The repurposing of Tebipenem pivoxil as alternative therapy for severe gastrointestinal infections caused by extensively drug-resistant Shigella spp
Figures
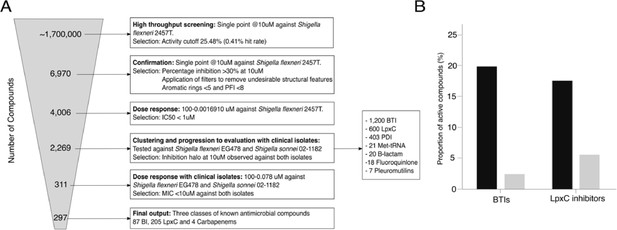
Screening compounds for antibacterial activity against Shigella spp.
(A) Flowchart outlining the screening process in six key stages: (1) identification of compounds with antibacterial activity against S. flexneri 2457T from an initial set of ~1.7 million chemicals at a concentration of 10 µM. (2) Confirmation of original hits with activity against S. flexneri 2457T at 10 µM with application of additional described filters. (3) Dose response against S. flexneri 2457T selecting compounds with IC50 of <1 µM. (4) Testing compounds with antibacterial activity against S. flexneri EG478 and S. sonnei 02–1181 (clinical isolates from Vietnam) at a concentration of 10 µM (leading to 2,269 compounds in seven classes). (5) Dose response against S. flexneri EG478 and S. sonnei 02–1181 selecting compounds with IC50 of <1 µM against both organisms. (6) Identification of the final 297 compounds in three classes. (B) Bar charts showing the activity profile (proportion of chemicals with antibacterial activity) of BTIs and LpxC inhibitors against the S. flexneri 2457T laboratory isolate (black) and both clinical isolates (S. flexneri EG478 and S. sonnei 02–1181; grey).
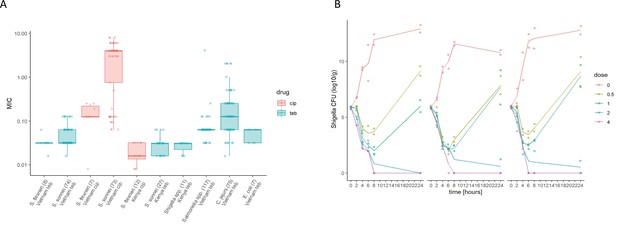
The in vitro activity of Tebipenem against various enteric pathogens.
(A) Boxplots showing the log of the minimum inhibitory concentrations (MIC; µg/ml) of Tebipenem (teb) (blue) and ciprofloxacin (cip) (red) for clinical isolates of Shigella spp. (sonnei and flexneri) from Vietnam and Kenya (highlighted) and a range of other enteric pathogens. The boxes show the interquartile range of MICs (median line), with individual data points, whiskers show the highest and lowest MICs; the number of isolates screened are highlighted on the x axis. MICs were performed in duplicate; a third replicate was performed if the initial data did not match. Time kill curves of 0 x (red), 0.5 x (light green), 1 x (dark green), 2 x (blue), and 4 x (pink) MIC Tebipenem over 24 hr against (B) S. flexneri EG478, (C) S. flexneri_01_0417, and (D) S. sonnei_02_1181; counts shown in CFU/ml and represent the mean ± the standard deviation of three biological replicates.
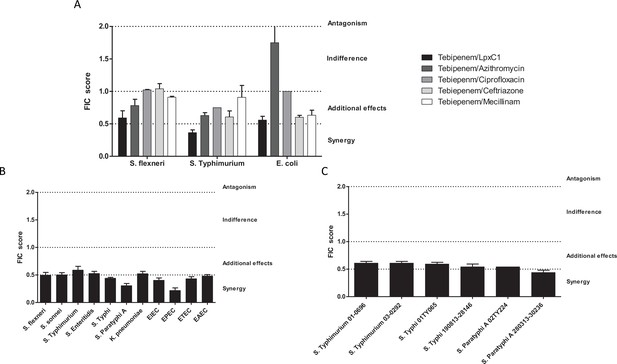
In vitro synergy of Tebipenem with common antimicrobials.
