Correlation between leukocyte phenotypes and prognosis of amyotrophic lateral sclerosis
Figures
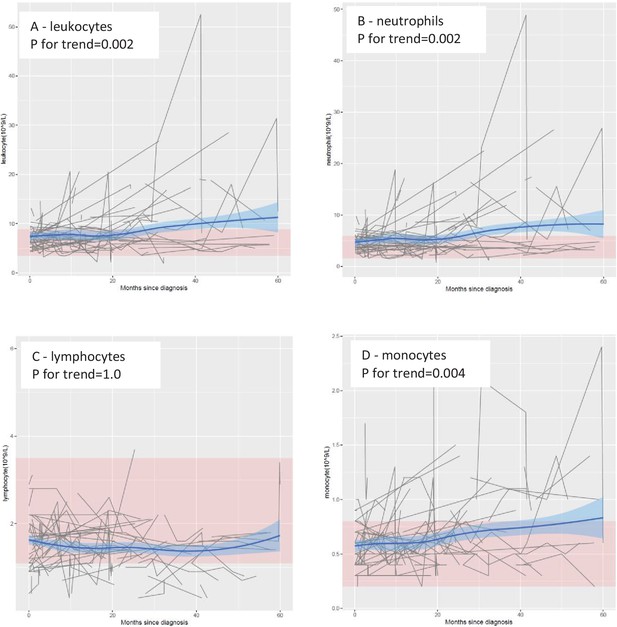
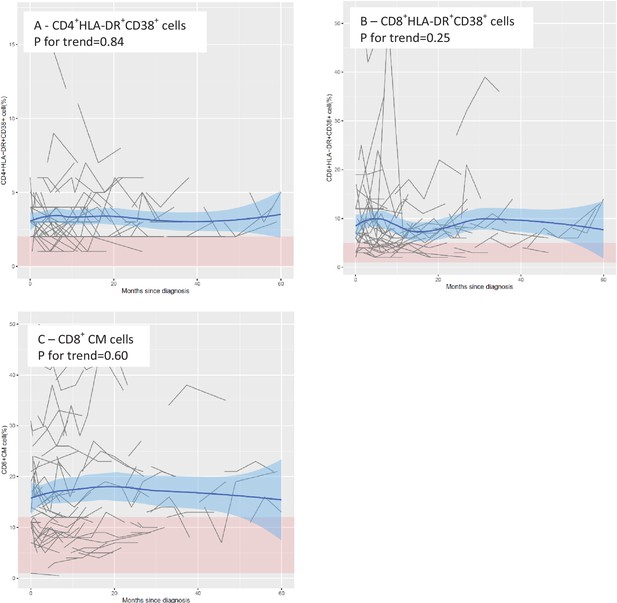
Mean levels of leukocyte populations after a diagnosis of amyotrophic lateral sclerosis (ALS).
The black lines show measured levels of leukocyte populations for each patient. The blue lines and shadow areas show the mean levels of leukocyte populations with 95% confidence intervals. Pink areas indicate normal range. p for trend shows the p value of within-individual temporal change of each cell population after taking into account the relatedness of repeated measurements.
-
Figure 1—source data 1
Levels of leukocyte populations from 3months before diagnosis of amyotrophic lateral sclerosis (ALS) onwards.
