Finger somatotopy is preserved after tetraplegia but deteriorates over time
Figures
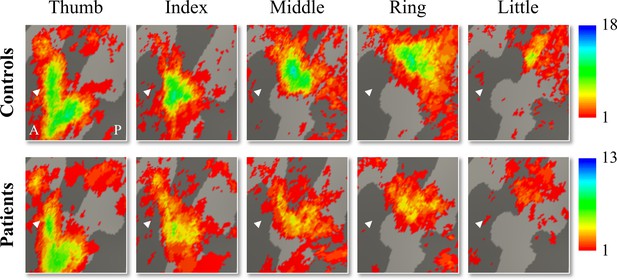
Inter-participant somatotopic finger-specific probability maps of the control and tetraplegic patient groups.
Colours indicate the number of participants (ranging from 1 [red] till 18 and 13 [blue] participants for the control and SCI patient group, respectively) who demonstrated finger selectivity for a given vertex. Characteristic finger selectivity is characterised by a progression of finger selectivity from the thumb (laterally) to the little finger (medially). These characteristic finger progressions can be observed in both the control (top) and the tetraplegic patient (bottom) group’s probability maps. Qualitative inspection suggests that inter-participant consistency was lowest for the little finger representation in both groups. It further appears that overall inter-participant consistency was reduced in the patient group compared to the control group. White arrows indicate the central sulcus. A: anterior; P: posterior.
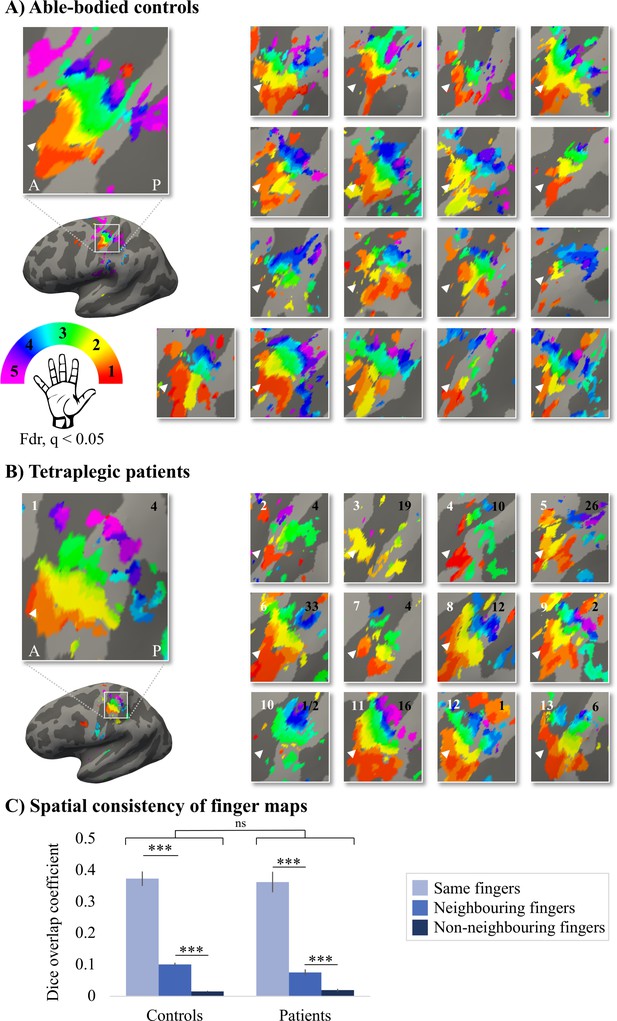
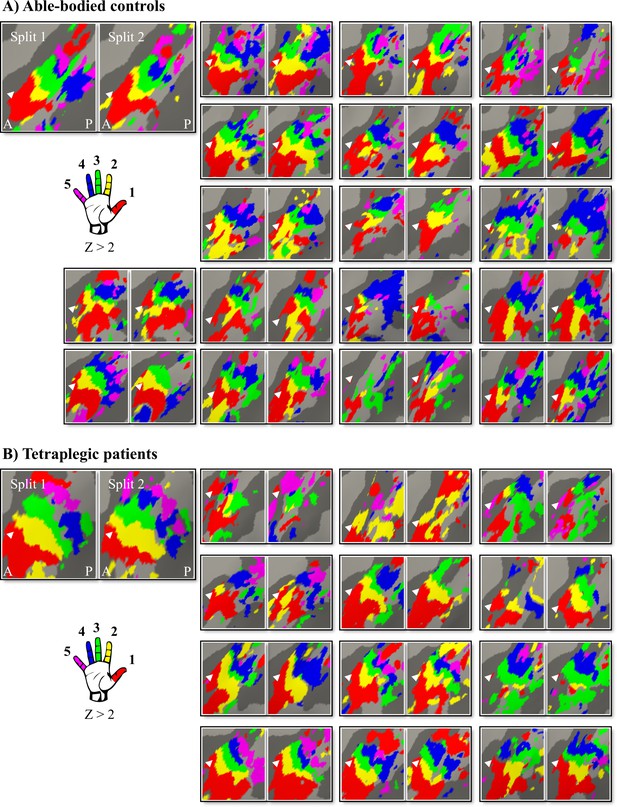
Finger selectivity is preserved in tetraplegic patients.
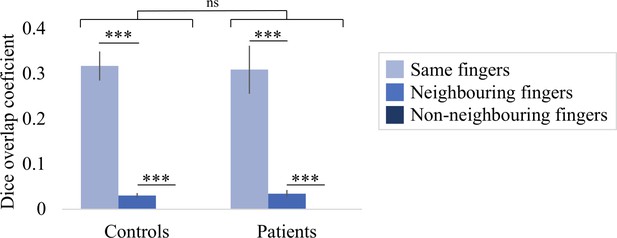
Colours indicate selectivity for the thumb (finger 1, red), index finger (finger 2, yellow), middle finger (finger 3, green), ring finger (finger 4, blue), and little finger (finger 5, purple). Maps of participants for whom the left hand was tested are horizontally mirrored for visualisation purposes. Typical finger selectivity is characterised by a gradient of finger preference, progressing from the thumb (laterally) to the little finger (medially). These characteristic gradients of finger selectivity can be observed in both the able-bodied controls (A) and the