1d), the ITC curves related to T141A/G142A and G146A mutants decrease more continuously with less marked plateaus, reflecting an impaired cooperative binding to GT repeats. Lower panel: Kd1/Kd2 ratios obtained from ITC data. T141A/G142A and G146A have lower Kd1/Kd2 ratio values than wild type and Q213A, reflecting an impaired cooperativity. ITC statistics with thermodynamic parameters are indicated in Supplementary file 4. (d) Zoom in on the superimposed 1H-15N SOFAST-HMQC spectra (see full NMR spectra in Figure 4—figure supplement 2) of 15N-labeled RRM1–2 mutants bound to (GU)6 (turquoise) or to (GU)12 (magenta). The residues affected during wild-type RRM1–2 dimerization (see Figure 2b,c) are highlighted (red). Q213A mutant shows the same CSPs as the wild-type protein. However, in the case of T141A/G142A and G146A, we no longer detected the CSPs associated to dimerization.
-
Figure 4—source data 1
EMSA experiments on T141A/G142A mutant (see legend of Figure 4a).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-data1-v3.zip
-
Figure 4—source data 2
EMSA experiments on G146A mutant (see legend of Figure 4a).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-data2-v3.zip
-
Figure 4—source data 3
EMSA experiments on Q213A mutant (see legend of Figure 4a).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-data3-v3.zip
-
Figure 4—source data 4
NMR data for RNA-binding residues along RRM1–2 mutants bound to (GU)6 compared to their free forms (see legend of Figure 4b).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-data4-v3.xlsx
-
Figure 4—source data 5
ITC data obtained from the binding of RRM1–2 mutants to (GT)6 or (GT)12 oligonucleotides (see legend of Figure 4c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-data5-v3.xlsx
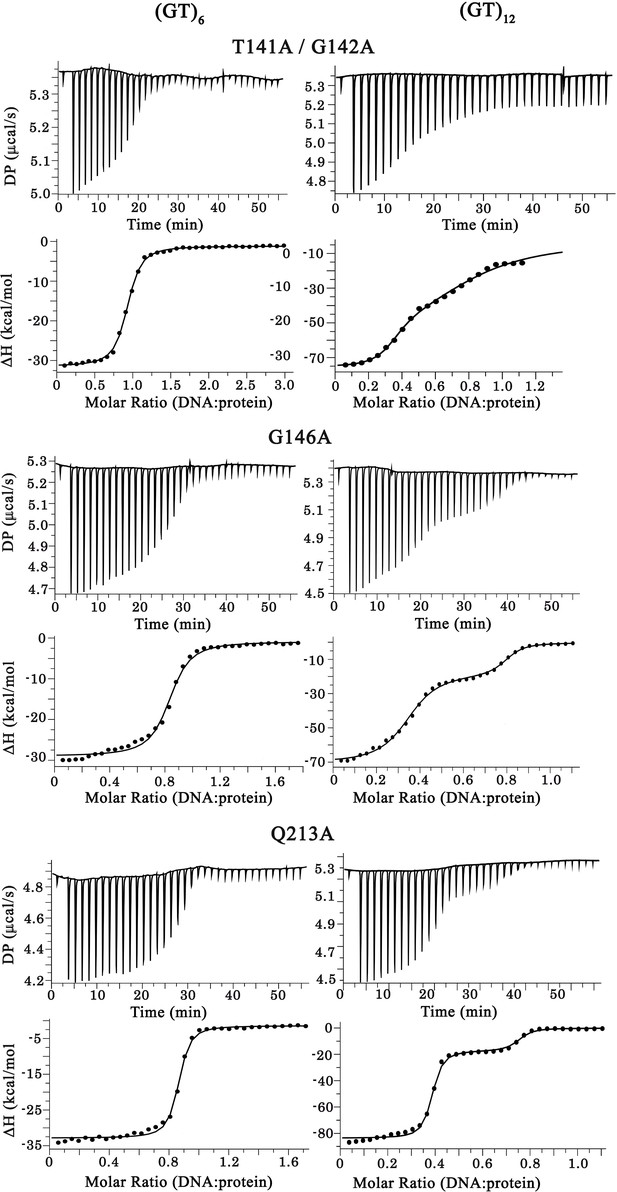
Raw calorigrams corresponding to the ITC curves displayed in Figure 4c.
-
Figure 4—figure supplement 1—source data 1
ITC data obtained from the binding of RRM1–2 mutants to (GT)6 or (GT)12 oligonucleotides (see legend of Figure 4—figure supplement 1).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-figsupp1-data1-v3.xlsx
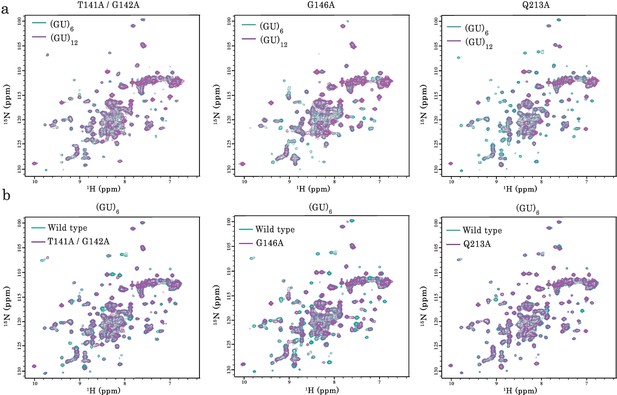
NMR analysis of the interaction of TDP-43 mutant fragments with GU-repeats.
(a) NMR spectra of monomeric and dimeric forms of RRM1–2 mutants (T141/G142, G146A, and Q213A) bound to GU-repeats. In all cases, the superimposition of 1H-15N SOFAST-HMQC spectra of 15N-labeled mutant bound to (GU)6 (turquoise) or (GU)12 (magenta) is shown. (b) Superimposition of 1H-15N SOFAST-HMQC spectra of 15N-labeled wild-type RRM1–2 (turquoise) and mutants (magenta) both bound to (GU)6.