- https://cdn.elifesciences.org/articles/74065/elife-74065-fig1-data1-v1.xlsx
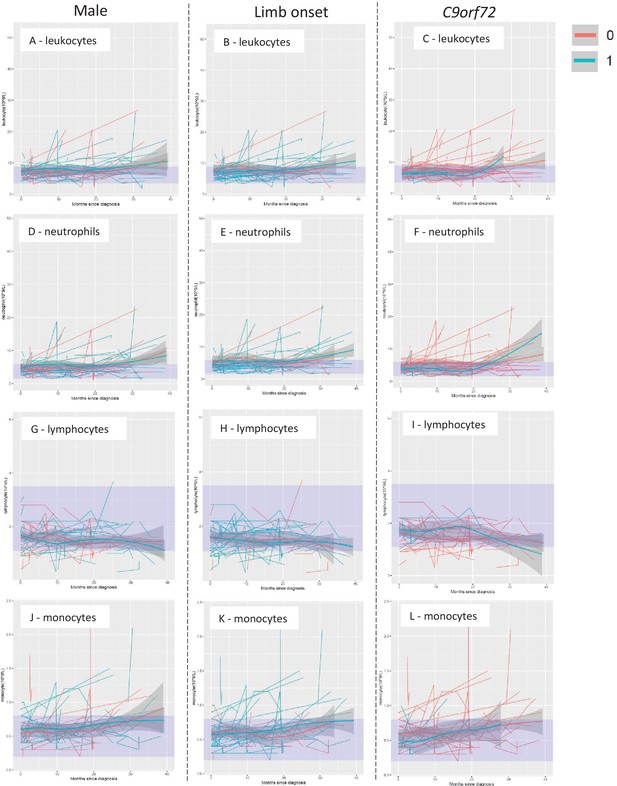
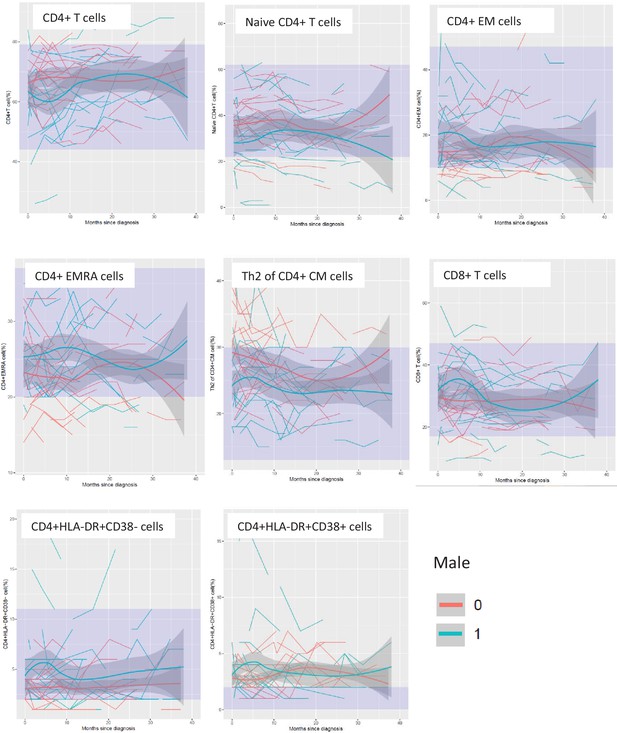
Temporal trend of leukocyte populations by sex, site of onset, and presence of C9orf72 expansions.
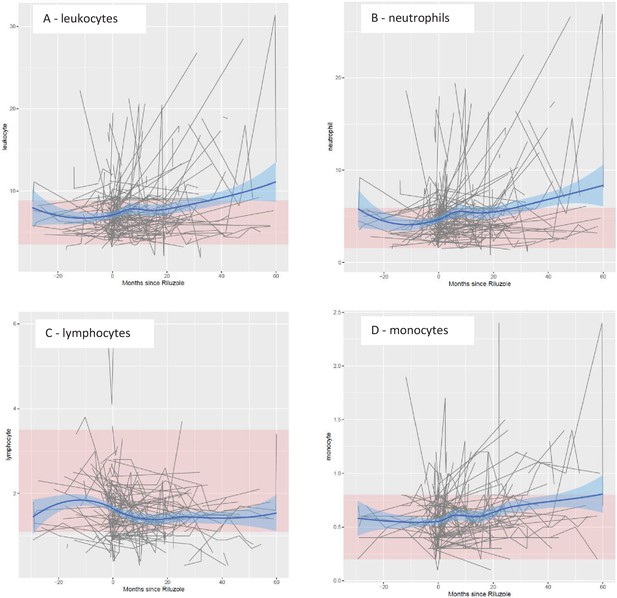
Temporal trend of leukocyte populations before and after Riluzole treatment.
