Adaptation of the periplasm to maintain spatial constraints essential for cell envelope processes and cell viability
Figures
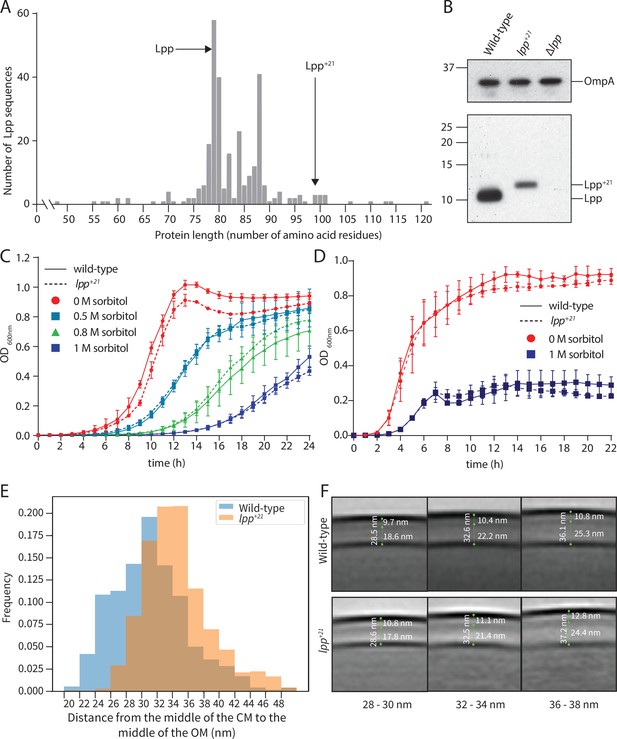
Phenotypes of E. coli cells encoding the Lpp+21 isoform.
(A) Non-redundant Lpp sequences were identified (Materials and methods) and the protein length charted on the x-axis. The number of non-redundant sequences showing that length is shown on the y-axis. The location of Lpp and the lengthened Lpp+21 are indicated. (B) Whole cell lysates were prepared from the indicated strains and subject to SDS-PAGE and immunoblot analysis with anti-Lpp antibodies and anti-OmpA antibodies. OmpA serves as a loading control. (C) The JW5028 – Keio BW25113 strain with kan gene replacing a pseudogene background and isogenic Lpp+21 strain (Figure 1—figure supplement 1) were grown over 24 hr. The growth medium is M9, containing the indicated concentration of sorbitol as an osmolyte. (D) Growth rates for the same strains were measured in rich (LB) growth media with and without sorbitol over 20 hr. (E) The periplasmic width distribution of the indicated strains in hyperosmotic conditions. The histogram depicts the frequency with which a given distance is observed between the OM and PG. (F) Subtomogram averages of cell envelopes in hyperosmotic conditions. Measurements from EM views evaluate the distance between OM and PG in the Lpp+21 strain. While the PG in the wild-type strain is a uniform thin electron dense layer, the PG layer in the Lpp+21 strain is more diffuse and thicker. Each panel represents averages of the subtomogram cell envelope section binned into the sizes shown.
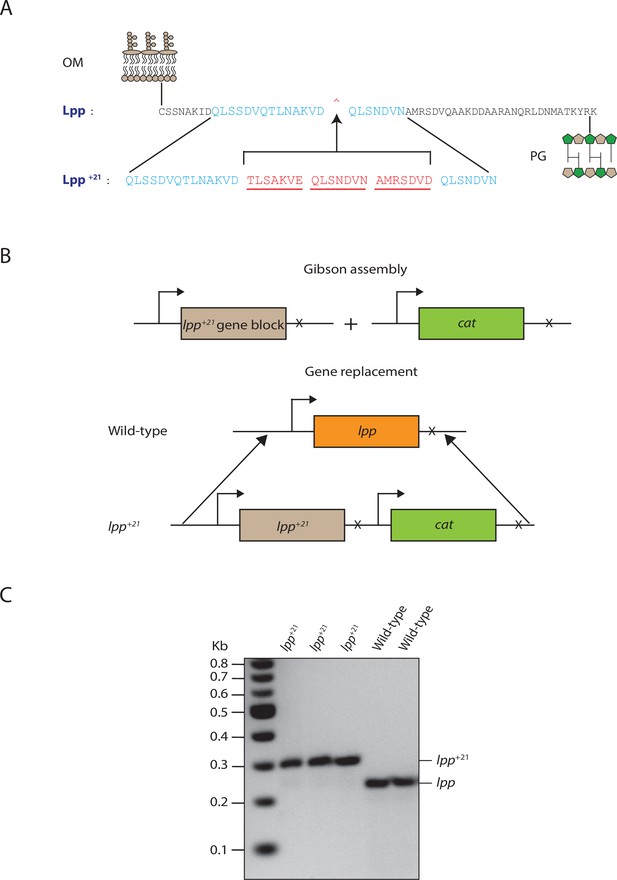
Construction and assessment of Lpp+21 E. coli.