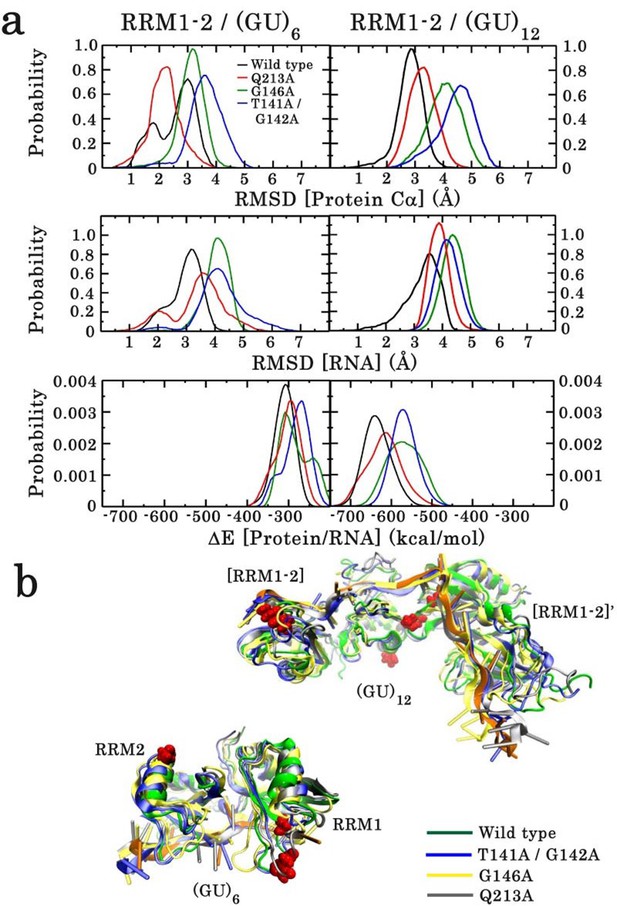
Effect of point mutations on the stability of the monomeric and dimeric structures of wild type and mutants of RRM1–2 fragment.
(a) In each case, the RMSD distribution of the protein Cα atoms (top panel) and RNA (middle panel) is shown. The lower panel illustrates the energy distribution of RNA/protein interactions. (b) Overlay of wild-type and mutant 3D structures sampled from the 100 ns MD simulation trajectories. The upper overlay corresponds to the dimeric form and the lower to the monomeric form, bound to (GU)12 and (GU)6, respectively.
-
Figure 4—figure supplement 3—source data 1
Effect of point mutations on the stability of the monomeric and dimeric structures of wild type and mutants of RRM1–2 fragment (see legend of Figure 4—figure supplement 3).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig4-figsupp3-data1-v3.xlsx
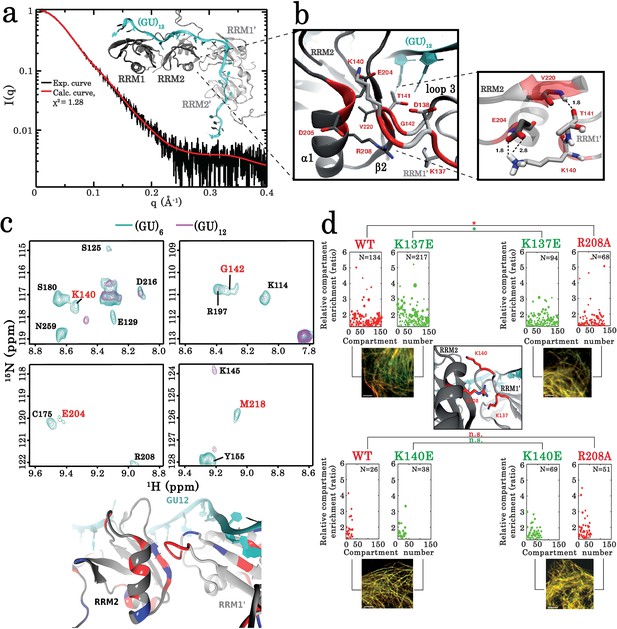
Key residues governing the RRM-dependent TDP-43 multimerization on RNA targets.
(a) Superimposition of calculated (red curve) and experimental (black dots) SAXS curves corresponding to RRM1–2 bound to (GU)12. SAXS curves were calculated from all-atoms model using the program GAJOE from the suite EOM. The corresponding χ2 values are indicated. The inset is a 3D representation of the model built using MD simulations from which the conformational state at equilibrium was considered. (b) Zoom in on the 3D model corresponding to the RRM1–2 bound to (GU)12 showing the protein-protein interface created by the interaction of residues located in the α -helix α1 and β-strand β2 belonging to the RRM2 (first monomer) with residues located in the RRM1 loop 3 (second monomer). Interacting couples are highlighted in red and interaction bonds are shown by dotted lines. Numbers in black reflect the distance (Å). (c) Upper panel shows a zoom in on the superimposed 1H-15N SEA-HSQC spectra (see full NMR spectra in Figure 5—figure supplement 5) of 15N-labeled RRM1–2 bound to (GU)6 (turquoise) or to (GU)12 (magenta). The residues present at RRM1–2 dimerization interface (highlighted in red) are no longer exposed to the solvent. Lower panel shows the global changes derived from SEA-experiment in solvent-exposed amides located at the protein-protein interface which are mapped on the 3D structure obtained from MD simulations (blue: exposed, red: not exposed). (d) As in Figure 3c, the microtubule bench assay was used to quantify the compartmentalization of different forms of TDP-43 co-expressed in HeLa cells. Center panel: view on the close proximity between R208 in RRM2 (first monomer) and K137 in RRM1 (second monomer). Upper panel shows a demixing phenotype between wild-type and K137E TDP-43. In contrast, R208 better mixes with K137E than wild-type TDP-43. Bottom panel, as a control, this behavior is not observed in the case of K140E. Scale bar: 10 µm. p<0.05*; p<0.01** (paired two-sample Kolmogorov–Smirnov test). n.s. non-significant. N, number of compartments.
-
Figure 5—source data 1
Small-angle X-ray scattering (SAXS) data of RRM1–2 bound to (GU)12 (see legend of Figure 5a).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-data1-v3.xlsx
-
Figure 5—source data 2
The microtubule bench assay was used to quantify the compartmentalization of different forms of TDP-43 co-expressed in HeLa cells (See legend of Figure 5d).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-data2-v3.xlsx
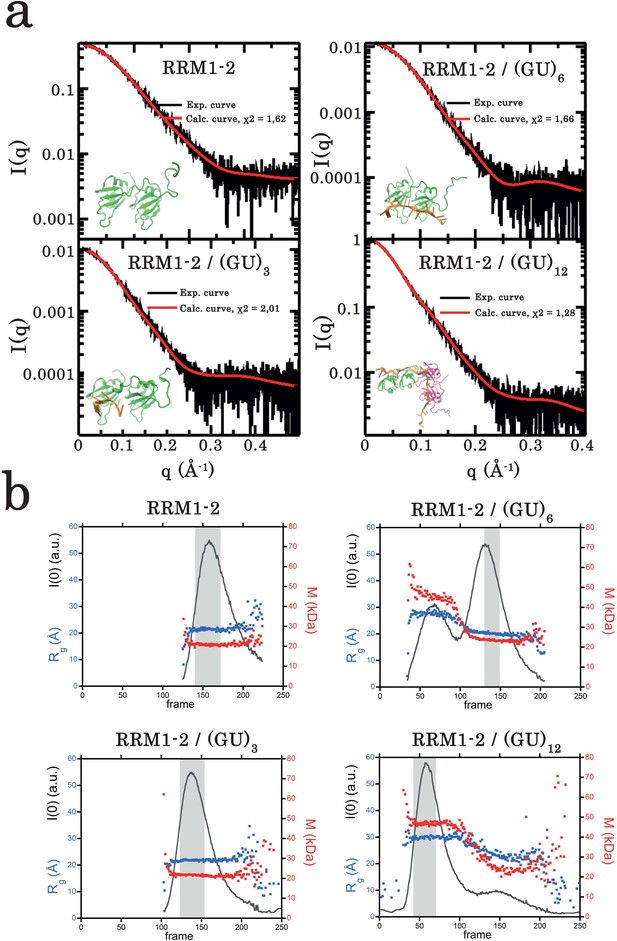
Small-angle X-ray scattering (SAXS) analysis of TDP-43 RRM1–2 fragment alone or bound to GU-repeats.