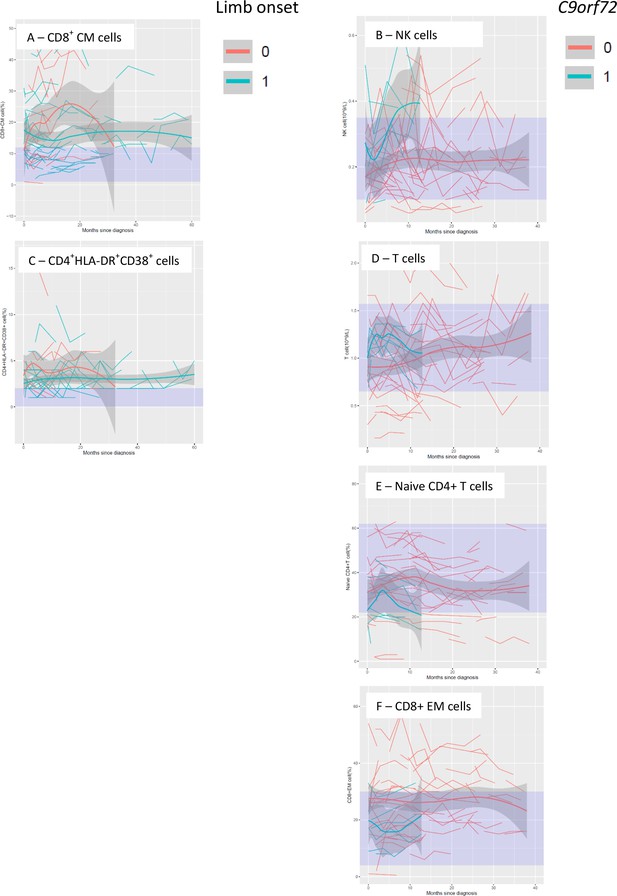
Temporal trend of lymphocyte populations that differed by site of onset and presence of C9orf72 expansions.
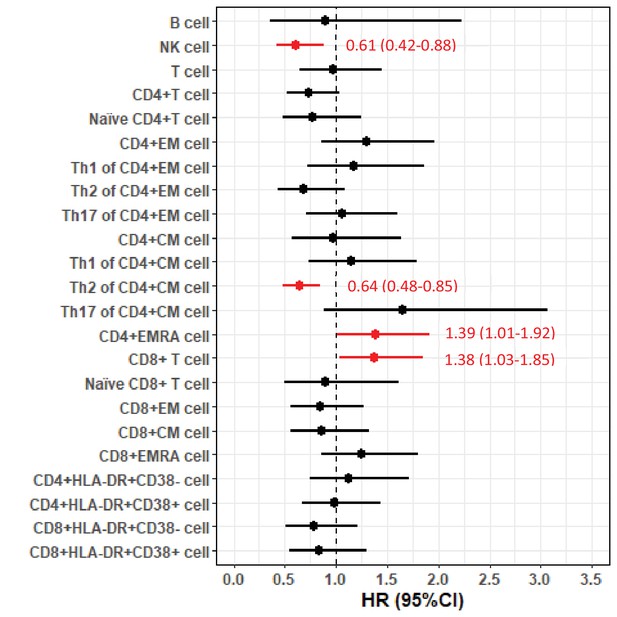
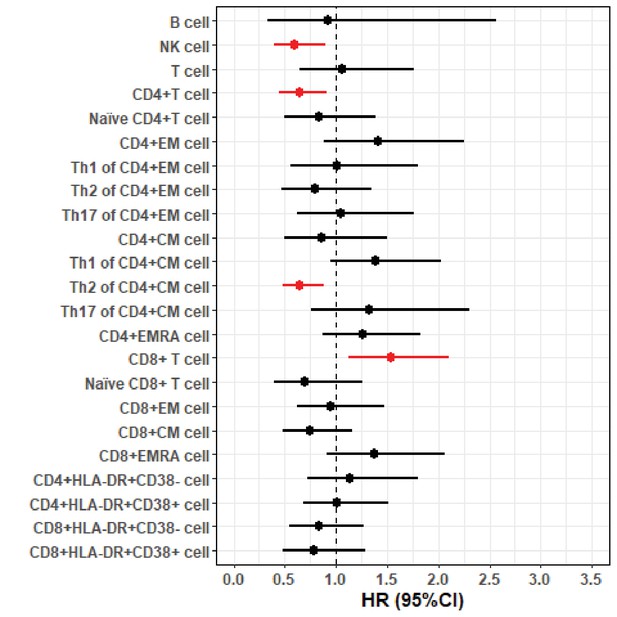
Forest plot of hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of lymphocyte populations with risk of death after a diagnosis of amyotrophic lateral sclerosis (ALS).