(A) Schematic representation of the elongated Lpp+21 isoform. The strain was constructed by inserting (red lettering) heptad repeats between residue D42 and Q43 of Lpp. (B) Schematic detailing the isogenic replacement of the lpp gene with the lpp+21 gene. (C) PCR confirmation of the lpp+21 mutant strain (Materials and methods).
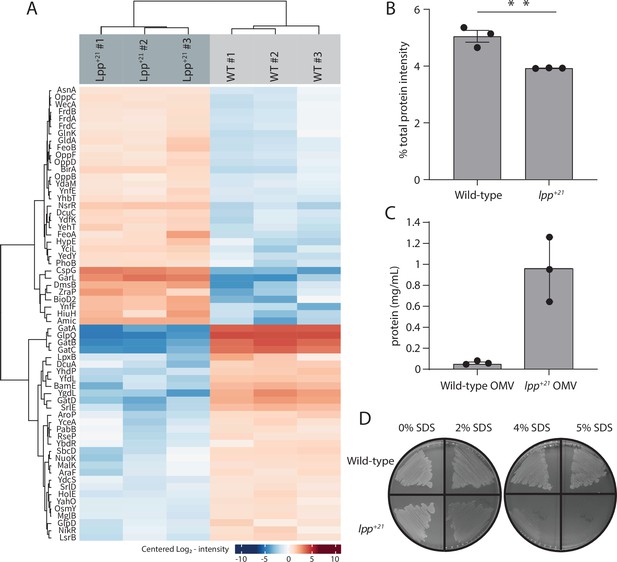
The Lpp+21 cells have altered outer membranes and increased blebbing.
(A) Heat map of the significant proteomic differences observed between the wild-type and Lpp+21 mutant. Blue boxes indicate a relative reduction and red indicates a relative increase in protein level, centred on the average of the replicate samples. The grouping of the proteins is based on the similarity of the change in expression observed. (B) The Lpp+21 mutant has an overall reduction in the level of periplasmic proteins. (C) The Lpp+21 mutant has an increase in protein secreted via OMV blebbing. (D) SDS sensitivity profiles of the Lpp+21 mutant compared to the wild-type in increasing concentration of SDS in LB (solid) media. Representative data are shown from experiments performed in triplicate.
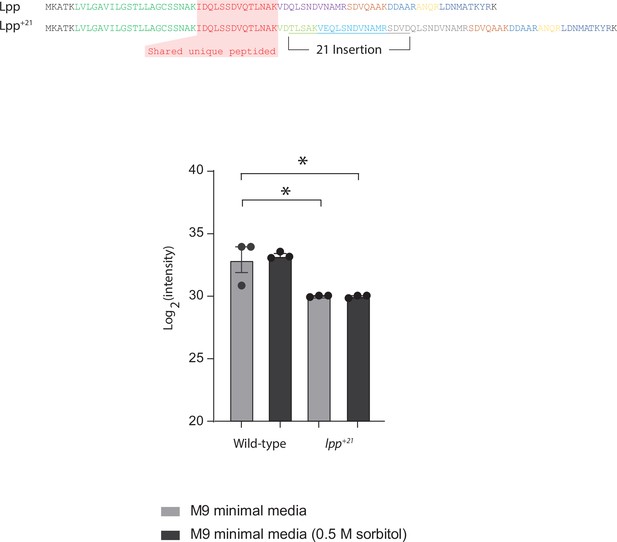
Quantitation of Lpp and Lpp+21 isoforms.
The sequence of the 21 residues inserted to create the Lpp+21 isoform is also indicated. Mass spectrometry data for Lpp vs Lpp+21 was reanalyzed after extraction from the whole cell proteomic data. Given the different tryptic peptides generated from the two isoforms of Lpp, a shared peptide (red) was used to quantify the relative levels of each Lpp isoform in each of the strains. The graphs document the relative levels of the peptide and show that the presence or absence of sorbitol in the growth medium has no effect on the level of Lpp+21 relative to Lpp.