(a) Superimposition of calculated (red curve) and experimental (black dots) SAXS curves for the free RRM1–2 (top left panel), RRM1–2 bound to (GU)3 (bottom left panel), to (GU)6 (top right panel), and to (GU)12 (bottom right panel). SAXS curves were calculated from all-atoms models using the program GAJOE from the suite EOM. The corresponding χ2 values are indicated. The insets are 3D representations of these models built using MD simulations (see Materials and Methods). (b) Plots illustrate, for each protein complex, the evolution of the gyration radius Rg (blue dots) during SEC-SAXS elution, the molar mass (red dots) and the forward scattered intensity I(0) (black curve). The dark gray rectangle corresponds to the zone of elution where the successive SAXS curves are rigorously identical. The average of these identical curves was used for analysis.
-
Figure 5—figure supplement 1—source data 1
SAXS analysis of TDP-43 RRM1–2 fragment alone or bound to GU-repeats (See legend of Figure 5—figure supplement 1a).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-figsupp1-data1-v3.xlsx
-
Figure 5—figure supplement 1—source data 2
SAXS analysis of TDP-43 RRM1–2 fragment alone or bound to GU-repeats (See legend of Figure 5—figure supplement 1b).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-figsupp1-data2-v3.xlsx
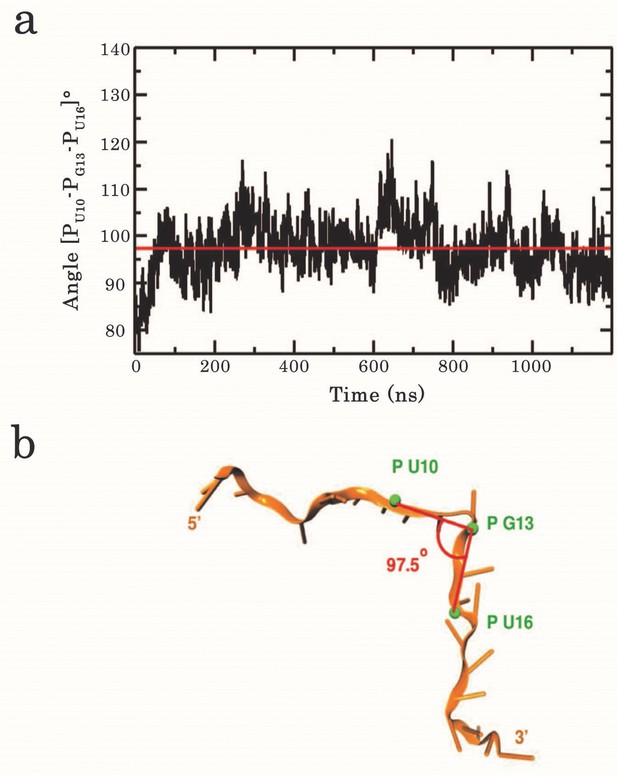
Analysis of the kink angle stability.
(a) Time series of the RNA backbone kink angle imposed at G13 by the dimeric assembly of RRM1–2 on (GU)12. The angle was calculated between the phosphate P atoms of U10, G13, and U16. The red line indicates the average angle value of 97.5°. (b) Position of the phosphates used to calculate the kink angle around G13.
-
Figure 5—figure supplement 2—source data 1
Analysis of the kink angle stability (See legend of Figure 5—figure supplement 2).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-figsupp2-data1-v3.xlsx
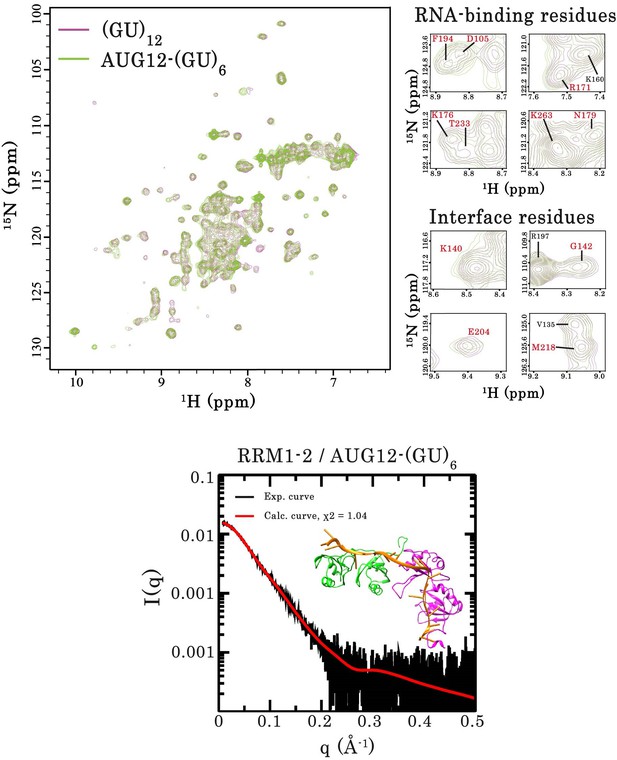
Interaction of RRM1–2 protein fragment with two different GU-rich oligonucleotides.
Top left panel, superimposition of 1H-15N SOFAST-HMQC spectra of 15N-labeled RRM1–2 bound to (GU)12 (magenta) or bound to 5′-GUGUGAAUGAAUGUGUGUGUGUGU-3’ (AUG12-(GU)6) (green). Top right panel, zoom in on NMR spectra showing unchanged resonances for representative RNA-binding residues as well as residues involved in the protein dimerization (highlighted in red). Bottom panel, Superimposition of calculated (red curve) and experimental (black dots) SAXS curves for the RRM1–2 bound to AUG12-(GU)6. SAXS curves were calculated from all-atoms models using the program GAJOE from the suite EOM. The corresponding χ2 values are indicated. The inset is a 3D representation of the model built using MD simulations.