tetraplegic patients (B). Despite most maps (except patient map 3) displaying aspects of characteristic finger maps, some finger representations were not visible in the thresholded patient and control maps. Patients’ hand maps are sorted according to their overall upper-limb impairments (assessed using the Graded Redefined Assessment of Strength, Sensibility and Prehension test [GRASSP]): from most to least impaired – as indicated by the white numbers. Black numbers indicate the years since spinal cord injury (SCI). Multiple comparisons were adjusted using a false discovery rate (FDR) with q < 0.05. Other figure annotations are as in Figure 1. (C) To ensure that the observed clusters were not representing noise, but rather true finger selectivity, we calculated split-half consistency between two halves of the minimally thresholded (Z > 2) travelling wave dataset (see Figure 2—figure supplement 1 for the travelling wave maps used to calculate split-half consistency). Both controls and patients showed higher split-half consistency (assessed using the Dice overlap coefficient) for comparison of the same fingers between two halves of the travelling wave dataset (light blue), compared to neighbouring (blue), and non-neighbouring fingers (dark blue). Moreover, neighbouring fingers showed greater overlap across the split-halves of the dataset then non-neighbouring fingers for both patients and controls. The same results were obtained when calculating split-half consistency on maps thresholded using FDR q < 0.05 (as was used for the maps in A, B; see Figure 2—figure supplement 2). Error bars show the standard error of the mean. *** = corrected p≤0.001, ns: non-significant.
Hard-edged split-half travelling wave maps used to calculate the intra-participant spatial consistency reported in Figure 2C.