(A) Bar chart showing the average (assays performed in duplicate) fractional Inhibitory Concentration (FIC) index value determining the synergy/antagonism potential of Tebipenem in combination with PF-5081090 (LpxC inhibitor), azithromycin, ciprofloxacin, ceftriaxone, and mecillinam (highlighted) against laboratory isolates of S. flexneri, S. typhimurium, and E. coli (labelled on x axis). The scale of interaction is highlighted on the right. (B) Bar chart showing the average FIC index value to assess the synergy/antagonism potential of Tebipenem in combination with an LpxC inhibitor against a range of clinical isolates of enteric bacteria (labelled on x axis) (assays performed in duplicate). (C) Bar chart showing the average FIC index value to assess the synergy/antagonism potential of Tebipenem in combination with azithromycin against a range of clinical isolates of invasive and non-invasive Salmonella (labelled on x axis) (assays performed in duplicate).
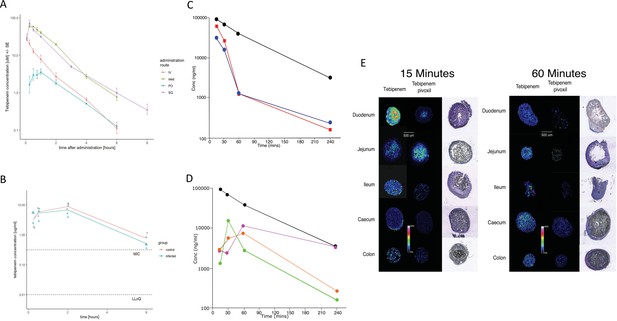
The pharmacodynamics of Tebipenem in blood and the gastrointestinal tract of experimental animals.
(A) The blood pharmacokinetics of Tebipenem in mice (n = 3 per treatment) over 6 hr after the administration of 5 mg/kg intravenous Tebipenem (red), 39 mg/kg subcutaneous Tebipenem (pink), 39 mg/kg oral Tebipenem, and 50 mg/kg oral Tebipenem pivoxil. Data points represent mean ± the standard deviation. Notably, the drug concentration in all preparations remained above the estimated MIC of S. flexneri 2457T (0.39 µM) over the course of the experiment. (B) The pharmacodynamics of Tebipenem in piglets over 6 hr after the administration of 50 mg/kg oral Tebipenem pivoxil in Shigella infected (blue) (n = 4) and uninfected (red) (n = 2) animals. The lower limit of quantitation (LLOQ) and the estimated MIC are shown. (C) Plots showing the concentration (ng/ml) of Tebipenem in the blood (black), duodenum (blue), and jejunum (red), of mice up to 240 min after oral administration of 50 mg/kg Tebipenem pivoxil. (D) Plots showing the concentration (ng/ml) of Tebipenem in the blood (black), ileum (green), caecum (purple), and colon (orange) of mice up to 240 min after oral administration of 50 mg/kg Tebipenem pivoxil. (E) MALDI imaging data showing the localisation of active Tebipenem in different sections of the gastrointestinal tract (duodenum to colon) of mice after oral administration of Tebipenem and Tebipenem pivoxil 15 min (left) and 60 min (right) after administration. Drug intensity scale (0–60%) and size scale are shown. A histology section of each compartment of the gastrointestinal tract is shown to highlight the localisation of active drug in the tissue or in the lumen.
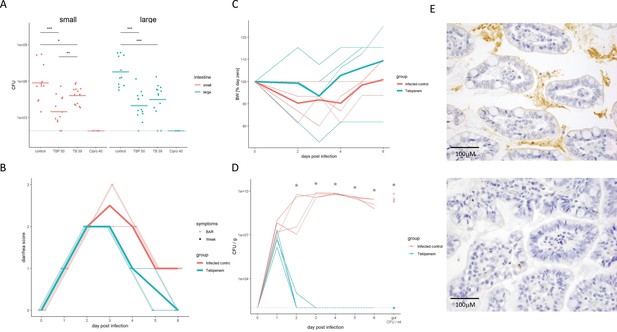
The efficacy of Tebipenem against Shigella in animal infections models.