-
Figure 5—figure supplement 3—source data 1
SAXS analysis of TDP-43 RRM1–2 fragment bound to AUG12-(GU)6 oligonucleotide (See legend of Figure 5—figure supplement 3).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-figsupp3-data1-v3.xlsx
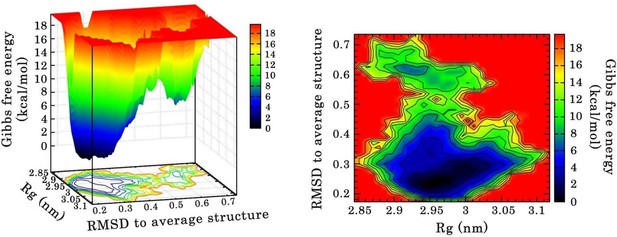
Free energy landscape (FEL) of RRM1–2 dimer in complex with (GU)12 sampled from 110 ns of MD simulation.
FEL is represented using two structural reaction coordinates: the radius of gyration of the system (Rg) and the root mean square deviation (RMSD) with respect to the average structure. The zero energy is at 0 kcal/mol and corresponds to the most stable conformational states. The free energy scale highlights energy differences (0–19 kcal/mol) relative to the global minimum. Radius of gyration and RMSD values are reported in nm. (a) The 3D representation shows ‘valleys’ of low-free energy corresponding to the metastable conformational states of the system, and ‘hills’ that account for the energetic barriers connecting these states. (b) The 2D representation shows the “contour plot” projecting the free-energy surface.
-
Figure 5—figure supplement 4—source data 1
Ø Free energy landscape (FEL) of RRM1–2 dimer in complex with (GU)12 (See legend of Figure 5—figure supplement 4).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig5-figsupp4-data1-v3.xlsx
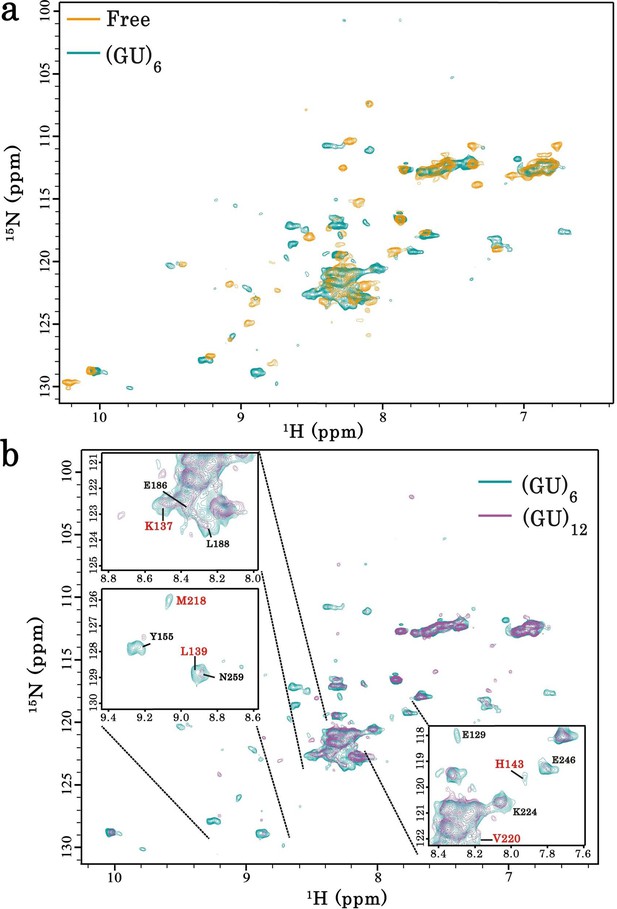
Interaction of RRM1–2 protein fragment with GU-repeats.
(a) Superimposition of 1H-15N SEA-HSQC spectra of 15N-labeled RRM1–2 alone (orange) or bound to (GU)6 (turquoise). (b) Superimposition of 1H-15N SEA-HSQC spectra of 15N-labeled RRM1–2 bound to (GU)6 (turquoise) or to (GU)12 (magenta). Zoom in on the superimposed 1H-15N SEA-HSQC spectra of 15N-labeled RRM1–2 bound to (GU)6 (turquoise) or to (GU)12 (magenta). The residues present at RRM1–2 dimerization interface (highlighted in red) are no longer exposed to the solvent.
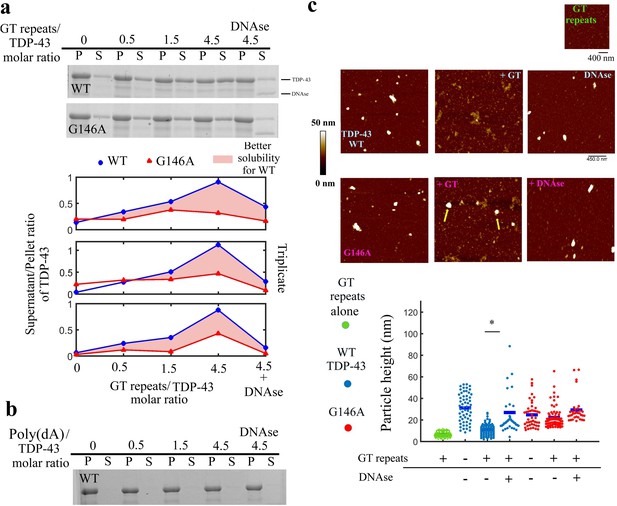
GT-repeats increase the solubility of full-length wild-type TDP-43 but to a lesser extent the solubility of G146A mutant.
(a) Higher panel: Sedimentation assays of full length recombinant TDP-43 and the G146A mutant in the absence or presence of 24 GT repeats. Recombinant proteins were diluted in 20 mM Tris–HCl, pH 7.4 containing 25 mM KCl, 0.5 mM DTT, and 2 mM MgCl2 (Buffer B) to a final concentration of 10 μM and incubated in the presence or absence of ssDNA for 5 min. The supernatant and pellet content after centrifugation were analyzed on SDS-PAGE and gels were stained with Coomassie blue. When indicated, the DNase treatment was performed for 5 min after a 5 min preincubation of TDP-43 with GT-repeats. Lower panel: Analysis of the ratio of wild-type and mutant TDP-43 found in the supernatant and in the pellet. Quantification was performed using an Amersham Typhoon Imagers. Three independent experiments were performed (gels are shown in Figure 6—figure supplement 1). (b) Same as (a) with 48 nt-long Poly(dA) DNA and wild-type TDP-43. Poly(dA) DNA failed to increase TDP-43 solubility. (c) Higher panel: AFM images of the higher order assembly of full length wild-type or mutant TDP-43. Before their deposition onto a mica surface, indicated proteins (2 μM) were incubated for 5 min in the buffer B with or without 24 GT repeats (10 μM) (and DNase when indicated). Arrows show the presence of spherical aggregates when G146A mutant was incubated with GT repeats. Lower panel: Quantification of the particle heights under the indicated conditions was performed with Bruker Nanoscope analysis software. p<0.05*; n.s. non-significant (paired t-test).