Control (A) and patient maps (B) are ordered as in Figure 2. Colours indicate selectivity for the thumb (finger 1, red), index finger (finger 2, yellow), middle finger (finger 3, green), ring finger (finger 4, blue), and little finger (finger 5, purple). Maps of participants for whom the left hand was tested are horizontally mirrored for visualisation purposes. We used minimally thresholded finger-specific clusters (Z > 2) for the Dice overlap coefficient (DOC) spatial consistency analysis to ensure we were sensitive to overlaps that would be missed when using high thresholds. A: anterior; P: posterior. Multiple comparisons were adjusted using a false discovery rate (FDR) with q < 0.05.
Spatial consistency of false discovery rate (FDR)-thresholded finger maps.
We repeated the split-half consistency analysis using an q < 0.05 FDR-thresholding criterion (as in the travelling wave maps in Figure 2A and B). Similar results were found as when we thresholded the split-half finger maps using Z > 2: overall, split-half consistency was not significantly different between patients and controls, as tested using a robust mixed ANOVA (F(1,17.69) = 0.08, p=0.79). There was a significant difference in split-half consistency between pairs of same, neighbouring, and non-neighbouring fingers (F(2,14.77) = 38.80, p<0.001). This neighbourhood relationship was not significantly different between the control and patient groups (i.e. there was no significant interaction; F(2,14.77) = 0.12, p=0.89). *** = corrected p≤0.001, ns: non-significant.
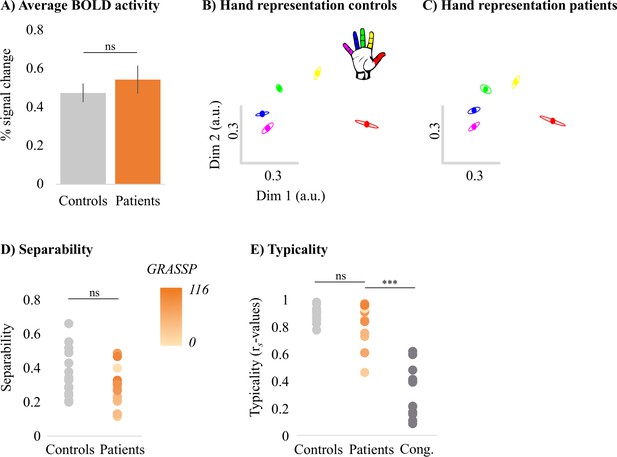
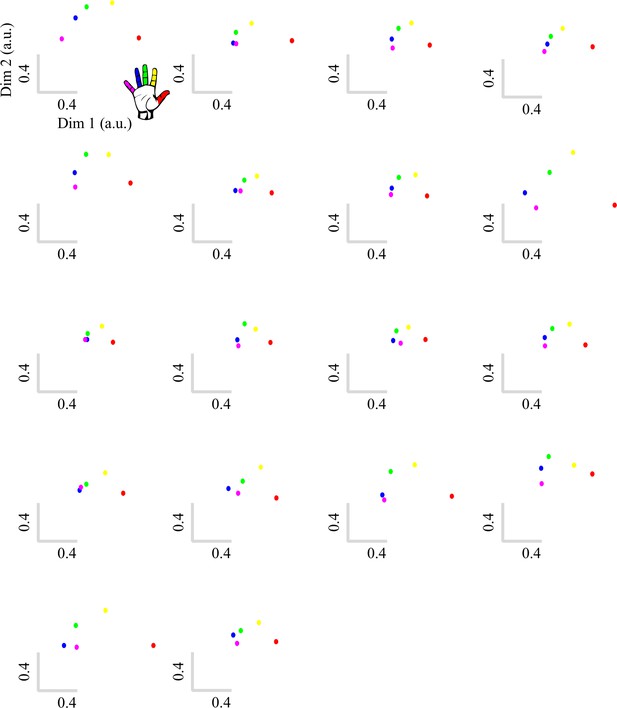
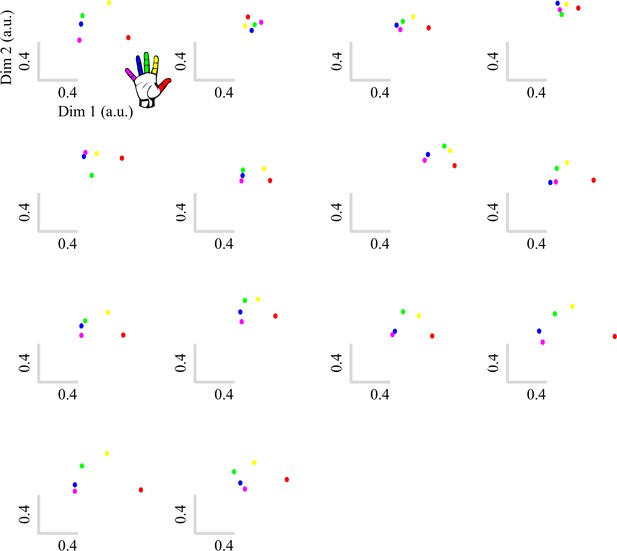
Typical multivariate hand somatotopy is preserved following tetraplegia.