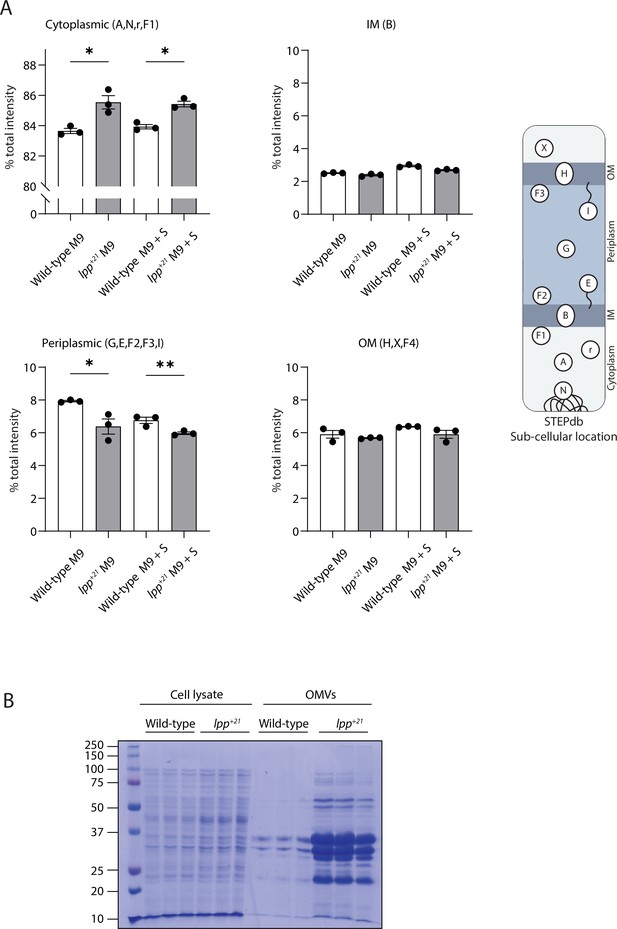
Subcellular proteomics of Lpp+21 E. coli.
(A) Relative amount of protein in different subcellular compartments, measured by the raw relative proportion of peptide intensities identified from proteins annotated to reside in cellular compartments defined in the STEPdb. (B) Comparative proteome profile of the wild-type and Lpp+21 strain for cell lysate and extracted outer membrane vesicles. Loading of each technical replicate was normalized to OD 600. Representative data are shown from experiments performed in biological triplicate.
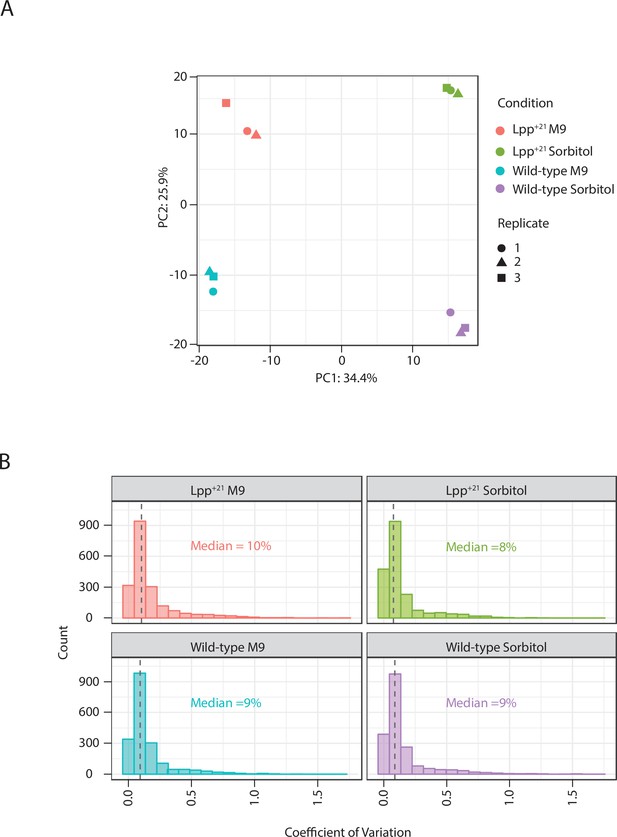
Proteomics quality control report.
(A) Principle Component Analysis (PCA) plot of data from triplicate samples of wild-type and Lpp+21 strains grown on M9 medium with or without supplementation by sorbitol. (B) Sample Coefficient of variation plots for the same four samples.
