Mechanical vibration patterns elicit behavioral transitions and habituation in crawling Drosophila larvae
Figures
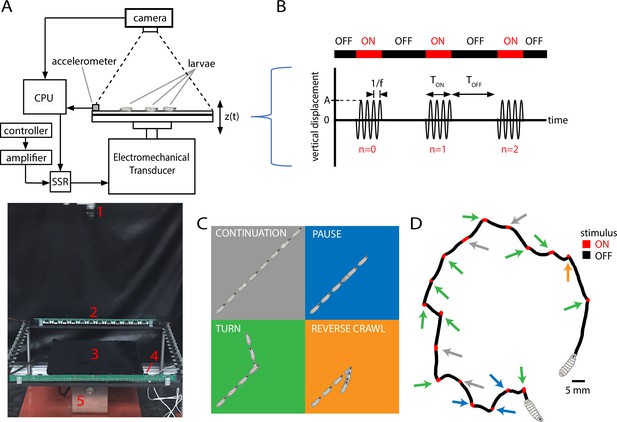
Vibration stimulus delivery and avoidance behavior classification.
(A) Top: schematic of the experimental setup, where larvae crawl on a vertically vibrated agar gel supported by aluminum and steel plates. An electromechanical transducer provides vibration, while a CCD camera records 2D crawling of red-light-illuminated animals simultaneously. See ‘Methods and materials’ for details. Bottom: photograph of the experimental setup, with red numbering labeling (1) camera, (2) LED array, (3) crawling substrate, (4) accelerometer, and (5) mechanical shaker. (B) Stimulus pattern in a typical experiment. Beginning at time , pulses of sinusoidal vibration are delivered for a duration of , and repeated at times , where is an integer referring to nth application of the stimulus. The initial vibration is referred to as the stimulus, the next as , etc. Vibration strength is described by the frequency and the peak (dimensionless) acceleration . In the top horizontal bar, red indicates stimulus ON, and black indicates stimulus OFF. (C) Schematic of four behavioral responses to non-nociceptive vibration: continuation (gray), pause (blue), turn (green), and reverse (orange). In each illustration, the larva crawls forward from the bottom left, and a stimulus is delivered in the center. Pictures in the sequence are equally spaced in time. (D) Representative trajectory of a single larva crawling for 300 s during a vibration experiment ( Hz, , s, s). The four behaviors are indicated by arrows matching the behavior’s color from (C). The regions with the stimulus ON appear shorter than the OFF segments because larvae come to a stop before adjusting direction, and this can take at least several seconds.
-
Figure 1—source code 1
Code from MATLAB that determines the behavioral state of crawling larvae.
The program uses input data from the MAGAT Analyzer software (Gershow et al., 2012).
- https://cdn.elifesciences.org/articles/69205/elife-69205-fig1-code1-v1.zip
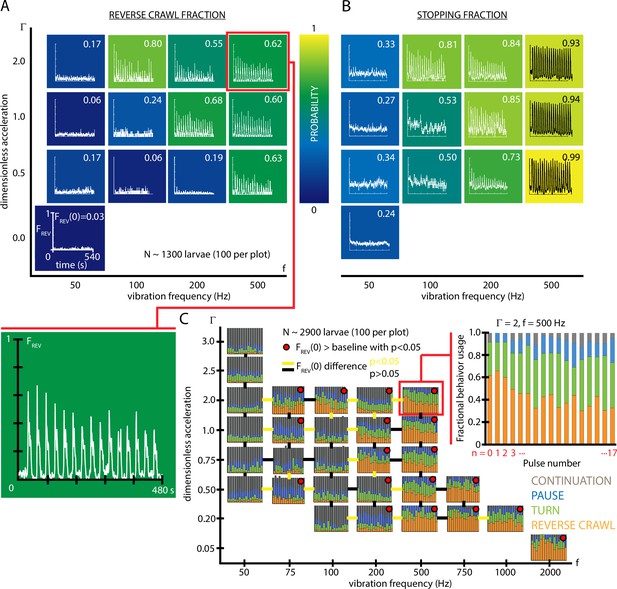
Fractional strong and weak behavioral responses depend on vibration strength.