-
Figure 2—source data 1
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of lymphocyte populations with risk of death after a diagnosis of amyotrophic lateral sclerosis (ALS).
- https://cdn.elifesciences.org/articles/74065/elife-74065-fig2-data1-v1.xlsx
Tables
Temporal changes of leukocyte populations after diagnosis of amyotrophic lateral sclerosis (ALS), a cohort study of 288 patients with ALS in Stockholm, Sweden†.
| Cell type | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|
| Coefficient | p value | FDR | Coefficient | p value | FDR | |
| Leukocyte (109/l) | 0.19 | 0.01 | 0.01 | 0.22 | 2.4E−03 | 4.7E−03 |
| Neutrophil (109/l) | 0.18 | 3.6E−03 | 0.01 | 0.21 | 1.5E−03 | 4.7E−03 |
| Lymphocyte (109/l) | 3.7E−03 | 0.73 | 0.73 | 4.1E−05 | 1.00 | 1.00 |
| Monocyte (109/l) | 0.01 | 0.03 | 0.04 | 0.01 | 4.2E−03 | 0.01 |
-
Bold values denote statistical significance of p < 0.05.
-
*
Adjusted for age at diagnosis and sex.
-
†
Linear mixed model was applied to derive the coefficient estimates, per year and p value for trend.
-
FDR: false discovery rate.
Cross-sectional correlations between leukocyte populations and amyotrophic lateral sclerosis (ALS) Functional Rating Scale-revised (ALSFRS-R) score and disease progression rate, a cohort study of 288 ALS patients in Stockholm, Sweden*.
| Cell type | ALSFRS-R | Progression rate | ||||
|---|---|---|---|---|---|---|
| Coefficient | p value | FDR | Coefficient | p value | FDR | |
| Leukocyte (109/l) | −2.80 | 4.0E−03 | 0.01 | 0.02 | 0.74 | 0.74 |
| Neutrophil (109/l) | −3.10 | 1.0E−03 | 4.0E−03 | 0.05 | 0.33 | 0.67 |
| Lymphocyte (109/l) | 1.48 | 0.15 | 0.15 | −0.08 | 0.32 | 0.67 |
| Monocyte (109/l) | −2.75 | 2.0E−03 | 4.0E−03 | −0.03 | 0.52 | 0.69 |
-
Bold values denote statistical significance of p < 0.05.
-
*
Generalized estimating equation model was applied to derive the coefficient estimates and p values, with adjustment for age at diagnosis and sex. ALSFRS-R score ranges from 0 to 48, with higher score showing better motor function status. Progression rate indicates the decline of motor function per month.
-
FDR: false discovery rate.
Associations between longitudinal changes in cell measures and longitudinal changes in Amyotrophic Lateral Sclerosis Functional Rating Scale – revised (ALSFRS-R) score, a cohort study of 288 patients with ALS in Stockholm, Sweden†.
| Cell type | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| Coefficient | p value | Coefficient | p value | |
| Leukocyte (109/l) | −5.72 | 0.010 | −5.41 | 0.012 |
| Neutrophil (109/l) | −4.05 | 0.020 | −3.85 | 0.023 |
| Lymphocyte (109/l) | −0.49 | 0.839 | −0.22 | 0.925 |
| Monocyte (109/l) | −12.90 | 0.001 | −12.14 | 0.001 |
-
Bold values denote statistical significance of p < 0.05.
-
*
Adjusted for age at diagnosis and sex.
-
†
Generalized estimating equation model was applied to derive the coefficient estimates and p values, per unit change of log-transformed leukocyte counts.