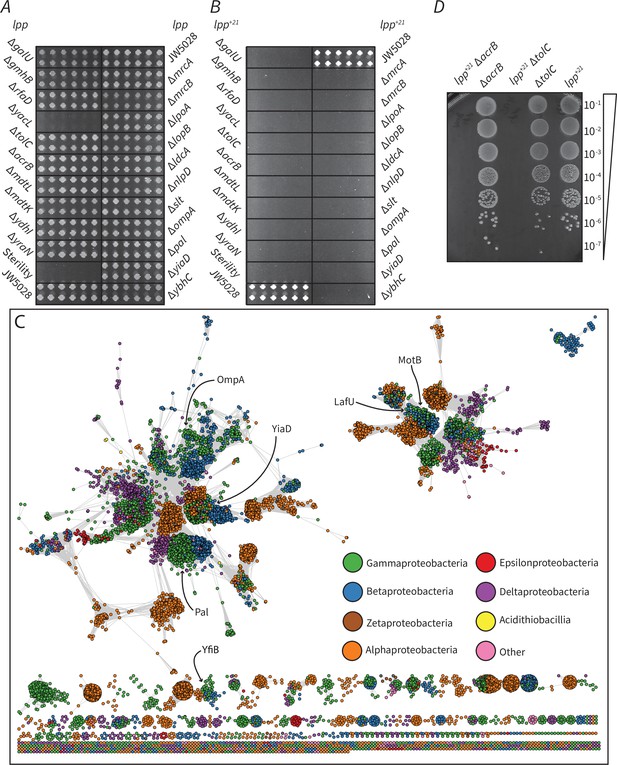
Factors that become essential to mediate OM-PG linkage in Lpp+21 E. coli.
(A) The growth phenotype in M9 minimal media (0.5 M sorbitol) of single gene knock outs that exhibit essentiality on Lpp+21 background (Table 1). (B) The growth phenotype in M9 minimal media (0.5 M sorbitol) of double gene mutants. The mutants are results of Hfr Cavalli lpp:lpp+21 cat crossed with 22 KanR recipients shown in panel A (Materials and methods). The double mutants are indicated and are arranged in four biological replicates (each having four technical replicates). (C) Sequence similarity network of domain (Pfam PF00691) containing proteins from across the Proteobacteria. Each circle represents a protein from a representative proteome (rp35) containing the PF00691 domain, connected by lines with a length imparted by their similarity score as defined by EFI - Enzyme Similarity Tool (Gerlt et al., 2015), with a cutoff of 30. Proteins are colored by their taxonomic class and the approximate location of the E. coli K12 six PF00691 proteins is indicated. (D) Synthetic lethal phenotype of the drug efflux mutants in the absence of selective antibiotics in M9 minimal media condition. Representative data are shown from experiments performed in biological triplicate.
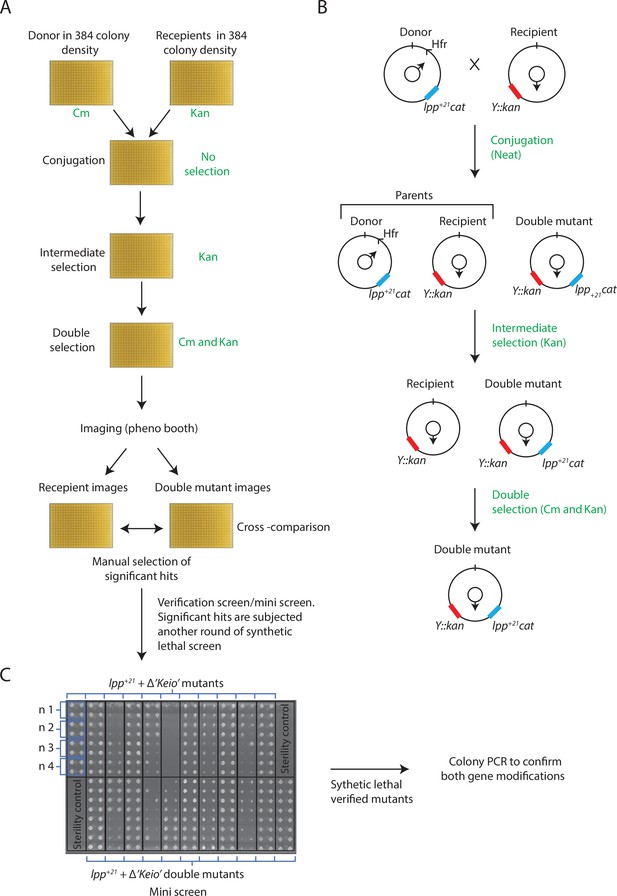
A synthetic lethal screen to determine genes essential to Lpp+21 E. coli.