(A) Reversal behavior heat map. Vibration parameters were s, s, with ranging from and frequency between 50 and 500 Hz. , the fraction of larvae that reverse crawl after the first () vibration pulse is printed for each square region, alongside graphs of , averaged over all experiments. Color indicates the value. All graphs have the same scale in and . Each () result is based on five experiments, each with larvae (total 1300 animals), and lasting 600 s. Note that the and axes are not on a linear scale. Uncertainties in are not listed, but are for all values. (B) Stopping behavior heat map. From the same experiments as (A), but considering , the fraction of larvae showing any avoidance behavior (pause, turn, or reversal). As vibration strength increases (along either the or axes), the fraction of avoidant larvae increases. (C) Inset: fractional deployment of the behavioral repertoire during habituation. (orange), (green), (blue), and (gray) during a 3-s window after pulse initiation, as a function of the pulse number . Over time the stronger avoidance behavior diminishes in favor of weaker avoidance and nonavoidance. , Hz. Larger graph: behavioral repertoire over a range of vibration space. Fractional use of behaviors as a function of vibration pulse number () for repeated vibrations ( s, s), for many specific , combinations. Each experimental condition is represented by a vs. plot, and the response of 100 larvae is averaged, for a total of 2900 animals. Lines bridging adjacent graphs indicate whether the two sets of vibration conditions induce a significantly different reverse crawl probability following the pulse, colored either yellow for p<0.05 and black for p>0.05 (Fisher’s exact test). Red dots on the graphs indicate a reverse crawl probability significantly greater than the baseline (zero vibration) response probability (Fisher’s exact test).
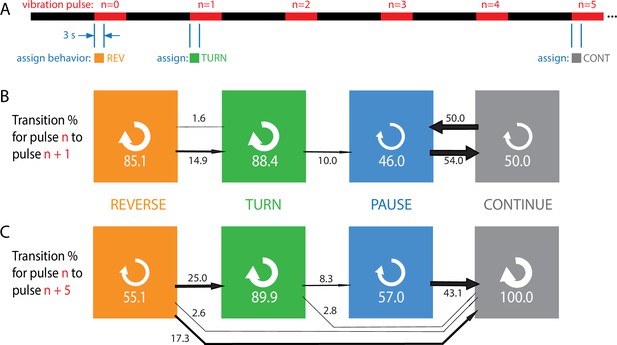
Habituation to repeated pulses is an essentially one-way process for individuals.
(A) Schematic of the stimulus pattern and example analysis. The stimulus consisted of vibration ( Hz, ) with repeated pulses of width s, repeated after s. The behavior of each individual, in the 3 s following the onset of each vibration pulse, was assigned to one of four categories: reverse crawl (orange), turn (green), pause (blue), or continuation (gray). In the example shown, a larva reverse crawls in response to the pulse, then turns in response to the next, and continues in response to the pulse. (B, C) Behavioral transitions during repeated stimuli for individual larvae. For a given behavior observed in response to pulse , the arrows represent the percentage of larvae that exhibit each of the four behaviors in response to pulse (B) or (C). White circular arrows represent repeating the same behavior, and the thickness of the black arrows is proportional to the fraction of animals that make the respective transition. The sum of the repeat arrows and all outgoing arrows is 100 for each behavior. Larvae were observed in five separate experiments, for a total of 107 animals making behavioral transitions.
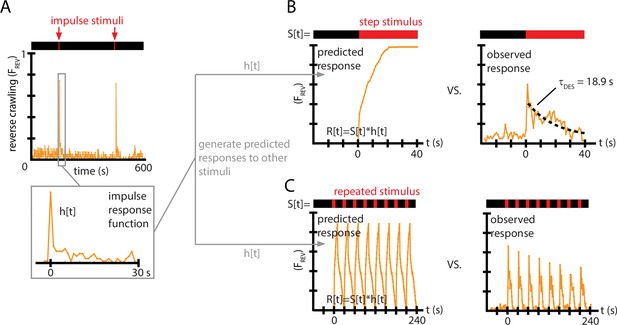
Impulse response experiments show that avoidance response to vibration is nonlinear and adaptive.