-
Figure 6—source data 1
GT-repeats increase the solubility of full-length wild-type TDP-43 but to a lesser extent the solubility of G146A mutant (See legend of Figure 6).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig6-data1-v3.xlsx
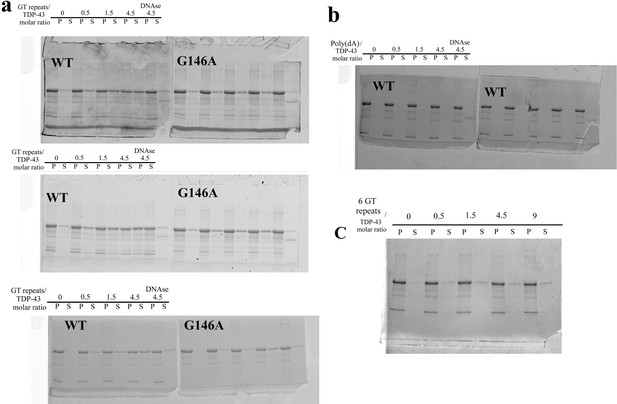
SDS-PAGE gels used for the quantification of fractions of full-length TDP-43 and G146A proteins found in the pellet and in the supernatant in sedimentation assays.
(a) Sedimentation assays of wild-type TDP-43 and G146A mutant in the presence of 24 GT repeats (triplicate). The three gels have been used for the quantification shown in Figure 6c (see the caption of Figure 6 for the experimental conditions). (b) Two independent experiment showing that poly-(dA) oligonucleotides are unable to improve the solubilization of TDP-43 (one of the two gels is shown in Figure 6a). (c) Representative experiment showing that 6 GT repeats do not increase the solubilization of TDP-43 as significantly as 24 GT repeats (a).
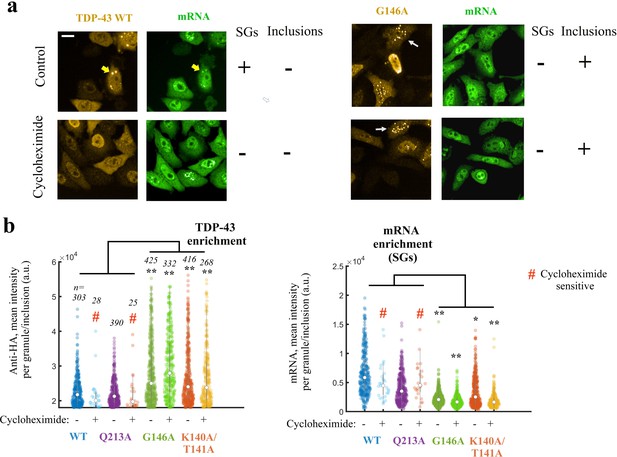
Expressing TDP-43 mutants with an altered cooperative binding mRNA in HeLa cells leads to the formation of mRNA-poor TDP-43 condensates in small fraction of cells.
(a) Subcellular distribution of wild-type TDP-43 or G146A mutant. Cycloheximide treatment was used to dissociate stress granules in the cell cytoplasm, when indicated in the figure. Wild-type TDP-43 is generally homogenously distributed in the cytoplasm but can be also found in cytoplasmic mRNA-rich stress granules (yellow head-arrows). G146A mutant is also generally homogenously distributed in the cytoplasm but can be found in brilliant condensates in the cytoplasm and G146A condensates do not colocalize with mRNAs (white head-arrows). Scale bar: 40 µm. Representative images of larger areas are shown in Figure 7—figure supplement 1b. (b) Violinplots representing TDP-43 (anti-HA) and mRNA (in situ hybridization with poly(dT) probes) fluorescence intensity in the cytoplasmic granules/aggregates detected under indicated conditions. Wild-type TDP-43 or Q213A mutant, a negative control, can be recruited in mRNA-rich stress granules that disappeared after cycloheximide treatment. On the other hand, K140A/T141A and G146A mutants are located in dense cytoplasmic condensates poorly enriched in mRNAs. Interestingly, K140A/T141A and G146A are not sensitive to cycloheximide. Cytoplasmic granules/aggregates were detected automatically by using Cell Profiler. n: number of granules/aggregates detected. p<0.05*; p<0.01** (paired two-sample t-test).
-
Figure 7—source data 1
Expressing TDP-43 mutants with an altered cooperative binding to mRNA in HeLa cells leads to the formation of mRNA-poor TDP-43 condensates in small fraction of cells (See legend of Figure 7b).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig7-data1-v3.xlsx
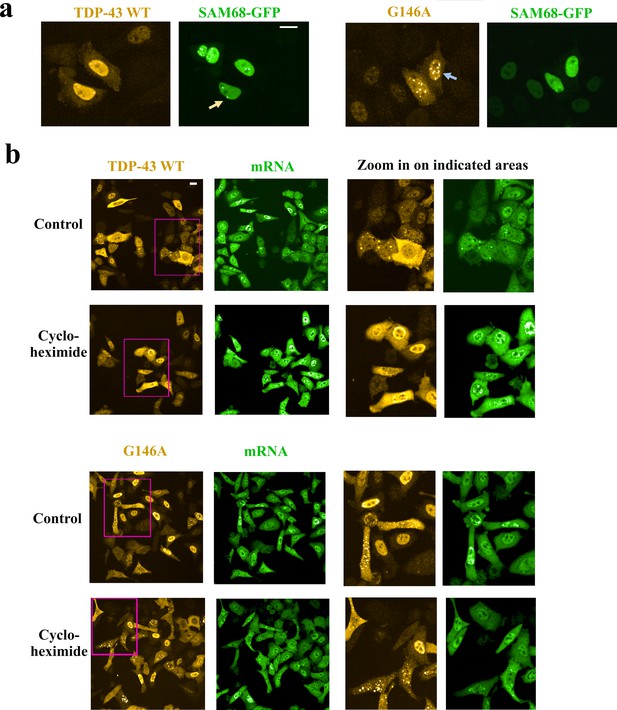
Cytoplasmic and nuclear G146A condensates are not stress granules.