(A) Percent signal change in the S1 hand area during finger movement for able-bodied controls (grey) and tetraplegic patients (orange). Similar results were found in the M1 hand ROI (see Figure 3—figure supplement 1). (B, C) Two-dimensional projection of the representational structure of inter-finger distances in the control (B) and tetraplegic patient groups (C). Inter-finger distance is reflected by the distance in the two dimensions. Individual fingers are represented by different colours: thumb, red; index finger, yellow; middle finger, green; ring finger, blue; little finger, purple. Ellipses represent the between-participants’ standard error after Procrustes alignment. Inter-finger distances across finger pairs were significantly different across finger pairs (as would be expected based on somatotopic mapping), but not between controls and tetraplegic patients (see Figure 3—figure supplement 2). Individual participant inter-finger distance patterns are visualised in Figure 3—figure supplement 3 and Figure 3—figure supplement 4 for the controls and patients, respectively. (D) Separability, measured as mean inter-finger distance, of the representational structure in the S1 hand area of controls and patients. Patients are presented on a colour scale representing the sensory and motor functioning of their tested upper limb, measured using the Graded Redefined Assessment of Strength, Sensibility and Prehension test (GRASSP) (0 = no upper limb function, 116 = normal upper limb function). (E) Typicality of the representational structure in controls, patients, and congenital one-handers (Cong. in the figure). *** p<0.001; ns: non-significant; Dim: dimension; a.u.: arbitrary unit; Cong: congenital one-handers.
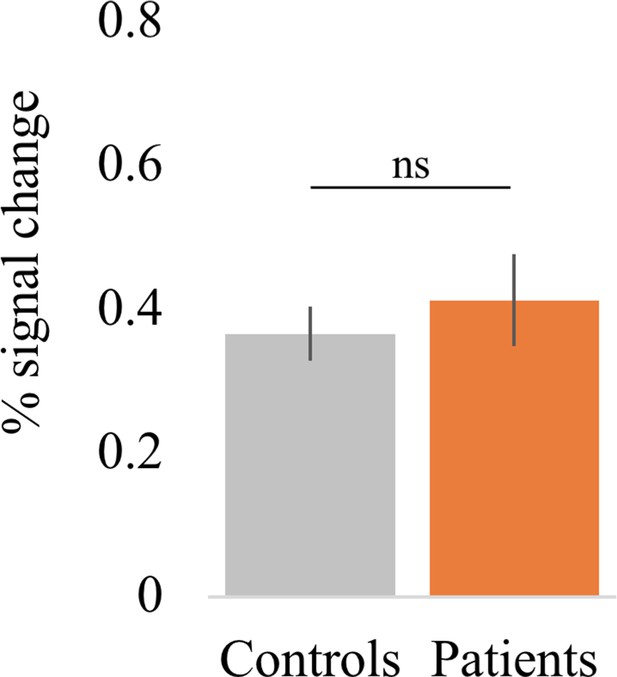
Percent signal change in the M1 hand area during finger movement.