An Hfr donor strain carrying a selectable marker (cat) fused to lpp+21, replacing the lpp ORF, is mated on agar plates with arrayed F- recipients (384) per plate carrying a selectable marker (kan) replacing other ORF. Upon mating, cells are subjected the first round of selection (intermediate selection) using antibiotic kanamycin and then further subjected to a second round of selection using both antibiotics; (A) depicts images of representative plates generated in each step of the procedure with imaging and manual analysis step, cross-referencing of single gene knock outs and double recombinants, included. (B) Depiction of the strains as cartoons generated in each step of the procedure. (C) A representative mini screen of manually selected genes from the main synthetic lethal screen. Sterility controls were included on each mini screen. The mini screen was performed in 384-pin density with each clone arrayed in four biological replicates, each having four technical replicates (blue boxes). Synthetic lethal mutants identified from the mini screen were further verified by PCR to confirm the presence of both gene modifications and rule out partial duplication events.
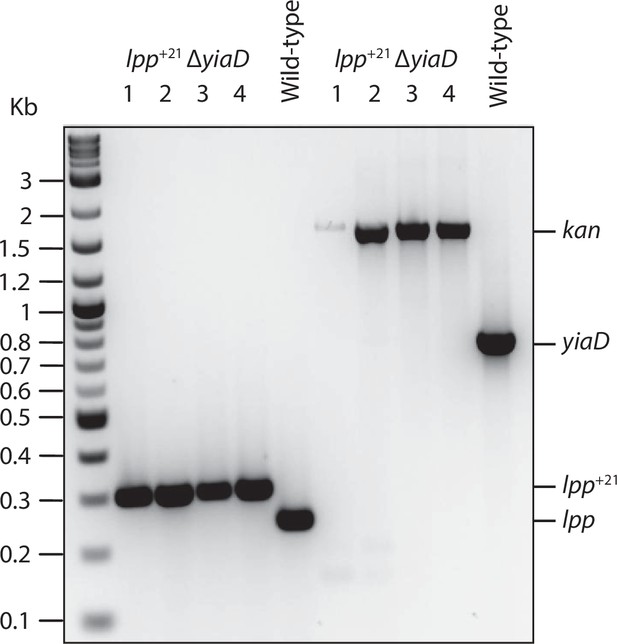
Construction and characterization of the validation yiaD mutant.
A kanamycin resistance cassette was amplified from pKD4 using primers with overhangs complementary to upstream and downstream of yiaD. The PCR fragment was electroporated in BW25113 cells harbouring the λ –red recombineering plasmid (pKD46). Transformants were selected on kanamycin-resistant plates and verified by PCR (methods). Primers flanking the yiaD gene confirm replacement of yiaD with kanamycin cassette and primers amplifying lpp confirm lpp+21 replacement of lpp. The sequence information for all primers used are included in Supplementary file 5.
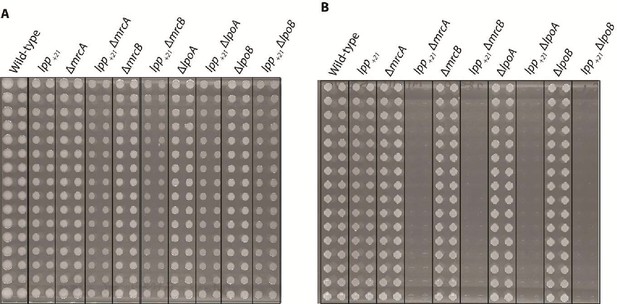
Synthetic lethality of major penicillin-binding proteins and their cognate lipoprotein activators with Lpp +21.
(A) The inidicated E. coli mutants plated on nutrient-rich (LB) media, and (B) mutants plated on in M9 minimal media supplemented with 0.2% (w/v) glucose. Four double recombinants in LB were generated then manually arranged: this provides four replicas for each genetic condition as well as single deletion mutant controls. The double recombinants and controls were then pinned onto both LB and M9 minimal media and their growth analysed. lpp+21 is synthetically lethal with ΔmrcA, ΔmrcB, ΔlpoA, and ΔlpoB in minimal media, unlike in nutrient-rich media. Both the single and the double-gene recombinants were pinned at n = 32 for each independent knock-out.