(a) Hela cells were co-transfected with indicated HA-tagged TDP-43 (G146A or wild type) and Sam68-GFP. Cells were then treated with hydrogen peroxide to promote the appearance of nuclear G146A condensates (blue arrow). We noticed the presence of Sam68 nuclear bodies (orange arrow). However, Sam68 is not recruited in G146A condensates in the nucleus. (b) Left-panel: Large scale images of wild-type TDP-43 and G146A expressed in HeLa cells. Right panel: zoom in on indicated areas (pink rectangles). Most of the cells display a homogenous distribution of wild-type TDP-43 or G146A in the cytoplasm and nucleus. However, in some cells, we can notice the recruitment of TDP-43 in mRNA-rich stress granules while G146A is rather associated to smaller and brighter condensates that are not enriched in mRNA. Cycloheximide makes TDP43-rich stress granules disappeared but not G146A condensates. Scale bar: 40 µm.
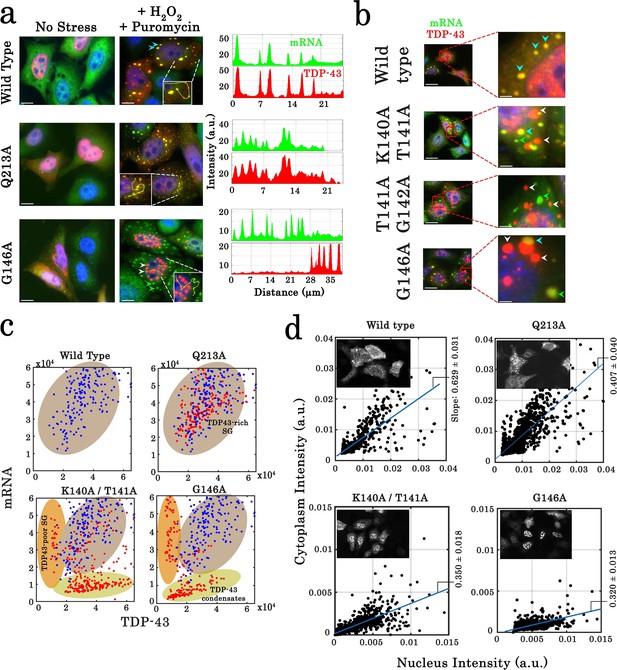
Cellular stress promotes the formation of condensates for cooperativity-defective mutants in cells that are distinct from stress granules.
(a) Subcellular distribution of wild-type TDP-43 or mutants (Q213A and G146A) upon H2O2/puromycin treatment. Wild-type TDP-43 and Q213A are found in cytoplasmic mRNA-rich stress granules (cyan head-arrows). G146A generates the formation of mostly nuclear TDP-43 condensates coexisting with TDP43-poor stress granules in the cytoplasm (white head-arrows). Scale bar: 10 µm. Right panels: line profiles of TDP-43 (red) and mRNA (green) intensities along the indicated path. (b) Cytoplasmic distribution of wild-type TDP-43 or mutants (K140A/T141A, T141A/G142A, and G146A) under stress conditions. Wild-type TDP-43 is only present in mRNA-rich stress granules (cyan head-arrows) but mutants also formed mRNA-poor TDP-43 condensates (white head-arrows) or mRNA-rich/TDP43-poor condensates (green head-arrows). (c) Statistical analysis of the subcellular compartments made of mRNA and TDP-43 in HeLa cells expressing wild-type TDP-43 or its mutants (K140A/T141A, T141A/G142A, G146A, and Q213A). Data from wild type (blue dots) are superimposed to data from mutants (red dots) in order to delineate formed subcellular compartments, TDP43-rich stress granules (brown), TDP43-poor stress granules (orange) and TDP43-condensates (green). Compartments were detected automatically using Cell Profiler. More than 100 compartments were analyzed for each condition. (d) Images of transfected HeLa cells (as displayed in the inset) were used to quantify the cytoplasmic and nuclear distribution of TDP-43 after cellular stress. The average slope for each distribution was then computed (blue line). For wild-type and Q213A mutant, the slope shows that TDP-43 is partly nuclear and cytoplasmic after its stress-induced nucleocytoplasmic shuttling. In contrast, for K140A/T141A and G146A mutants, the reduced slope indicates a nuclear retention of TDP-43. Cell cytoplasm and nucleus were detected automatically by using Cell Profiler. Ncell > 150.
-
Figure 8—source data 1
Statistical analysis of the subcellular compartments made of mRNA and TDP-43 in HeLa cells expressing wild-type or TDP-43 mutants (See legend of Figure 8c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig8-data1-v3.xlsx
-
Figure 8—source data 2
Assessment of the cytoplasmic and nuclear distribution of TDP-43 after cellular stress (See legend of Figure 8d).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig8-data2-v3.xlsx
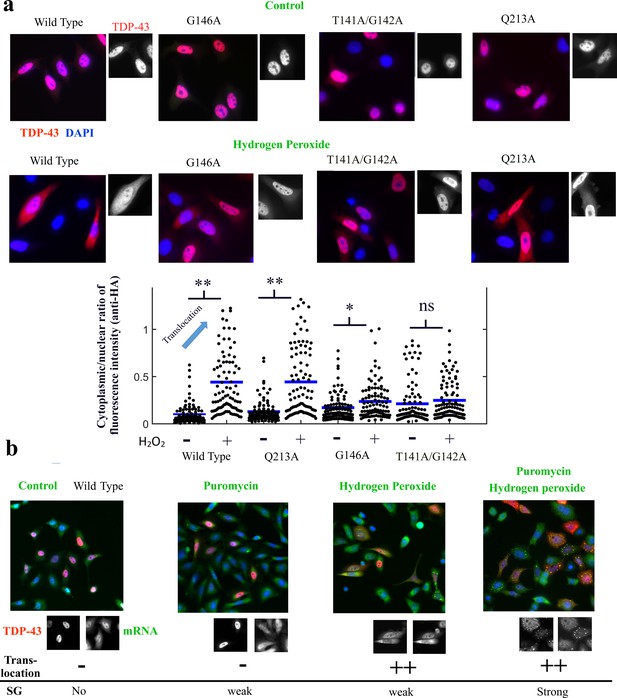
The cooperative binding of TDP-43 to mRNA is critical for TDP-43 translocation.