Overall, all tetraplegic patients were able to engage their M1 hand area by moving (or attempting to move) individual fingers (t(13) = 6.44, p<0.001; BF10 = 1.09 e + 3), as did controls (t(17) = 9.73, p<0.001; BF10 = 5.65 e + 5). Furthermore, patients’ task-related activity was not significantly different from controls (t(30) = –0.66, p=0.52; BF10 = 0.40), with the Bayes factor (BF) showing anecdotal evidence in favour of the null hypothesis.
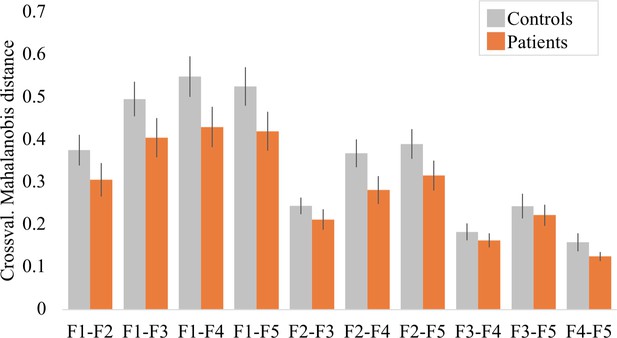
Inter-finger distances across finger pairs for controls and tetraplegic patients.
A robust mixed ANOVA with a within-participants factor for finger pair (10 levels) and a between-participants factor for group (two levels: controls and tetraplegic patients) revealed a significant main effect for finger pair, as would be expected based on somatotopic mapping (F(9,15.38) = 27.22, p<0.001). We did not find a significant group (F(1,21.66) = 1.50, p=0.23) or finger pair by group interaction (F(9,15.38) = 1.05, p=0.45). When testing for group differences per finger pair, the Bayes factor (BF) only revealed inconclusive evidence (BF >0.37 and < 1.11; note that we could not run a Bayesian ANOVA due to normality violations). Crossval.: crossvalidated; F: finger; F1: thumb; F2: index finger; F3: middle finger; F4: ring finger; F5: little finger.
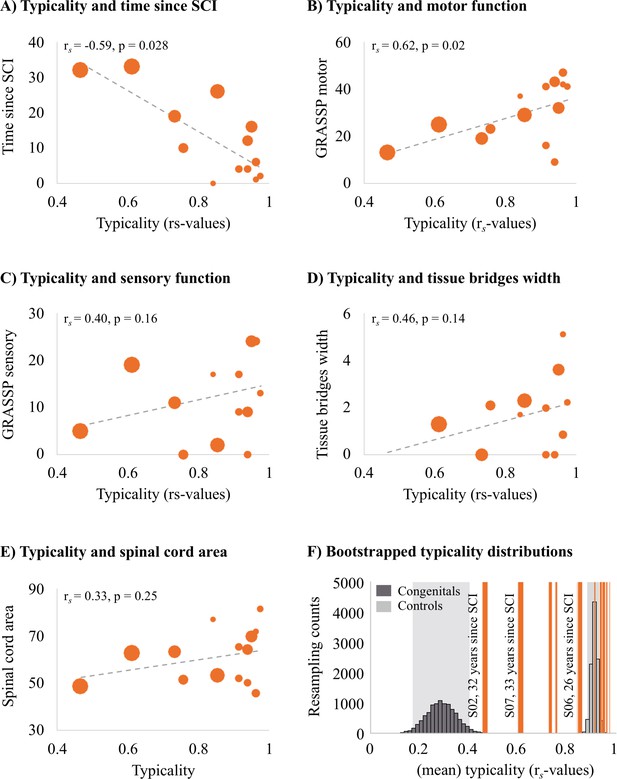
Years since spinal cord injury and retained motor function correlate with hand representation typicality in the primary somatosensory cortex (S1).