(A) The fraction of larvae performing reverse crawling ((t)) while exposed to very short bursts of strong vertical vibration ( Hz, , s, s). Inset shows a time-expanded view of the response, labeled as to denote the impulse response function (IRF) used to make predictions for other stimulus inputs. (B) Avoidant response () to continuous vibration, as predicted by a linear, time-invariant (LTI) model using the impulse responses from (A) (left), and as observed empirically (right) (i.e., ) with Hz and . (C) Avoidant response () to repeated pulse vibration ( Hz, , s, s), as predicted by a linear, LTI model using the impulse responses from (A) (left), and as observed empirically (right). The LTI calculation fails to predict the empirical behavior due to de-sensitization (B) and slow re-sensitization (C). Each plot is the average from five experiments using 20 larvae each (total 100 animals).
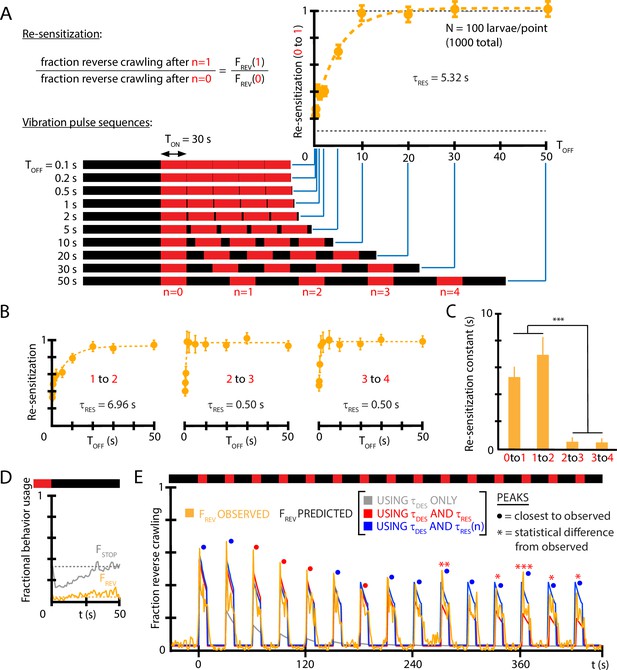
Re-sensitization after removal of mechanical stimulus depends on prior vibration pulses.
(A) Visualization of vibration pulse sequence experiments used to determine re-sensitization to the stimulus. Time indicates the start of the initial () vibration pulse period, lasting s (red). The stimulus is removed between pulses for varying amounts of time . Recovery of sensitization is determined for each pulse by computing the ratio to , normalized to account for incomplete recovery for short times. Lower dashed line indicates baseline (no stimulus) reversal fraction. (B) Re-sensitization as a function of the time , determined for the , , , and pulses. Vibration intensity was Hz, . Each data point is the average from five experiments of animals each, for a total of 1000 larvae from 50 experiments. Error bars are SEM. (C) Re-sensitization time constants as a function of vibration pulse number . was determined from fits of the data in (A) and (B) (Equation 4). After two vibration pulses, the re-sensitization is significantly faster (***p<0.001, Student’s t-test). (D) Behavioral response to the ON→OFF stimulus transition: and , where indicates the stimulus OFF transition. is unaffected. Vibration conditions ( Hz, , s, s). Data points are the average of (gray) and (orange) up to the pulse. Dashed lines indicate the baseline behavior fractions while the stimulus is ON. (E) Comparison of habituation models with , , and vs. dependence (blue) to empirical strong avoidance behavior (orange), as well as more limited models with only (gray) or only and (red). Colored circle markers above each peak indicate which model was closest to the empirical peak value, while * symbols indicate whether the model peak value was significantly different than the empirical peak value (Fisher’s exact test, *p<0.05, **p<0.01, ***p<0.001, gray trace not included).
-
Figure 5—source code 1
Code in Igor Pro for performing fits to determine desensitization and re-sensitization time constants from reversal count data.