(a) Lower panel, mutants and wild-type TDP-43 were expressed in Hela cells with a HA-tag as indicated. Cells were then exposed to H2O2 for 90 min. Representative images of the subcellular distribution of TDP-43 are shown. Upper panel, Images of transfected HeLa cells (as displayed in the inset) were used to quantify the cytoplasmic and nuclear distribution of TDP-43 after H2O2. Cell cytoplasm and nucleus were detected automatically by using Cell Profiler. Ncell = 100. p<0.01** (paired two-sample t-test), n.s. non-significant. Mutations interfering with the cooperative binding of TDP-43 to mRNA reduce the presence of TDP-43 in the cytoplasm after hydrogen peroxide treatment. (b) Representative images showing the spatial distribution of wild-type TDP-43 expressed in HeLa cells after the indicated treatments. Puromycin alone has no remarkable effect on TDP-43 distribution except the scare presence of stress granules in a limited number of cells. H2O2 treatment promotes TDP-43 translocation as shown in (a) with only few cells displaying stress granules. However, puromycin and H2O2 treatment leads to an increased presence of TDP-43 in the cytoplasm combined to a marked formation of stress granules.
-
Figure 8—figure supplement 1—source data 1
Assessment of TDP-43 translocation (See legend of Figure 8—figure supplement 1).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig8-figsupp1-data1-v3.xlsx
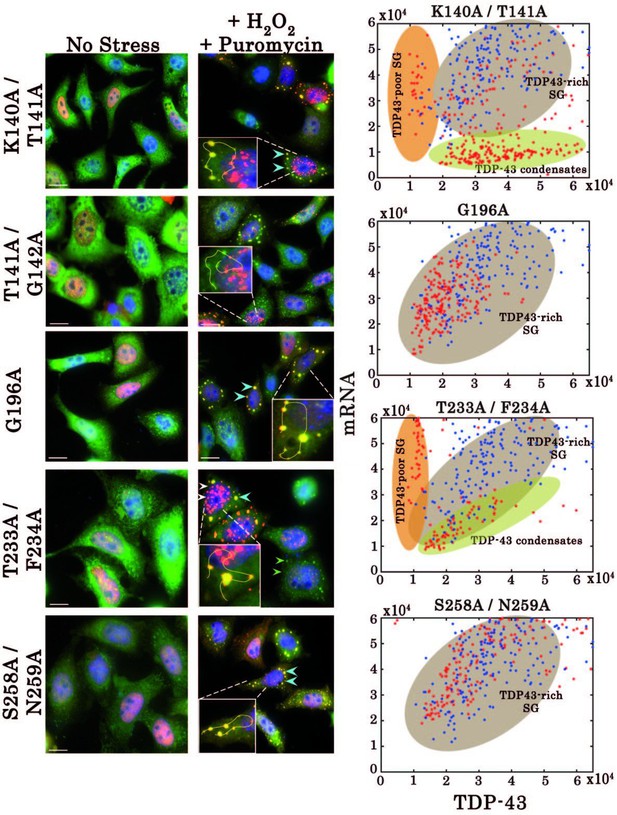
Effect of mutations on subcellular distribution of TDP-43 in HeLa cells.
Left, Subcellular distribution of wild-type TDP-43 or mutants (K140A/T141A, G196A, T233A/F234A, and S258A/N259A) upon H2O2/puromycin treatment. Zooms containing solid lines represent how intensities for TDP-43 (red) and mRNA (green) were quantified on ImageJ. Right, statistical analysis on the subcellular distribution of free mRNA and TDP-43 in HeLa cells expressing wild-type TDP-43 or its mutants. Data from wild type (blue dots) are superimposed to data from mutants (red dots) in order to delineate formed subcellular compartments, TDP43-rich stress granules (brown), TDP43-poor stress granules (orange) and TDP43-condensates (green). Scale bar: 10 µm.
-
Figure 8—figure supplement 2—source data 1
Effect of mutations on subcellular distribution of TDP-43 mutants in HeLa cells (See legend of Figure 8—figure supplement 2).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig8-figsupp2-data1-v3.xlsx
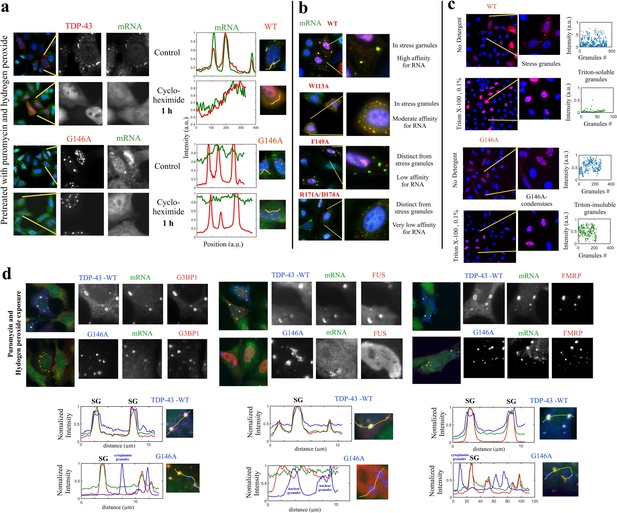
G146A mutation leads to the formation of insoluble condensates.
(a) HeLa cells expressing indicated proteins (in red) were treated with puromycin and H2O2 to generate the formation of stress granules. HeLa cells were then exposed to cycloheximide to dissociate stress granules for 1 hr. Wild-type TDP-43 then displayed a homogenous distribution in the cytoplasm. However, G146A condensates were resistant to cycloheximide. (b) Different TDP-43 mutants having a different affinity according to reference (Lukavsky et al., 2013), as indicated in the figure, were expressed in HeLa cells. These mutations are located in conserved residues which interact directly to mRNA. Their cytoplasmic distribution in cells is then shown after puromycin and H2O2 treatment using representative images. Mutations strongly interfering with the RNA binding prevent the association of TDP-43 with stress granules. (c) The reversibility of G146A condensates was then probed by Triton X-100 treatment (5 min, 0.1%). Stress granules were largely dissociated in agreement with their dynamic nature. However, G146A condensates are detergent-insoluble. The scatter plots represent the number and fluorescence intensity (anti-HA) of granular structures detected automatically by using Cell Profiler. Similar results as also shown in (a) and (c) were obtained with T141A/G142A (data not shown). (d) TDP-43 condensates obtained with G146A mutant do not contain other RNA-binding proteins including G3BP-1, FUS, and FRMP. HeLa cells expressing indicated proteins (in blue) were treated with puromycin and H2O2. The co-localization of wild-type and G146A TDP-43 with indicated BPS was then analyzed (lower panels) along the indicated lines. Wild-type TDP-43 co-localizes with mRNA and other RBPs such as G3BP-1, FMRP, and FUS in stress granules. On the other hand, G146A mutant does not co-localize with mRNA, G3BP-1, FMRP, and FUS whatever in the cytoplasm or in the nucleus.