We examined clinical, behavioural, and spinal cord structural correlates for hand representation typicality. Increasing marker sizes represent increasing years since spinal cord injury (SCI) in graphs A–E. (A) There was a negative correlation between years since SCI and hand representation typicality. (B) We found a positive correlation between motor function of the tested upper limb (measured using the Graded Redefined Assessment of Strength, Sensibility and Prehension test [GRASSP]) and hand representation typicality. There was no significant correlation between hand representation typicality and sensory function of the tested upper limb (C; measured using the GRASSP), spared midsagittal spinal tissue bridges (D), and cross-sectional spinal cord area (E). (F) Bootstrapped distribution of controls’ and congenital one-handers’ mean S1 hand representation typicality. Dark grey bars indicate the distribution of congenital one-handers (data taken from an independent study; Wesselink et al., 2019), and light grey bars indicate the distribution of the able-bodied controls (tested for this study). The typicality scores of the SCI patients are plotted as orange lines. Increasing line thickness represent increasing years since SCI. Grey shaded areas indicate the 95% confidence intervals of the mean for congenital one-handers and able-bodied controls.
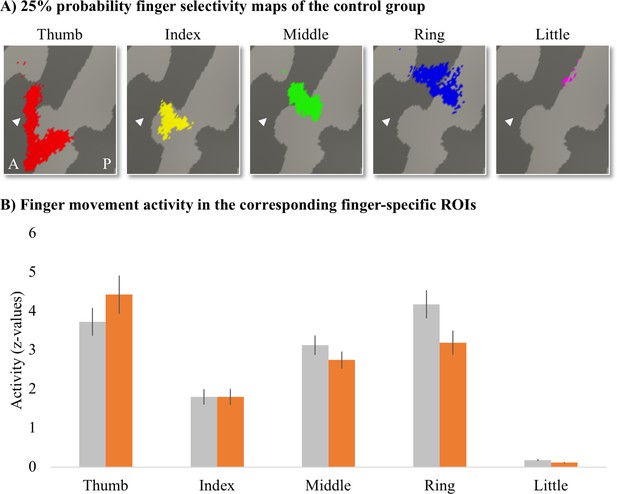
Finger-specific activity levels in finger-specific regions of interest (ROIs).
(A) Finger-specific ROIs were based on the control group’s binarised 25% probability travelling wave finger selectivity maps. White arrows indicate the central sulcus. A: anterior; P: posterior. (B) Finger movement activity levels in the corresponding finger-specific ROIs. There were no significant differences in activity levels between thetetraplegic patient and control groups. Controls are projected in grey; patients are projected in orange. Error bars show the standard error of the mean.
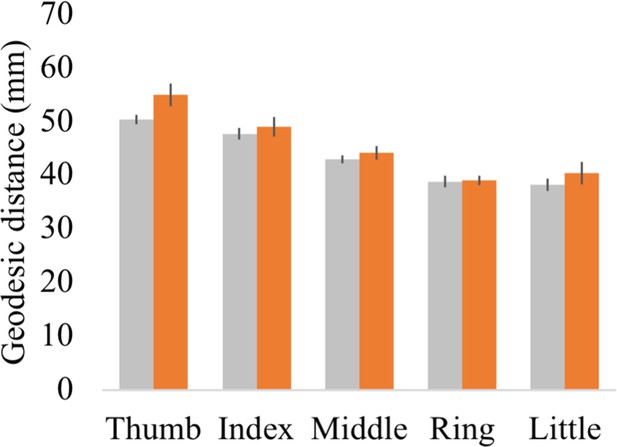
Geodesic distance between each finger’s peak activated vertex and a cortical anchor.
Cortical geodesic distances were calculated between a reference anchor in the S1 foot cortex (MNI coordinates: −16.93, –32.06, 73.80) and the peak activated vertex per finger movement for each participant. Controls are projected in grey; spinal cord injury (SCI) patients are projected in orange. Error bars show the standard error of the mean.
Tables
Demographic and clinical details.