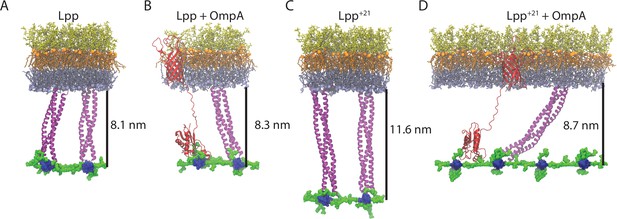
Final states of the OM-PG linkage from MD simulations.
(A–B). A patch of OM with the LPS molecules depicted in orange (lipid A moiety) and yellow (core oligosaccharides), and the phospholipids in the inner leaflet of the OM depicted in gray. The PG layer (blue for glycans and green for peptide crosslinks) is attached to the OM via two trimers of Lpp (A), or a trimer of Lpp and the PG-binding domain of OmpA (red). The β-barrel anchor of OmpA is shown embedded in the OM. (C–D) Equivalent scenarios formed with Lpp+21 trimers. The distances shown are calculated from the inner face of the OM to the centre of the PG layer and represent the average over the last 100 ns of a 200-ns simulation.
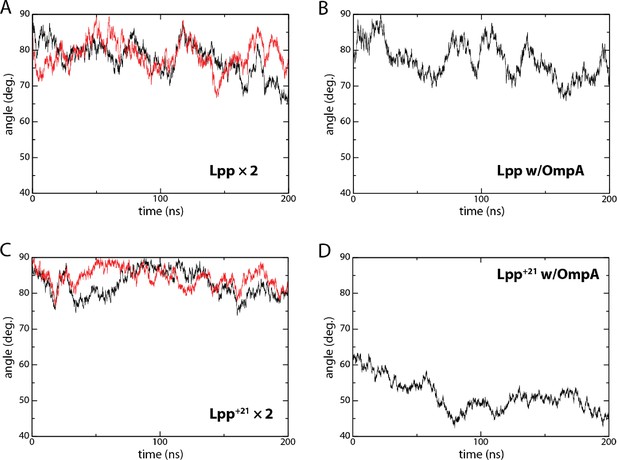
Molecular dynamics plots of Lpp tilt angles over time.
Plots of the angles over time, from which the averages and standard deviations were derived. Simulation were run for 200 ns and average tilt angles from the last 100 ns were calculated. To check for convergence, the Lpp simulation ran for an additional 200 ns. The angle from the last 200 ns is 78.3±4.8°, nearly identical (within 1.4°) to that from the 100–200 ns window.
Tables
Essential genes in the Lpp+21 strain.
| Cellular process | Lpp+21-essential genes | Function |
|---|---|---|
| LPS biosynthesis | galU | UDP-glucose metabolic process |
| gmhB/yaeD | ADP-L-glycero-β-D-manno-heptose biosynthetic process | |
| rfaD/hldD/waaD | ADP-L-glycero-β-D-manno-heptose biosynthetic process | |
| Peptidoglycan biosynthesis, turnover, and remodeling | lpoA, lpoB, | Regulators of PG synthases |
| mrcA, mrcB | PG synthases | |
| ldcA | L,D-carboxypeptidase involved in PG recycling/turnover | |
| nlpD | Regulator of AmiC PG hydrolase | |
| slt | Lytic PG transglycosylase, degradation of uncrosslinked glycan strands | |
| PG-OM linkage | acrB, mdtL*, mdtK*, ydhl* | IM components of drug efflux pumps. |
| tolC | OM component of drug efflux pump | |
| ompA | β-barrel protein with PG binding domain | |
| pal | Lipoprotein with PG binding domain | |
| yiaD | Lipoprotein with PG binding domain |
-
*
Putative IM drug efflux machinery, TolC independent with as yet unknown OM component (Bay et al., 2017).
Additional files
-
Supplementary file 1
Proteomic results.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp1-v2.xlsx
-
Supplementary file 2
Substantive changes in steady-state protein levels in cell envelope of Lpp+21.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp2-v2.docx
-
Supplementary file 3
Proteins used in the generation of the sequence similarity network.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp3-v2.xlsx
-
Supplementary file 4
Representative Lpp protein information.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp4-v2.xlsx
-
Supplementary file 5
Bacterial strains and primers used in the study.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp5-v2.xlsx
-
Supplementary file 6
Genes up-regulated in Lpp +21 strain.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp6-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/73516/elife-73516-transrepform1-v2.pdf
-
Source data 1
Raw gel image files used for the generation of Figure 1, Figure S1, Figure S3, and Figure S5.
- https://cdn.elifesciences.org/articles/73516/elife-73516-supp7-v2.zip