-
Figure 8—figure supplement 3—source data 1
G146A mutation leads to the formation of insoluble condensates (See legend of Figure 8—figure supplement 3c).
- https://cdn.elifesciences.org/articles/67605/elife-67605-fig8-figsupp3-data1-v3.xlsx
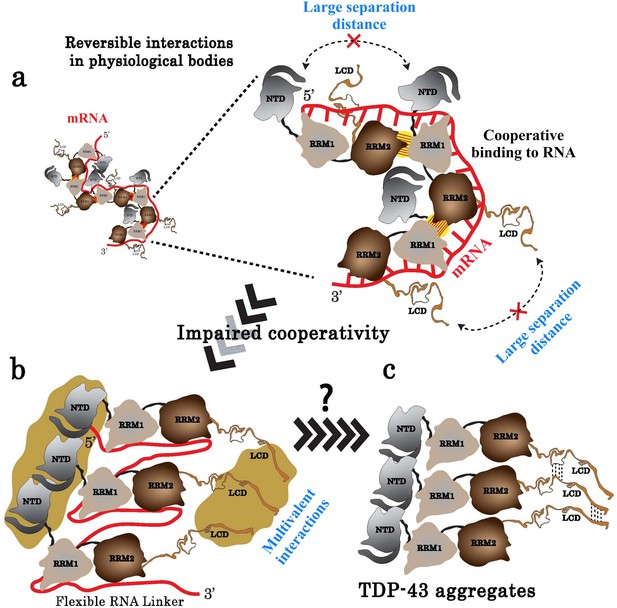
Model of TDP-43 assemblies in the presence of mRNA.
(a) Under physiological conditions, an inter-protein interaction (yellow with hatches) involving RRM2 and RRM1 prevents self-attractions of both the N-terminal domain (NTD) and the low complexity domain (LCD) between adjacent TDP-43. (b) When TDP-43 assemblies are no longer stabilized by the RRM-dependent inter-protein interaction, the presence of a RNA linker separating two consecutive TDP-43 leaves room for multivalent interactions between NTDs and LCDs, rendering possible a head-to-tail assembly of TDP-43. (c) The reduced access of mRNA to RRMs in the head-to-tail assembly may explain the transition towards a mRNA-free TDP-43 aggregation.
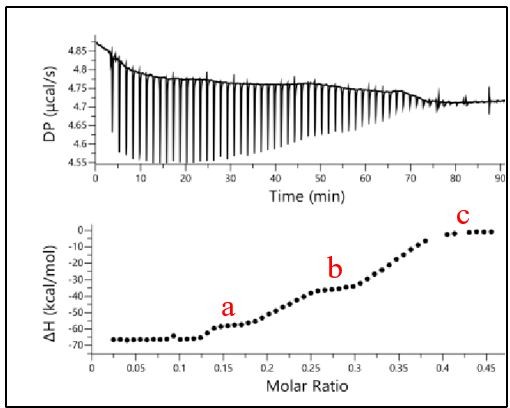
Binding of RRM1-2 to CLIP24nt oligonucleotide.
The binding profile was monitored by ITC and the titration curve was fitted (bottom). The plateaus observed for each oligonucleotide are indicated in red.
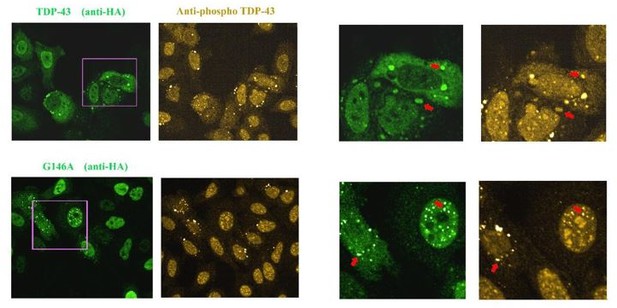
Images of HeLa cells expressing HA-tagged wild Type TDP-43 or G146A.
As indicated by the red arrow, we detected the presence of TDP-43 in stress granules that appear to be positive for phosphorylation. When G146A is expressed, both cytoplasmic and nuclear aggregates are also positive for phosphorylation (red arrows). Similar results were obtained with K140A/T141A. We also noted the presence of bright anti-phospho TDP-43 spots that are negative to G146A or wild type TDP-43 in some cells, for unknown reasons.
Additional files
-
Supplementary file 1
SAXS experimental conditions and deduced parameters from collected data.
Characteristic dimensions, Rg and Dmax, and molar mass (MMcorrelation volume) were obtained from data analysis. The theoretical masses (MMsequence) were calculated from the amino acid sequence.
- https://cdn.elifesciences.org/articles/67605/elife-67605-supp1-v3.docx
-
Supplementary file 2
Bonds involved in the multimerization of TDP-43 as deduced from the complex RRM1–2/(GU)12 MD model.
Physical parameters of the established interactions between atoms of residues of RRM2 pocket around V220 and those located in RRM1 loop 3 from monomer 1 and 2, respectively, are shown. Values in brackets indicates the energy contribution (in kcal/mol) of amino acid residues to the protein-protein interface stability. Energies were averaged over 100 ns of MD simulation and values are reported in kcal/mol with variant of fluctuations being ±0.1 kcal/mol. 1bb and vdW correspond to backbone and van der Waals, respectively.
- https://cdn.elifesciences.org/articles/67605/elife-67605-supp2-v3.docx
-
Supplementary file 3
Poly (GT) repeats binding capacity (N), apparent dissociation constant (KD), and thermodynamic parameters for RRM fragments of TDP-43, as determined by ITC.
a and b correspond to apparent dissociation constants KD1 and KD2, respectively. The thermodynamic parameters (ΔH, TΔS, ΔG) and χ2 values were expressed in kcal/mol and (kcal/mol)2, respectively.
- https://cdn.elifesciences.org/articles/67605/elife-67605-supp3-v3.docx
-
Supplementary file 4
Poly (GT) repeats binding capacity (N), apparent dissociation constant (KD), and thermodynamic parameters for RRM1–2 protein mutants, as determined by ITC.
a and b correspond to apparent dissociation constants KD1 and KD2, respectively. The thermodynamic parameters (ΔH, TΔS, ΔG) and χ2 values were expressed in kcal/mol and (kcal/mol)2, respectively.
- https://cdn.elifesciences.org/articles/67605/elife-67605-supp4-v3.docx
-
Supplementary file 5
Cell line authentication.
- https://cdn.elifesciences.org/articles/67605/elife-67605-supp5-v3.pdf
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/67605/elife-67605-transrepform1-v3.pdf