Tetraplegic patients are ordered according to their retained upper-limb sensory and motor function (assessed using the Graded Redefined Assessment of Strength, Sensibility and Prehension test [GRASSP]). Sex: F, female; M, male; Age, age in years; AIS grade, American Spinal Injury Association (ASIA) Impairment Scale grade defined based on the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI); A, complete; B, sensory incomplete; C, motor incomplete; D, motor incomplete; E, normal; Neurological level of injury, defined based on the ISNCSCI; dominant hand, defined using the Edinburgh handedness inventory: L, left; R, right; GRASSP, Graded Redefined Assessment of Strength, Sensibility and Prehension (maximum score: 232 points); tested side, side with the lowest score on the GRASSP measurement; GRASSP motor/sensory score of the tested upper limb (maximum scores: 50/24; see Table 2 for further details).
| Sex | Age | Years since injury | AIS grade | Cause of injury | Neurological level of injury | Dominant hand | GRASSP score | Hand tested | GRASSP tested side motor/sensory | |
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | M | 32 | 4 | A | Trauma | C4 | L | 21 | L | 9/0 |
| S02 | M | 52 | 32 | A | Trauma | C5 | R | 78 | L | 16/5 |
| S03 | M | 35 | 4 | A | Trauma | C4 | R | 90 | L | 16/17 |
| S04 | M | 41 | 19 | A | Trauma | C6 | L | 105 | L | 19/11 |
| S05 | M | 52 | 10 | A | Trauma | C2 | L | 118 | R | 23/0 |
| S06 | M | 67 | 26 | A | Trauma | C4 | R | 119 | L | 29/2 |
| S07 | M | 57 | 33 | C | Trauma | C5 | L | 145 | R | 25/19 |
| S08 | F | 67 | 4 | D | Trauma | C5 | L | 173 | L | 41/9 |
| S09 | M | 59 | 12 | D | Trauma | C2 | R | 187 | R | 43/9 |
| S10 | M | 42 | 2 | D | Trauma | C4 | R | 187 | R | 41/13 |
| S11 | M | 58 | 0.5 | D | Ischaemic | C4 | R | 194 | R | 37/17 |
| S12 | M | 71 | 16 | D | Trauma | C7 | R | 196 | R | 32/24 |
| S13 | M | 65 | 1 | D | Trauma | C2 | R | 218 | R | 42/24 |
| S14 | M | 74 | 6 | D | Surgery | C4 | L | 220 | R | 47/24 |
GRASSP motor sub-scores.
Each muscle was tested with resistance through its full range of motion and given a muscle grade between 0 and 5: 0, flaccid motion; 1, flicker motion; 2, full range of motion with gravity eliminated; 3, full range of motion against gravity; 4, full range of motion with moderate resistance; 5, full range of motion with maximal resistance (Kalsi-Ryan et al., 2012).
| Elbow flexion | Shoulder (deltoideus) | Wrist extension | Elbow extension | Fingers 2–5 extension | Thumb opposition | Thumb flexion | Middle finger flexion | Little finger abduction | Index finger abduction | |
|---|---|---|---|---|---|---|---|---|---|---|
| S01 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S02 | 5 | 5 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| S03 | 4 | 4 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| S04 | 5 | 5 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| S05 | 4 | 1 | 3 | 4 | 3 | 3 | 1 | 1 | 1 | 2 |
| S06 | 5 | 5 | 5 | 5 | 1 | 1 | 4 | 3 | 0 | 0 |
| S07 | 5 | 5 | 5 | 5 | 1 | 1 | 1 | 0 | 1 | 1 |
| S08 | 5 | 4 | 5 | 4 | 4 | 5 | 5 | 4 | 4 | 1 |
| S09 | 5 | 4 | 4 | 4 | 4 | 4 | 5 | 5 | 4 | 4 |
| S10 | 5 | 5 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 |
| S11 | 5 | 5 | 4 | 2 | 5 | 4 | 5 | 5 | 1 | 1 |
| S12 | 5 | 5 | 5 | 5 | 4 | 1 | 4 | 1 | 1 | 1 |
| S13 | 5 | 4 | 4 | 4 | 5 | 4 | 5 | 3 | 4 | 4 |
| S14 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 4 |
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/67713/elife-67713-transrepform1-v2.pdf
-
Reporting standard 1
STROBE checklist.
- https://cdn.elifesciences.org/articles/67713/elife-67713-repstand1-v2.pdf