Characteristics of the 288 patients with amyotrophic lateral sclerosis (ALS) included in the study, compared with the entire population of ALS patients during the study period in Stockholm, Sweden.
| Characteristics | Patients included in the study (N = 288) | All patients in Stockholm (N = 420) | p value for difference* |
|---|---|---|---|
| Sex, N (%) | 0.48 | ||
| Female | 134 (47%) | 201 (48%) | |
| Male | 154 (53%) | 219 (52%) | |
| Age at diagnosis, years | 0.02 | ||
| Median (Q1, Q3) | 65 (56, 71) | 66 (57, 72) | |
| Diagnostic delay, months | 0.94 | ||
| Median (Q1, Q3) | 12.30 (7.88, 19.93) | 12.35 (7.59, 20.54) | |
| Gene mutation, N (%)† | 1.00 | ||
| SOD1 | 7 (2.88%) | 9 (2.56%) | |
| C9orf72 | 22 (9.05%) | 30 (8.55%) | |
| Other | 4 (1.65%) | 5 (1.42%) | |
| Site of onset, N (%) | 0.26 | ||
| Limb | 182 (63%) | 250 (60%) | |
| Bulbar | 78 (27%) | 118 (28%) | |
| Other | 20 (7%) | 32 (8%) | |
| Missing | 8 (3%) | 20 (5%) | |
| Family history, N (%) | 0.19 | ||
| Yes | 19 (7%) | 30 (7%) | |
| No | 144 (50%) | 201 (48%) | |
| Not clear | 3 (1%) | 7 (2%) | |
| Missing | 122 (42%) | 182 (43%) | |
| No. of measurements for cell count (%) | – | ||
| One | 146 (51%) | – | |
| Two | 75 (26%) | – | |
| Three | 35 (12%) | – | |
| Four or more | 32 (11%) | – | |
-
*
p value for the differences between patients included in the study and patients not included in the study; Wilcoxon rank sum test was used for the comparison of continuous variables whereas chi-square test was used for the comparison of categorical variables.
-
†
Results available for 243 of the 288 patients included in the study, and 351 of the entire 420 patients in Stockholm.
Additional files
-
Supplementary file 1
Characteristics of the 92 patients with amyotrophic lateral sclerosis (ALS) included in the analysis of FlowC test, compared with the entire population of ALS patients during the study period in Stockholm, Sweden.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp1-v1.docx
-
Supplementary file 2
Mean levels of leukocyte subpopulations (N = 288 patients) and lymphocyte subpopulations (N = 92 patients) across all measures.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp2-v1.docx
-
Supplementary file 3
Temporal changes of lymphocyte populations after diagnosis of amyotrophic lateral sclerosis (ALS), analysis of 92 patients with FlowC test.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp3-v1.docx
-
Supplementary file 4
Associations of leukocyte populations with the risk of death after a diagnosis of amyotrophic lateral sclerosis (ALS), a cohort study of 288 patients with ALS in Stockholm, Sweden.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp4-v1.docx
-
Supplementary file 5
Sensitivity analyses of the associations of leukocyte populations with risk of death after a diagnosis of amyotrophic lateral sclerosis (ALS), focusing on newly diagnosed ALS patients, first cell measure only, or excluding patients with C9orf72 expansions*.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp5-v1.docx
-
Supplementary file 6
Cross-sectional correlations between lymphocyte populations and Amyotrophic Lateral Sclerosis Functional Rating Scale – revised (ALSFRS-R) score and disease progression rate, a cohort study of 92 ALS patients in Stockholm, Sweden*.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp6-v1.docx
-
Supplementary file 7
Sensitivity analyses of the associations of leukocyte populations with Amyotrophic Lateral Sclerosis Functional Rating Scale – revised (ALSFRS-R) score and disease progression rate, after removing the the blood samples with potential ongoing infection*.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp7-v1.docx
-
Supplementary file 8
Sensitivity analyses of associations of leukocyte populations with the risk of death after a diagnosis of amyotrophic lateral sclerosis (ALS), after removing the blood samples with potential ongoing infection*.
- https://cdn.elifesciences.org/articles/74065/elife-74065-supp8-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/74065/elife-74065-transrepform1-v1.docx
-
Source code 1
Source code for Table 1 and 2.
- https://cdn.elifesciences.org/articles/74065/elife-74065-code1-v1.zip