(A) Measuring the efficacy of 50 mg/kg oral Tebipenem pivoxil and 39 mg/kg oral Tebipenem by measuring CFU of S. flexneri 2457T in the small intestine (left) and large intestine (right) 24 hr after infection with a x107 intraperitoneal dose of S. flexneri 2457T in comparison to no drug and 40 mg/kg ciprofloxacin (10 animals per group). Data shown represent mean ± the standard deviation and were determined to be normally distributed and analysed using t-tests. (B) Plot showing the diarrhoea score of treated (green) and untreated (red) piglets over the 6-day infection. Individual animal profiles are shown by the individual fine lines with heavy lines used to show the corresponding daily group means. The two groups differ statistically (p < 0.001) by AUC. (C) Plot showing the change in weight of the treated (green) and untreated (red) piglets over the six-day infection. The thin lines show individual pigs whilst the thick lines the corresponding averages. There was no significant different in weight loss between the two groups over the course of the experiment. (D) The efficacy of 50 mg/kg oral Tebipenem pivoxil (n = 4 animals, green) given daily for 5 days in reducing the CFU/g of faeces (every 24 hr) after infection with a 1 × 109 oral dose of S. flexneri 2457T compared to no drug (n=4 animals, black) over 6 days. Data shown represents mean ± standard deviation. (E) Sections of the large intestine taken on day six post infection in a gnotobiotic piglet treated with (top) or without (lower) 50 mg/kg oral Tebipenem pivoxil after infection with S. flexneri 2457T observed by Immunohistochemical (IHC) staining (scale shown).
Tables
MIC values of different antimicrobials against Shigella flexneri ATCC 700930.
| Antimicrobial | IC90 (µg/ml) * |
|---|---|
| Ciprofloxacin | 0.020 |
| Mecillinam | 0.058 |
| Azithromycin | 7.49 |
| Ceftriaxone | 0.067 |
| Tebipenem | 0.010 |
-
*
Data shown are the mean of triplicates.
The pharmacokinetics of Tebipenem and Tebipenem pivoxil.
| Value | Mice | Piglets | Paediatric population (Sato et al., 2008) | ||
|---|---|---|---|---|---|
| Tebipenem SQ (n = 3) | Tebipenem PO(n = 3) | Tebipenem-Pivoxil PO(n = 3) | Tebipenem-Pivoxil PO(n = 2) | Tebipenem-Pivoxil PO | |
| Cmax (µg/ml) | 71.0 ± 15.3 | 3.7 ± 0.9 | 61.6 ± 3.9 | 9.0 ± 0.8 | 7.5 ± 3.9 |
| Tmax [Range] (hr) | [0.25 0.25] | [0.5 1] | [0.25 0.5] | [2 2] | 0.7 ± 0.2 |
| AUC0-tlast (hr*µg/mL) | 80.4 ± 7.7 | 7.5 ± 1.5 | 93.8 ± 2.7 | 32.6 ± 3.9 | 16.1 ± 3.3 |
| Bioavailability (%) | 44 ± 4 | 4 ± 1 | 52 ± 1 | ND | ND |
Additional files
-
Supplementary file 1
Antimicrobial susceptibility profile of experimental organisms.
- https://cdn.elifesciences.org/articles/69798/elife-69798-supp1-v3.xlsx
-
Supplementary file 2
Profile and MIC ranges of marketed penem antimicrobials evaluated against S. flexneri EG478 and S. sonnei 02–1181.
- https://cdn.elifesciences.org/articles/69798/elife-69798-supp2-v3.xlsx
-
Supplementary file 3
Rates of spontaneous in vitro resistance for S. flexneri 2457T against a range of antimicrobials.
- https://cdn.elifesciences.org/articles/69798/elife-69798-supp3-v3.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/69798/elife-69798-transrepform1-v3.docx