- https://cdn.elifesciences.org/articles/69205/elife-69205-fig5-code1-v1.zip
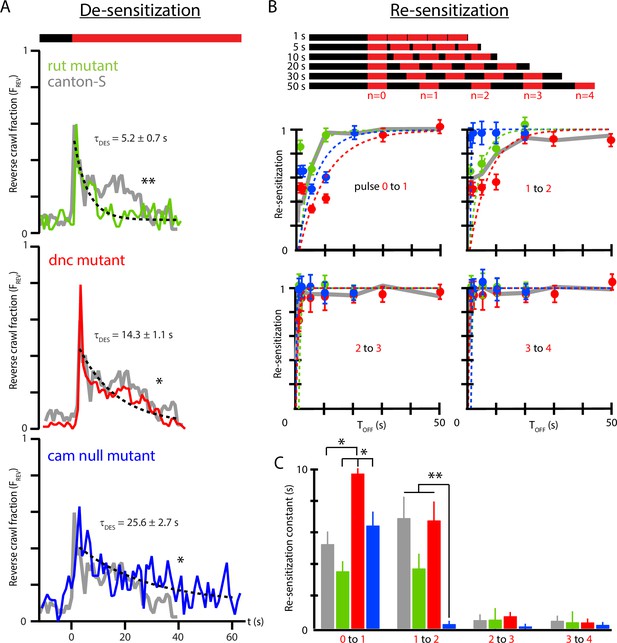
Memory-deficient mutants have distinct habituation and de-habituation time constants.
(A) De-sensitization: reverse crawl behavior usage in response to continuous vibration stimulation. vs. (where marks the vibration onset) for three mutants: rut (green), dnc (red), and cam0 (blue). Gray traces are the Canton-S wild-type response from Figure 4B. Vibrations were Hz and . Each trace is based on five experiments, with 20 larvae in each. (B) Re-sensitization to vibration following repeated pulses. Top: schematic of experiments performed. Bottom: plots of vs. after the nth pulse for rut (green), dnc (red), and cam0 (blue). Gray traces are the Canton-S wild-type response from Figure 5. Vibrations were Hz and . Each point is based on 5 experiments, with 20 larvae in each, for a total of 75 experiments and larvae. Error bars indicate SEM. (C) Desensitization and Re-sensitization time constants as functions of pulse number for the same three mutants, based on fits to the data in (B). Error bars indicate SEM. *p<0.05 and **p<0.01.
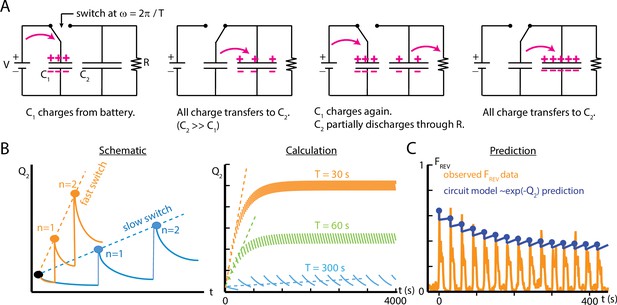
An electric circuit models possible mechanisms for larval habituation.
(A) The capacitor switch circuit, where a small capacitor C1 is continually charged by a battery , and discharges to a larger capacitor C2 each time the switch changes. The charge Q2 is related to the probability of observing an external event (). (B) Functions created by varying the duration of the charging phase of the circuit, , while holding the circuit elements constant. Left: a visual schematic of such functions. Right: generated by simulating the circuit behavior. In each case, after enough switches, the charge saturates when the charging from C1 to C2 balances the charge dissipated through for each cycle. For values of much smaller than , this saturation will only occur at a large . (C) Comparison of the circuit model to empirical data of reverse crawl probability ( Hz, , s, s, same data shown in Figure 4C). The circuit values are , , , , with the switch operating with period . The peak values from the circuit model do not significantly differ from the empirical peaks (p>0.05, Fisher’s exact test).
Additional files
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/69205/elife-69205-transrepform1-v1.docx
-
Source code 1
Matlab script, which should be run in the MAGAT Analyzer environment after loading movie files, that determines the behavioral state of crawling larvae, in particular identifying reverse-crawl behavior.
- https://cdn.elifesciences.org/articles/69205/elife-69205-code1-v1.zip
-
Source code 2
Functions in Igor Pro that perform fits to larva crawling data, used to extract desensitization and re-sensitization time constants.
- https://cdn.elifesciences.org/articles/69205/elife-69205-code2-v1.zip





